One- and two-photon luminescence in graphene oxide quantum dots†
Hai-Xing
Zhao
ab,
Yu-Chen
Wang
ab,
Lian-Ying
Zhang
ab and
Min
Wang
*ab
aInstitute for Clean Energy & Advanced Materials, Faculty of Materials and Energy, Southwest University, Chongqing 400715, P. R. China. E-mail: minwang@swu.edu.cn
bChongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, Chongqing 400715, P. R. China
First published on 25th September 2014
Abstract
The synthesized single- and few-layer graphene oxide quantum dots (GOQDs) with an average size of 3 nm can be dissolved in many solvents. Good photoluminescence (PL) properties based on one- and two-photon absorption can be observed. The treatment with acid and alkali can cause blue- and red-shift phenomena in the PL. The solvent and the excitation wavelength can also affect the PL.
Graphene has attracted much interest because of its unique properties.1,2 However, luminescence in graphene has a short lifetime due to the lack of an intrinsic band gap, thus improvements in its luminescent properties are necessary to meet the requirements for many of the practical optical and optoelectronic applications. To extend these luminescence properties, different modifications have been applied, including doping,3,4 defects5 and etching.6,7 Graphene quantum dots (GQDs) are considerable band-gap materials with the sizes smaller than 100 nm. GQDs display good light absorption and one-photon luminescent emissions8–24 for potential photovoltaic applications,25–27 and their non-toxicity and biocompatibility also allow biological applications28 such as cell imaging29 and biosensing.30
Moreover, two-photon luminescent carbon-based nanomaterials, including surface-passivated carbon dots,31 polyethylene glycol linked graphene oxide nanoparticles32 and nitrogen-doped GQDs,33 are beginning to attract increasing interest, since the two-photon properties have great potential for applications in bioimaging with a large tissue penetration depth in living organisms.34,35 Top-down approaches such as cutting graphite materials using hydrothermal,36 electrochemical26,37 and strong acid38 treatments, and bottom-up methods including solution chemistry starting from small organic compounds,25,39 can help to synthesize different sizes and shapes of GQDs and also tune the properties. Meanwhile, graphene oxide (GO) is one of the most investigated graphene materials. Hummers method40 is widely used for the syntheses of GO41,42 and good photoluminescence43 can be observed. In this work, we synthesize graphene oxide quantum dots (GOQDs) and investigate their optical properties including one- and two-photon luminescence and the effects of treatment with acid/alkali, solvent and light at different excitation wavelengths.
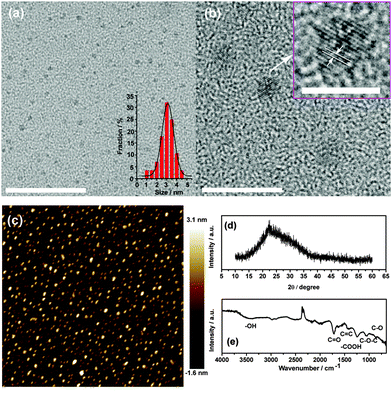
GOQDs are obtained using strong nitric acid treatment of CX-72 carbon black (ESI,† S1). The synthesized GOQDs are dissolved in water and other organic solvents, such as dimethylformamide (DMF) and ethanol. By analyzing the characteristics of the high resolution transmission electron microscopy (HRTEM) images, it can be observed that the synthesized GOQDs are uniform and the average size is about 3 nm (Fig. 1(a) and (b)). The inset of Fig. 1(b) shows that the (002) lattice spacing of the GOQDs is around 0.36 nm. The AFM image shows that the height range is around 0.5–3 nm (Fig. 1(c)). A very broad (002) peak centred at 22.4° was characterized using X-ray powder diffraction (XRD) (Fig. 1(d)) and the interlayer spacing is around 0.39 nm. The sample possesses many oxygen groups, including hydroxyl, epoxy and carboxyl groups, supported by the Fourier transform infrared (FTIR) spectrum (Fig. 1(e)). Thus, the synthesized GOQDs are a mixture of single- and few-layer structures.
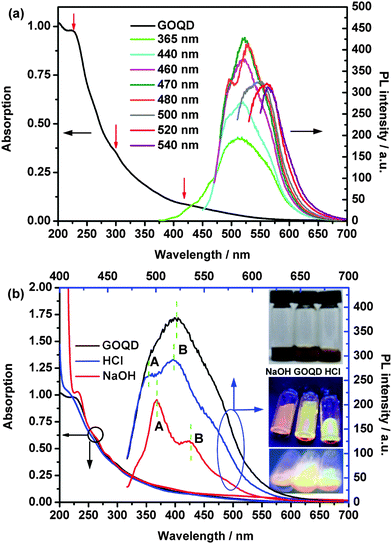
Due to the small and uniform size of the GOQDs, optical properties could be expected. The ultraviolet-visible (UV-vis) absorption and the photoluminescence (PL) were investigated (Fig. 2(a)). The GOQD aqueous solution displays a much broader UV-vis absorption from 200 nm to 700 nm with three shoulders at 228 nm, 300 nm and 420 nm, which are also pointed out by red arrows in Fig. 2(a). When the excitation wavelength is smaller than 470 nm, all PL emission peaks strongly locate around 520 nm. However, with the increase in excitation wavelength (480 nm to 560 nm), a red-shift of the PL peaks can be observed in the range of 527 to 565 nm. The excitation-dependent PL was extensively reported in fluorescent nano-sized carbon materials including GQDs and was considered to maybe come from the optical selection of quantum sizes and defects in the GOQDs.29,38,44 The PL quantum yield (QY)29 of the GOQD aqueous solution was measured to be 15% with an excitation wavelength of 470 nm, using fluorescein in 0.1 M NaOH (QY = 0.79) as a reference. The measured values of QY at other different excitation wavelengths (440–500 nm) are smaller and in the range of 9–14%. Meanwhile, lifetime data (τ = 1.8 ns) can be obtained by the exponential fitting of the decay curve (see Fig. S6, ESI†), which is obtained by monitoring the emission at 550 nm upon excitation at 488 nm. Moreover, the PL peaks of the GOQDs shift in DMF and ethanol (Fig. S4, ESI†), demonstrating a solvent effect induced by the attachment of solvent.44 This PL phenomenon indicates a possible application for optoelectronic devices.
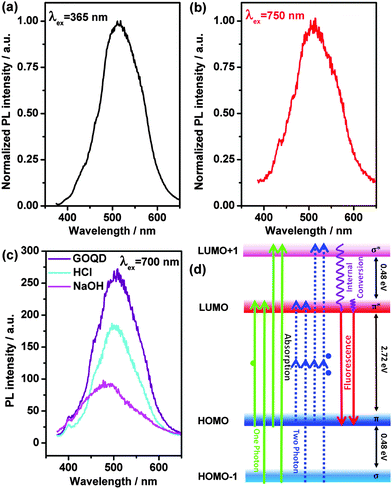
The photoluminescence excitation (PLE) spectra of the GOQDs (λem = 520 nm) are shown in Fig. S5 (ESI†). The PLE spectra show one strong transition peak at 456 nm and three shoulders at 388 nm, 337 nm and 282 nm, marked by green arrows in Fig. S5 (ESI†). The electronic transition of 456 nm (2.72 eV) in the PLE spectra can be considered as a transition from the π orbital (highest occupied molecular orbital, HOMO) to π* (lowest unoccupied molecular orbital, LUMO), as shown in Fig. 3(d). The electronic transition of 388 nm (3.20 eV) can be considered as a transition from the σ orbital (HOMO − 1) to π* (LUMO) or from the π orbital (HOMO) to σ* (LUMO + 1). Meanwhile, the transition of 337 nm (3.68 eV) can be considered as a transition from the σ orbital (HOMO − 1) to σ* (LUMO + 1). The transition of 282 nm is not plotted in Fig. 3(d), since its intensity in the PLE spectra is much smaller.
The optical properties of the GOQDs are influenced by the addition of acid or alkali. At visible light, the HCl GOQD solution has nearly the same red-orange colour as that of the neutral one, but the NaOH GOQD solution becomes darker (inset of Fig. 2(b)). The UV-vis absorption spectra of the HCl and NaOH GOQD solutions are slightly blue- and red-shifted, respectively (Fig. 2(a)), indicating that the band gap of the former is enlarged and that of the latter is reduced. With an excitation of 365 nm, the solutions exhibit light-red, light-yellow, and light-green colours for the NaOH, neutral and HCl GOQD solutions, respectively (inset of Fig. 2(b)), revealing the same blue- and red-shift phenomena. The electron transitions of the PLE spectra for the HCl and NaOH GOQD solutions (Fig. S5, ESI†) exhibit similar phenomena. The blue-/red-shift observations demonstrate that introducing or removing a proton from an oxygen group of the GOQDs affects the band gaps, resulting in a colour shift. First-principle calculations confirm this effect on the band gaps (ESI,† S5), and, moreover, the calculations reveal that the orbital distribution could also be affected (Fig. S7, ESI†). During the preparation of our work, Kozawa et al.45 reported similar one-photon luminescence observations in graphene oxides, originated from nanodisc states, and also demonstrated the effect of protonation and deprotonation experimentally and theoretically.
Moreover, two PL peaks can be seen in both HCl and NaOH curves, compared to only one large PL peak for the neutral GOQD solution. Additionally, the intensity of peak A is lower than that of peak B in the HCl PL curve, while peak A of the NaOH PL curve possesses a higher intensity than peak B. The phenomenon can be understood using the PLE spectra (Fig. S5, ESI†), due to the quite different locations of the PLE peaks and their intensities for the HCl and NaOH GOQD solutions.
Additionally, the PL intensities are reduced with an increase in the amount of acid or alkali, but the peaks do not move (Fig. 2 and Fig. S2, ESI†). Based on our theoretical calculations (ESI,† S5), it is noticeable that with the decrease in protonation of the GOQDs, the electronic properties predominantly originate from the oxygen region rather than the carbon sp2 network (Fig. S7, ESI†). Thus, the PL intensity is influenced. Meanwhile, the quantum size effect,29 zigzag sites36,38 and surface defects29 are all considered to influence the intensity, which can also not be ignored.
Importantly, luminescence can also be observed using light with excitation energies lower than the band gaps (Fig. 3(b) and (c)). The phenomenon is attributed to the unusually strong two-photon absorption. A schematic diagram is plotted in Fig. 3(d). An emission at λ2PL ≈ 513 nm, with an excitation light with λex = 750 nm, can be observed (Fig. 3(b)), where the peak shape and position are similar to those obtained by illuminating at λex = 365 nm (Fig. 3(a)). The emission curves for the excitation wavelengths of 650 nm and 700 nm are also quite similar to the latter ones (Fig. S3, ESI†). A schematic diagram for the one- and two-photon processes is illustrated in Fig. 3(d). Additionally, with the treatment of acid and alkali, the two-photon luminescence intensities are lower than for the neutral one (Fig. 3(c)). With the addition of alkali, a blue-shift two-photon luminescence can be observed, which is different from the red-shift phenomenon of the one-photon process, while the addition of acid does not result in any obvious shift. Moreover, it is noticeable that the two-photon luminescence (Fig. 3(b)) has the same order as the one-photon process (Fig. 3(a)), in comparison to the reported carbon-based QDs possessing much lower intensities for the two-photon one.46
In summary, the synthesized uniform GOQDs have an average size of 3 nm and can be dissolved in several solvents. The GOQD aqueous solution displays a broad UV-vis absorption and good one- and two-photon luminescence properties. The treatment with acid and alkali can cause blue- and red-shift phenomena for the optical properties. Based on the analysis and theoretical calculations, the protonated and deprotonated oxygen groups can affect the GOQDs' band gaps and the orbital distribution, resulting in blue- and red-shift optical phenomena. The solvent and the excitation wavelength can also affect the PL. Due to good luminescence properties, including a high PL quantum yield and a nanosecond PL lifetime, GOQDs are demonstrated to be good candidates for bioimaging and biosensing applications. Moreover, the two-photon process can potentially lead to future applications in deep-tissue imaging.
Acknowledgements
This work is supported by the Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies (Chongqing, China), the Chongqing Science and Technology Commission under cstc2012gjhz90002 (Chongqing, China), the National Natural Science Foundation of China (Grant No. 21203154 and 21375108) and the Fundamental Research Funds for the Central Universities (Grant No. XDJK2013C041). Computation resources are supported by the Faculty of Materials and Energy (Southwest University).Notes and references
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- L. T. Qu, Y. Liu, J. B. Baek and L. M. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed.
- M. Wang and C. M. Li, Phys. Chem. Chem. Phys., 2013, 15, 3786 RSC.
- F. Banhart, J. Kotakoski and A. V. Krasheninnikov, ACS Nano, 2011, 5, 26 CrossRef CAS PubMed.
- R. Yang, L. C. Zhang, Y. Wang, Z. W. Shi, D. X. Shi, H. J. Gao, E. G. Wang and G. Y. Zhang, Adv. Mater., 2010, 22, 4014 CrossRef CAS PubMed.
- M. Wang and C. M. Li, Nanoscale, 2012, 4, 1044 RSC.
- L. L. Li, J. Ji, R. Fei, C. Z. Wang, Q. Lu, J. R. Zhang, L. P. Jiang and J. J. Zhu, Adv. Funct. Mater., 2012, 22, 2971 CrossRef CAS.
- K. Lingam, R. Podila, H. J. Qian, S. Serkiz and A. M. Rao, Adv. Funct. Mater., 2013, 23, 5062 CrossRef CAS.
- S. J. Zhu, J. H. Zhang, S. J. Tang, C. Y. Qiao, L. Wang, H. Y. Wang, X. Liu, B. Li, Y. F. Li, W. L. Yu, X. F. Wang, H. C. Sun and B. Yang, Adv. Funct. Mater., 2012, 22, 4732 CrossRef CAS.
- H. Ding, L. W. Cheng, Y. Y. Ma, J. L. Kong and H. M. Xiong, New J. Chem., 2013, 37, 2515 RSC.
- Z. Ma, H. Ming, H. Huang, Y. Liu and Z. H. Kang, New J. Chem., 2012, 36, 861 RSC.
- J. H. Shen, Y. H. Zhu, X. L. Yang, J. Zong, J. M. Zhang and C. Z. Li, New J. Chem., 2012, 36, 97 RSC.
- H. Huang, Y. Xu, C. J. Tang, J. R. Chen, A. J. Wang and J. J. Feng, New J. Chem., 2014, 38, 784 RSC.
- Y. Han, H. Huang, H. Zhang, Y. Liu, X. Han, R. Liu, H. Li and Z. Kang, ACS Catal., 2014, 4, 781 CrossRef CAS.
- R. Liu, H. Huang, H. Li, Y. Liu, J. Zhong, Y. Li, S. Zhang and Z. Kang, ACS Catal., 2014, 4, 328 CrossRef CAS.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S.-T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430 CrossRef CAS PubMed.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S.-T. Lee and Z. Kang, Carbon, 2011, 49, 605 CrossRef CAS PubMed.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526 RSC.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- H. Li, X. He, Y. Liu, H. Yu, Z. Kang and S.-T. Lee, Mater. Res. Bull., 2011, 46, 147 CrossRef CAS PubMed.
- H. Li, R. Liu, W. Kong, J. Liu, Y. Liu, L. Zhou, X. Zhang, S.-T. Lee and Z. Kang, Nanoscale, 2014, 6, 867 RSC.
- H. Li, R. Liu, S. Lian, Y. Liu, H. Huang and Z. Kang, Nanoscale, 2013, 5, 3289 RSC.
- Z. Ma, H. Ming, H. Huang, Y. Liu and Z. Kang, New J. Chem., 2012, 36, 861 RSC.
- X. Yan, X. Cui, B. S. Li and L. S. Li, Nano Lett., 2010, 10, 1869 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Q. Shi, L. E. Deng, Y. B. Hou and L. T. Qu, Adv. Mater., 2011, 23, 776 CrossRef CAS PubMed.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960 CrossRef CAS PubMed.
- J. H. Shen, Y. H. Zhu, X. L. Yang and C. Z. Li, Chem. Commun., 2012, 48, 3686 RSC.
- S. J. Zhu, J. H. Zhang, C. Y. Qiao, S. J. Tang, Y. F. Li, W. J. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. N. Gao, H. T. Wei, H. Zhang, H. C. Sun and B. Yang, Chem. Commun., 2011, 47, 6858 RSC.
- H. J. Jiang, Small, 2011, 7, 2413 CAS.
- L. Cao, X. Wang, M. J. Meziani, F. S. Lu, H. F. Wang, P. J. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS PubMed.
- J. L. Li, H. C. Bao, X. L. Hou, L. Sun, X. G. Wang and M. Gu, Angew. Chem., Int. Ed., 2012, 51, 1830 CrossRef CAS PubMed.
- Q. Liu, B. D. Guo, Z. Y. Rao, B. H. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436 CrossRef CAS PubMed.
- F. Helmchen and W. Denk, Nat. Methods, 2005, 2, 932 CrossRef CAS PubMed.
- D. Kobat, M. E. Durst, N. Nishimura, A. W. Wong, C. B. Schaffer and C. Xu, Opt. Express, 2009, 17, 13354 CrossRef.
- D. Y. Pan, J. C. Zhang, Z. Li and M. H. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed.
- L. Bao, Z. L. Zhang, Z. Q. Tian, L. Zhang, C. Liu, Y. Lin, B. P. Qi and D. W. Pang, Adv. Mater., 2011, 23, 5801 CrossRef CAS PubMed.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. H. Ge, L. Song, L. B. Alemany, X. B. Zhan, G. H. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844 CrossRef CAS PubMed.
- X. Yan, X. Cui and L. S. Li, J. Am. Chem. Soc., 2010, 132, 5944 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- K. P. Loh, Q. L. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015 CrossRef CAS PubMed.
- D. Y. Pan, J. C. Zhang, Z. Li, C. Wu, X. M. Yan and M. H. Wu, Chem. Commun., 2010, 46, 3681 RSC.
- D. Kozawa, X. Zhu, Y. Miyauchi, S. Mouri, M. Ichida, H. B. Su and K. Matsuda, J. Phys. Chem. Lett., 2014, 5, 1754 CrossRef CAS.
- S. J. Zhuo, M. W. Shao and S. T. Lee, ACS Nano, 2012, 6, 1059 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The detailed experimental synthesis, characterization, PL in different solvents, PL with different amounts of acid and alkali, decay curve, PLE, two-photon processes and the theoretical calculations and analysis. See DOI: 10.1039/c4nj01104j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |