Tetragonal tungsten bronzes Nb8−xW9+xO47−δ: optimization strategies and transport properties of a new n-type thermoelectric oxide†
Christophe P.
Heinrich‡
ab,
Matthias
Schrade‡
c,
Giacomo
Cerretti
a,
Ingo
Lieberwirth
d,
Patrick
Leidich
a,
Andreas
Schmitz
e,
Harald
Fjeld
c,
Eckhard
Mueller
ef,
Terje G.
Finstad
c,
Truls
Norby
c and
Wolfgang
Tremel
*a
aInstitut für Anorganische Chemie und Analytische Chemie der Johannes Gutenberg-Universität, Duesbergweg 10-14, D-55099 Mainz, Germany. E-mail: tremel@uni-mainz.de
bGraduate School Materials Science in Mainz, Johannes Gutenberg-Universität, Staudingerweg 9, D-55128 Mainz, Germany
cCentre for Materials Science and Nanotechnology, University of Oslo, FERMiO, Gaustadalléen 21, NO-0349 Oslo, Norway
dMax Planck-Institut für Polymerforschung, Ackermannweg 10, D-55128 Mainz, Germany
eCentre for Materials Science and Nanotechnology, Department of Chemistry, University of Oslo, FERMiO, Gaustadalléen 21, NO-0349 Oslo, Norway
fJustus Liebig University Giessen, Institute of Materials Research, German Aerospace Center (DLR), Inorganic and Analytical Chemistry, Heinrich-Buff-Ring 58, D-51170 Cologne, 35392 Giessen, Germany
First published on 3rd July 2015
Abstract
Engineering of nanoscaled structures may help controlling the electrical and thermal transport in solids, in particular for thermoelectric applications that require the combination of low thermal conductivity and low electrical resistivity. The tetragonal tungsten bronzes Nb8−xW9+xO47 (TTB) allow a continuous variation of the charge carrier concentration while fulfilling at the same time the concept of a “phonon-glass electron-crystal” through a layered nanostructure defined by intrinsic crystallographic shear planes. The thermoelectric properties of the tetragonal tungsten bronzes Nb8−xW9+xO47−δ (0 < x < 2) were studied in the temperature range from 373 to 973 K. Structural defects and the thermal stability under various oxygen partial pressure pO2 were investigated by means of thermogravimetry, HR-TEM, and XRD. Nb8W9O47−δ was found stable at 973 K and a pO2 of ≈10−15 atm. The oxygen nonstoichiometry δ can reach up to 0.3, depending on the applied atmosphere. By increasing the substitution level x, the electrical resistivity ρ and the Seebeck coefficient S decreased. For x = 2, ρ reached 20 mΩ cm at 973 K, combined with a Seebeck coefficient of approximately −120 μV K−1. The thermal conductivity was low for all samples, ranging from 1.6 to 2.0 W K−1 m−1, attributed to the complex crystal structure. The best thermoelectric figure of merit zT of the investigated samples was 0.043, obtained for x = 2 at 973 K, but it is expected to increase significantly upon a further increase of x. The control of the oxygen non-stoichiometry δ opens a second independent optimization strategy for tetragonal tungsten bronzes.
Conceptual insightsThermoelectric materials have the potential to reduce energy currently lost in the form of waste heat. However, to make widespread implementation possible, improvements to both the efficiency and toxicity of thermoelectric materials are necessary. Oxides are promising materials for thermoelectric applications because they are composed of environmentally benign elements and chemically stable at high temperature. However, most metal oxides are inferior to thermoelectric alloys because they display low carrier mobilities. Even more importantly, large lattice energies and the small atomic mass of oxygen lead to a high velocity of the elastic waves and therefore to a high lattice thermal conductivity. These inherent characteristics of metal oxides seem to preclude their use as thermoelectrics. Here we show that tetragonal tungsten bronzes Nb8−xW9+xO47 (TTB) conceptually allow a continuous variation of the charge carrier concentration while fulfilling at the same time the concept of a “phonon-glas electron-crystal” through a layered nanostructure defined by intrinsic crystallographic shear planes. |
Introduction
Solid state energy conversion between heat and electricity using thermoelectric effects is attractive for waste heat recovery and environment friendly refrigeration. The efficiency of a thermoelectric generator scales with the dimensionless figure of merit zT = (S2/ρκ)T of the employed materials, where S is the Seebeck coefficient, ρ the electrical resistivity, κ the total thermal conductivity, and T the absolute temperature. Good thermoelectric materials behave as electron crystals and phonon glasses.1,2 However, such materials are rare in nature and difficult to engineer in the laboratory.Oxides exhibit great thermoelectric potential due to their abundance, high chemical and physical stability and low toxicity. However, oxide thermoelectrics exhibit in general lower zT values than competing non-oxide materials.3,4 In thermoelectric generators (TEGs) both n-type and p-type thermoelectric materials must be combined. Among the oxides, the highest zT values have been reported for layered cobaltates (p-type),5–8 whereas different materials have been suggested as n-type thermoelectrics. Among the best performing n-type materials so far most are wide band-gap semiconductors like ZnO,9,10 In2O310,11 or SrTiO312 with power factors comparable to those of non-oxide state of the art materials. However, their high crystallographic symmetry and small unit cells lead to relatively high thermal conductivity, which seriously limits their zT values.9,12,13
Hicks and Dresselhaus proposed the possibility to enhance zT by nanostructuring,14–19 the zT enhancement being caused by low thermal conductivity due to phonon scattering at interfaces.1,20 Nanoscaled materials with grain sizes below the phonon mean free path can effectively lower the thermal conductivity.1,19–21 Still, many experimental efforts failed because the small grains also reduced the power factor S2/ρ. In addition, the resulting materials are usually unstable after long-term operation at the targeted high application temperatures.22
Therefore, materials containing inherent phonon scattering centers at the atomic level have attracted scientific attention recently. Strategies include the generation of point defects23 such as cation vacancies,24 or isovalent doping by heavy elements to increase mass and strain contrast.25 In analogy to scattering at disordered interfaces (e.g. grain boundaries), phonons can efficiently be scattered at ordered interfaces e.g. in misfit-layered materials26–32 or materials with crystallographic shear (CS) planes.33 For n-type materials, this concept has recently been demonstrated for Magnéli phases of the oxides of titanium34–36 and tungsten.37,38 Upon reduction of WO3 to WnO3n−1 and WnO3n−2 the crystal structure changes on the nanoscale from a small (ReO3 type) to a large unit cell with well defined {102} and {103} crystallographic shear planes.39 In addition, chemical reduction is accompanied by an increase of the charge carrier concentration. However, as only specific compositions (i.e. reduction levels WO2.95, WO2.90, WO2.80, WO2.72) are stable, Magnéli phases do not allow for a simultaneous and independent optimization of the electronic and phononic transport properties, which is required for tailoring thermoelectric materials. The tetragonal tungsten bronzes Nb8W9O47 (TTB) overcome this limitation by combining the structurally complex character of the Magnéli phases with the possibility to independently vary the charge carrier concentration in a continuous manner. TTBs are structurally related to the Magnéli phases, but the cation composition and thereby the charge carrier concentration can be varied over a broad range without significant structural changes.42 High power factors have been reported for the niobium bronze SrxBa1−xNb2O6−δ,40 and recently it was pointed out that the TTB family might allow for a substantial improvement of the thermoelectric properties of some representatives.41
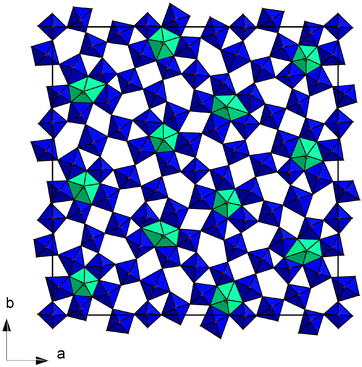
Therefore, the TTBs Nb8−xW9+xO47, which crystallize in the orthogonal space group Pbam, are a highly relevant target. The structure of the complete series was investigated previously by HR-TEM and electron diffraction by Krumeich et al.42–45 The so called 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 phase46 (Nb8W9O47 = 4 × Nb2O5 + 9 × WO3) and the complete series of Nb8−xW9+xO47, with x ranging from 0 to 5, crystallize in a threefold superstructure (lattice parameters a = 36.570 Å; b = 36.690 Å, c = 3.945 Å) of the basic TTB structure (Fig. 1).47,48 The crystal structure contains corner sharing octahedra with pentagonal tunnels. Due to the very large unit cell (Z = 6; ≈5300 Å3) low values of the lattice thermal conductivity are to be expected. Starting from the composition Nb8W9O47 with Nb and W in their highest oxidation states, substitution of Nb5+ by W6+ is formally compensated by a reduction of the cation valence, assuming that the oxygen content of the lattice remains unaltered. Using the Kröger–Vink notation,49 the substitution can be written as
9 phase46 (Nb8W9O47 = 4 × Nb2O5 + 9 × WO3) and the complete series of Nb8−xW9+xO47, with x ranging from 0 to 5, crystallize in a threefold superstructure (lattice parameters a = 36.570 Å; b = 36.690 Å, c = 3.945 Å) of the basic TTB structure (Fig. 1).47,48 The crystal structure contains corner sharing octahedra with pentagonal tunnels. Due to the very large unit cell (Z = 6; ≈5300 Å3) low values of the lattice thermal conductivity are to be expected. Starting from the composition Nb8W9O47 with Nb and W in their highest oxidation states, substitution of Nb5+ by W6+ is formally compensated by a reduction of the cation valence, assuming that the oxygen content of the lattice remains unaltered. Using the Kröger–Vink notation,49 the substitution can be written as
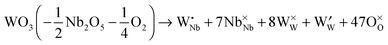 | (1) |
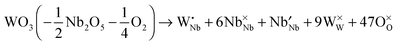 | (2) |
In both cases, the amount of transition metal ions in a lower oxidation state as compared to the pristine structure (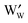 or
or 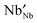 ) increases with increasing x, which corresponds to an increase in the electronic charge carrier concentration. In the following, we will for simplicity refer to electrons e′, without specification of the reduced cation. The tetragonal tungsten bronzes Nb8−xW9+xO47 with 0 < x < 5 thereby allow for the manipulation of the carrier concentration over a wide range and electronic optimization independent of the phononic properties.
) increases with increasing x, which corresponds to an increase in the electronic charge carrier concentration. In the following, we will for simplicity refer to electrons e′, without specification of the reduced cation. The tetragonal tungsten bronzes Nb8−xW9+xO47 with 0 < x < 5 thereby allow for the manipulation of the carrier concentration over a wide range and electronic optimization independent of the phononic properties.
In addition, similar to the scenario of the Magnéli phases or other TTBs50,51 the formation of oxygen vacancies may lead to both the generation of additional charge carriers according to
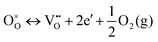 | (3) |
In this paper, we show the potential of tetragonal tungsten bronzes as thermoelectric materials: the materials synthesis and powder processing, the structural characterization, the thermal stability, the control of oxygen vacancy concentration, and the thermoelectric properties of Nb8−xW9+xO47−δ. Further, we outline the potential of the different thermoelectric optimization strategies for the TTB family.
Experimental
Synthesis
Bulk samples of polycrystalline Nb8−xW9+xO47 with compositions x = 0, 0.075, 0.1, 1, 2 were prepared by solid state reactions using powders of NbO2 (Alfa Aesar, 99+%), Nb2O5 (ABCR, 99.5%), and WO3 (Alfa Aesar, 99.8%). The phase purity of all starting materials was verified by X-ray diffraction. Annealing was performed in evacuated quartz ampoules, which were preheated at 1073 K under dynamic vacuum for 5 hours to ensure dry conditions. The stoichiometric amounts of the starting compounds were thoroughly ground, sealed in quartz ampoules, and annealed in a first step at 1173 K for 12 hours. In a second step, the harvested powders were ground again, re-sealed and re-annealed for 12 hours at 1173 K to ensure a homogeneous element distribution. Heating and cooling rates for all procedures in the horizontal tube furnaces were 5 K min−1. The quartz ampoules were 10–12 cm in length and 11 mm in inner diameter with a maximum of 4 g starting materials per ampoule. The resulting powders of Nb8−xW9+xO47 were manually ground. The phase purity was verified by powder X-ray diffraction prior to consolidation and characterization.Powder processing
Batches of bulk powders with different degrees of substitution were used for consolidation into 1–1.5 mm thick, 12.7 mm diameter disks at 1173 K under vacuum and a pressure of 56 MPa by current-assisted short-time sintering utilizing a DSP (“Direktsinterpresse”). Boron nitride coated high density graphite was used as die material. In the first consolidation segment the pressure was applied, followed by a 6 min heating segment to 1173 K. After 10 min sintering time the pressure was released to 2 MPa, and the sample was cooled down over a period of 6 min to obtain strain-free materials. The resulting pellets had >95% theoretical density, as determined by the Archimedes method.Characterization details
Room temperature powder X-ray diffraction measurements were performed on a Siemens D5000 powder diffractometer in transmission geometry with a Braun M50 position sensitive detector, Ge(220) monochromator and CuKα radiation, with a step size of 0.0078° in 2Θ. Le Bail fits were performed of all diffraction data with TOPAS Academic V4.152 applying the fundamental parameter approach using the crystallographic data from Craig and Stephenson as structure model.48Thermogravimetric (TG) measurements were conducted using a CI Electronics MK2 microbalance. The atmospheric composition, in particular the oxygen partial pressure pO2 was controlled by an in-house-built gas mixer, of a type described elsewhere.53 The sample and counterweight were attached to the arms of the balance with platinum wires. The sample was hanging in an alumina tube in a vertical tube furnace and the counterweight was hanging in a glass tube kept at room temperature. To ensure equilibrium, the weight was monitored isothermally at constant pO2 values. Mixtures of O2 and Ar were used to obtain an oxygen partial pressure between ≈10−5 and 1 atm, whereas lower oxygen partial pressures of up to 10−15 atm could be obtained by diluting CO in a carrier gas (CO + 1/2 O2 = CO2). All thermogravimetric reductions and oxidizations were performed under conditions where the tetragonal tungsten bronzes are stable as confirmed by X-ray diffraction. To measure the electrical conductivity and the Seebeck coefficient at high temperatures, we used a custom-built assembly mounted into a NorECs ProboStat measurement cell as described elsewhere.54,55 The gas atmosphere in the measurement cell was controlled using a similar gas mixer as for the thermogravimetric investigation. The maximum temperature (973 K) and the atmospheric composition during the electrical measurements were chosen to prevent oxidation or reduction of the sample during the measurement. Based on previous TG experiments, this could be achieved by measuring in argon (pO2 ≈ 10−5 atm) for T < 773 K and diluted CO (pO2 ≈ 10−15 atm) for T > 773 K.
Thermal diffusivity was measured in N2 flow using a Netzsch laser flash diffusivity instrument (LFA micro flash 457).
The thermal conductivity was calculated using the measured thermal diffusivity and sample density in combination with the specific heat (Cp) calculated from the Dulong–Petit law.56
Scanning electron microscopy (SEM) images and energy dispersive X-ray spectroscopy (EDX) measurements of the as-synthesized powders and the sintered pellets were recorded using a FEI Nova NanoSEM600 equipped with an Everhart-Thornley detector (ETD) and a low voltage high contrast detector (vCD) in high vacuum mode. For energy dispersive X-ray spectroscopy, a built-in EDAX-Genesis detector was used.
High-resolution transmission electron microscopy (HR-TEM) images were taken using a FEI Tecnai F20 with an acceleration voltage of 200 kV. Samples were either finely crushed in an agate mortar or cut with a microtome into 1–10 nm thick slices, then dispersed in ethanol and drop casted on a carbon–lacey grid.
Results and discussion
Synthesis
Starting from the binary oxides, the solid state synthesis of the complete series Nb8−xW9+xO47 with x = 0,…,5 is possible.42 The screening of reaction parameters showed that the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 phase and the complete series of tetragonal tungsten bronzes can easily be obtained in larger batches of several grams at temperatures as low as 1173 K with short reaction times of 12 h. To ensure a homogeneous cation distribution, all samples were subjected to a second annealing step at 1173 K for 12 h after the powder was thoroughly ground and re-sealed in an ampoule. Cation substituted, hence reduced, samples are known to be oxidized at high temperatures under structural changes and/or decomposition, for example 8Nb7W10O47 + 2O2 → 7Nb8W9O47 + 17WO3.57 Therefore, both annealing steps were conducted under vacuum to prevent oxidation at higher temperatures. The obtained powder of the unsubstituted end member Nb8W9O47 exhibits a gray color, whereas all cation substituted samples exhibit a dark blue to purple color. This dark color is an indication of substantial concentrations of electronic defects, most likely itinerant electrons, confirming that the cation substitution is compensated to a large extent by electrons, thereby increasing the charge carrier concentration. The light gray color of the unsubstituted Nb8W9O47 suggests that after synthesis already a few electron charge carriers are present, induced by the formation of oxygen vacancies during synthesis.
9 phase and the complete series of tetragonal tungsten bronzes can easily be obtained in larger batches of several grams at temperatures as low as 1173 K with short reaction times of 12 h. To ensure a homogeneous cation distribution, all samples were subjected to a second annealing step at 1173 K for 12 h after the powder was thoroughly ground and re-sealed in an ampoule. Cation substituted, hence reduced, samples are known to be oxidized at high temperatures under structural changes and/or decomposition, for example 8Nb7W10O47 + 2O2 → 7Nb8W9O47 + 17WO3.57 Therefore, both annealing steps were conducted under vacuum to prevent oxidation at higher temperatures. The obtained powder of the unsubstituted end member Nb8W9O47 exhibits a gray color, whereas all cation substituted samples exhibit a dark blue to purple color. This dark color is an indication of substantial concentrations of electronic defects, most likely itinerant electrons, confirming that the cation substitution is compensated to a large extent by electrons, thereby increasing the charge carrier concentration. The light gray color of the unsubstituted Nb8W9O47 suggests that after synthesis already a few electron charge carriers are present, induced by the formation of oxygen vacancies during synthesis.
A series of samples with a low degree of substitution and nominal composition Nb8−xW9+xO47 with x = 0, 0.075, 0.1, 1, and 2 were prepared to obtain a first assessment of the thermal stability, the thermoelectric transport properties and the electronic optimization potential of cation substituted TTBs.
Powder processing
In order to conduct proper thermoelectric characterization, the powders were consolidated into dense pellets by hot-pressing or current-assisted short-time sintering (also called spark plasma sintering, SPS). In the case of hot-pressing, the high temperatures, reducing conditions, and long consolidation times needed to obtain dense pellets induced material deterioration, as verified by PXRD of the consolidated pellets. Therefore, these pellets were not suited for further characterization. The use of current-assisted sintering, however, allowed the combination of short sintering times at a relatively low consolidation temperature of 1173 K. No sample deterioration was observed via PXRD. The resulting short-time sintered pellets all had densities >95%. Scanning electron microscopy images were taken of the as-synthesized TTB powders and consolidated pellets (Fig. S1, ESI†). The as-synthesized powders consisted of anisotropic match-like crystals with prismatic crystal habit, few micrometers in diameter and several micrometers in length. The consolidated pellets showed high density with only few cavities visible on the breaking edge. The polished surface showed no signs of contrast corroborating a homogeneous elemental distribution, as confirmed by EDX measurements. A low magnification image of a consolidated pellet and zoomed-in image of the cavities are provided in the ESI,† Fig. S1 and S2, illustrating the absence of preferred crystal orientation within the pellet. No serious anisotropic contributions to the transport properties are expected, and therefore the consolidated pellets were used as such for further characterizations.Structural characterization
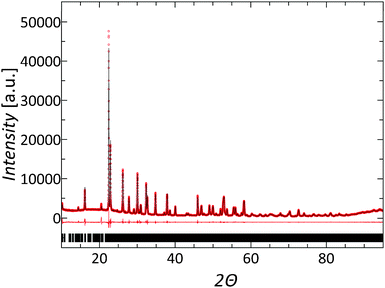
Due to the large unit cell, complex structure, and low symmetry of the TTBs, structure refinement of the as-synthesized powders and consolidated pellets by the Rietveld method was not possible with standard laboratory X-ray diffraction data. Therefore, the computationally more simple Le Bail fits were used to match the diffraction data. The refined diffraction data are shown in Fig. 2. No structural changes were observed upon substitution, in agreement with the infinitely adaptive nature of the TTB. The data obtained from all as-synthesized samples and consolidated pellets could be fitted using the crystallographic structure model of Nb8W9O47 with Rwp values <5%. In addition, data obtained from consolidated samples and pellets, which were subsequently reduced or oxidized, showed no significant difference in structure within the experimental resolution limit.The presence of prevalent oxygen defects was studied by HR-TEM imaging. As-synthesized pellets of composition Nb8W9O47 and Nb7W10O47 were analyzed to investigate the influence of cation substitution on the micro- and nanostructure. In addition, an as-synthesized pellet of composition Nb8W9O47 was cut into two pieces, which were subsequently either oxidized or reduced, to investigate the effect of oxygen deficiency on the structure. After oxidation the sample color changed from dark gray to yellow/white, which indicates the absence of charge carriers corroborating the annihilation of oxygen vacancies according to the back reaction of eqn (3).
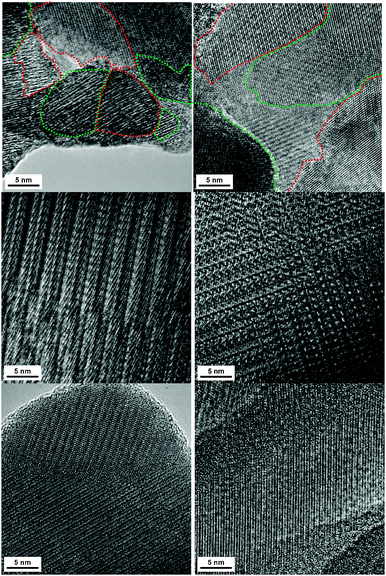
A HR-TEM investigation revealed that all samples – independent of the cation composition and the reduction state – contain a rich variety of defects, including point defects, Wadsley defects and CS planes. Additionally, a broad grain size distribution from a few nm to several μm was observed. An overview of different defects and grain sizes is shown in Fig. 3.
Due to the presence of both ordered CS planes and disordered grains of varying size, the investigated TTB materials may be considered as nanostructured as well as nanoscaled materials. As a consequence, the lattice thermal conductivity is expected to be relatively low.
Thermal stability and oxygen deficiency
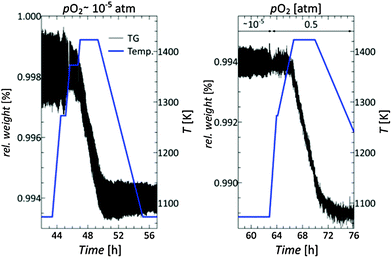
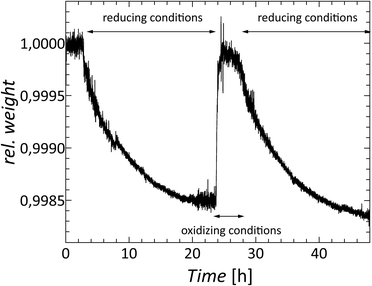
To obtain information on the thermal stability and the oxygen vacancy concentration at elevated temperatures, TG measurements under controlled atmospheres were conducted. Initially, the thermal stability was investigated under varying atmospheres. The entire TG run as a function of time can be seen in the ESI,† Fig. S3, enlarged sections are displayed in Fig. 4. A slight initial weight loss of 0.2% was encountered from room temperature up to 650 K, probably due to loss of adsorbed water. The measurement was conducted at pO2 values of 10−5 atm (left image in Fig. 4) and 0.5 atm (right image in Fig. 4), respectively. In both cases, the onset of an irreversible weight loss occurred at ≈1373 K, which is probably associated to the volatilization of WO3. No weight gain was observed while increasing pO2 at 1273 K (right image in Fig. 4), indicating no significant oxygen non-stoichiometry under these conditions. During the final cooling to room temperature, the pO2 was again reduced to 10−5 atm at 920 K without any significant weight change. During this TG study, the sample color again had changed from dark gray to light yellow, due to the annihilation of oxygen vacancies. From these experiments, we conclude that the application of Nb8−xW9+xO47 is limited to temperatures below 1373 K.The residual oxygen partial pressure of argon (pO2 ≈ 10−5 atm) was not low enough to cause reduction of the oxidized sample. Therefore, other more reducing gas mixtures were investigated as well to obtain higher reduction potentials. The use of wet H2 as a reductive gas (pO2 typically between 10−25 and 10−20 atm depending on temperature) did not lead to a reduction of Nb8W9O47, but to sample decomposition, which could be verified by X-ray diffraction. Therefore, milder reducing gas mixtures were tested. A reduction of Nb8W9O47 was possible with the use of a CO/CO2/Ar gas mixture. It was found that Nb8W9O47 can be reduced at 1173 K and a pO2 of ≈10−15 atm, while preserving the overall TTB structure. Due to the weight loss correlated to oxygen vacancy formation, the reduction can be monitored gravimetrically. The subtle structural changes correlated with the defect formation, although detectable in the HR-TEM images, could not be resolved by standard laboratory X-ray diffraction. Fig. 5 shows the reversible weight loss and gain, associated with the reduction and oxidation of an as-synthesized Nb8W9O47 sample.
The oxidation reaction is much faster than the reduction, demonstrated by the rapid weight gain at t ≈ 24 h. The reduction–oxidation cycle was reversible and could be repeated without any sign of sample degradation. By thermal quenching to room temperature, the oxygen vacancy and the corresponding charge carrier concentrations could be preserved.58 Assuming a completely oxidized sample (δ = 0) before reduction, the monitored relative weight loss of 0.15% can be translated to an oxygen deficiency δ of 0.3 in Nb8W9O47−δ. According to eqn (3), each oxygen vacancy donates two electrons. Therefore, the effect of the maximum oxygen deficiency of 0.3 on the thermoelectric properties should be comparable to a substitution degree of x = 0.6.
Thermoelectric transport properties and optimization strategies
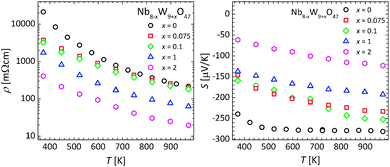
Starting from non-substituted, fully oxidized Nb8W9O47, the charge carrier concentration must be increased to enhance the thermoelectric properties. An increase of the charge carrier concentration can be achieved either by cation substitution or – to a lower extent – by an increase of the oxygen vacancy concentration viaeqn (3). To obtain a first assessment of the thermoelectric application potential, a series of cation substituted compounds with compositions Nb8−xW9+xO47, x = 0, 0.075, 0.1, 1, and 2 was investigated for their thermoelectric transport properties. The results of the electronic transport measurements are plotted in Fig. 6.The overall electrical resistivities of the samples were high compared to other thermoelectric materials, but decreased considerably with rising temperature, probably due to a higher degree of charge carrier delocalization at high temperature.
In agreement with eqn (1) and (2), an increasing degree of substitution leads to a higher charge carrier concentration and consequently to a lower electrical resistivity, reaching a value of 20 mΩ cm at 973 K for a sample of composition Nb6W11O47. The samples with a lower degree of substitution (x = 0, 0.075, 0.1), however, exhibit a similar electrical resistivity. A possible reason for this may be the dominating influence of oxygen vacancies on the charge carrier concentration at lower substitution degree.
In line with the high electrical resistivity, the measured Seebeck coefficients are rather large. At room temperature the Seebeck values range from −240 to −65 μV K−1 for samples of composition Nb8W9O47 and Nb6W11O47, respectively. This corroborates the assumption of an n-type material with a charge carrier concentration increasing with an increasing substitution level. All substituted samples showed an increase of the absolute value of the Seebeck coefficient with increasing temperature, probably due to an increase of the internal overall entropy of the system at higher temperatures. However, further studies are needed to fully understand the underlying principles of the electronic transport properties of the tungsten bronzes.
When substituting niobium by tungsten, or when creating oxygen vacancies, the change in all transport parameters can be understood by a variation in the charge carrier concentration according to eqn (1)–(3).
The measured thermal diffusivities and calculated thermal conductivities of the cation-substituted series of TTBs are shown in Fig. 7. The thermal diffusivity values of all samples are very similar to each other. Beside a subtle increase upon substitution, no clear trend was observed. With increasing temperature, the thermal diffusivity decreases slightly. The obtained thermal conductivities are all low, with values ranging from 1.6 to 2.0 W K−1 m−1 for the unsubstituted and substituted samples, respectively. The glass-like thermal conductivities can be attributed to the nanosized grains and the complex disordered structure,45 and are similar to values reported for other niobium tungsten compounds.59 The similar thermal conductivities for all samples can be rationalized as (i) the structure of the TTB does not change upon substitution, (ii) the change in mass contrast (from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 7
9 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for Nb8−xW9+xO47 with x = 0 and 2) is only moderate, and (iii) strain contrast should be low considering the ionic radii (0.64 and 0.60 Å) of Nb5+ and W6+ in octahedral coordination.60
10 for Nb8−xW9+xO47 with x = 0 and 2) is only moderate, and (iii) strain contrast should be low considering the ionic radii (0.64 and 0.60 Å) of Nb5+ and W6+ in octahedral coordination.60
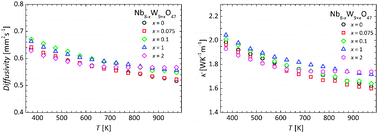 | ||
| Fig. 7 Temperature dependence of the thermal diffusivity (left) and conductivity (right) in cation substituted TTBs of composition Nb8−xW9+xO47 with x = 0, 0.075, 0.1, 1, and 2. | ||
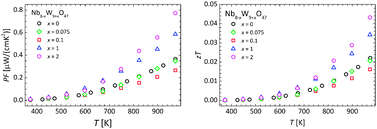
Due to the high electrical resistivity, the electronic contribution to the thermal conductivity is negligible for all studied compositions. Using these initial measurements, the power factor and the thermoelectric figure of merit of the cation substituted series were calculated. The results are summarized in Fig. 8.
As a consequence of the rather high electrical resistivity, the power factor is not very large, but increases with rising temperature and substitution degree. Indeed, a substantial increase of the power factor to a value of 0.8 μW cm−1 K−2 at 973 K could be obtained for the composition Nb6W11O47 with respect to the lower substituted samples. This trend is also reflected in the thermoelectric figure of merit, where a value of 0.043 was reached at 973 K for the highest substituted sample (x = 2) studied in this contribution. In comparison to state of the art optimized n-type oxide thermoelectric materials, the figure of merit is still lower by a factor of ten. However, in this initial study, TTBs with a maximal substitution level of x = 2 have been investigated, although substitution levels of up to x = 5 (Nb3W14O47) are reported in literature without significant structural changes, and are currently under investigation. With increasing substitution level, the electrical properties are expected to improve even more, while keeping a similar, low thermal conductivity. Therefore, the figure of merit is expected to be enhanced further with increasing substitution level.
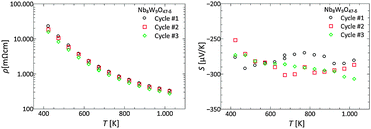
In addition to cation substitution, a second optimization strategy is to adjust the charge carrier concentration and phonon scattering centers by the control of the oxygen deficiency. According to eqn (3), an oxygen vacancy carriers an effectively positive charge, and thereby acts as a donor of two electrons to the system. As shown in section Thermal stability and oxygen deficiency, the oxygen content of Nb8−xW9+xO47 can reversibly be varied at elevated temperatures by controlling the atmospheric composition (see Fig. 5). In order to demonstrate that the control of the oxygen deficiency corroborates the control of the electronic properties, a simple experiment was conducted. A sample of composition Nb8W9O47 was repeatedly reduced and oxidized over three cycles under controlled, almost identical thermodynamic conditions (temperature, pO2) while monitoring the weight change. After equilibrium had been reached, the sample was quenched to room temperature. The corresponding electronic properties were determined after each cycle. In the oxidized state the color was white/slightly yellow. The sample resistivity was very high and could not be determined with our setup. In the reduced state, the sample was dark blue in color. The results of the electronic transport measurements after each red/ox-cycle are shown in Fig. 9.
In consideration of the substantial changes from the white, oxidized, insulating sample to the dark blue, reduced, semiconducting sample, the measured electronic properties are very similar. The measured electric resistivities and the Seebeck coefficients in the three cycles are reproducible within a relative error of 17% and 7%, respectively, and they are fully within the limits of reproducibility based on a round-robin testing of transport properties of bulk thermoelectrics.61,62 The variation may be caused by changes in the nanostructure or slightly different oxygen vacancy concentrations after each quenching. However, it is evident that control of the oxygen non-stoichiometry and the associated charge carrier concentration can be achieved. Analogue to the electronic properties no significant variation of the thermal conductivity upon oxidation/reduction cycling has been noted. These outcomes confirm that the control of oxygen vacancies can be considered as a viable further optimization strategy for tetragonal tungsten bronzes and even other thermoelectric oxides.
Conclusions
A first assessment of tetragonal tungsten bronzes as thermoelectric energy conversion materials was conducted. They were chosen because of their structural complexity and their possible electronic tuning by cation substitution x and oxygen deficiency δ. To this end, a series of compounds with low substitution degrees x = 0, 0.075, 0.1, 1, and 2, was synthesized and characterized structurally by X-ray diffraction and high-resolution transmission electron microscopy. In view of thermoelectric transport measurements, optimal sintering parameters, thermal stability and redox behavior at elevated temperatures were investigated.The materials exhibit a complex atomic structure. HR-TEM investigations revealed varying grain sizes down to few nanometers and a variety of different oxygen defects, including point defects, Wadsley defects and crystallographic shear planes. Thermogravimetric analyses showed an irreversible decomposition at 1323 K, presumably due to evaporation of WO3, which sets an upper limit for use in thermoelectric devices.
The oxygen deficiency could be reversibly controlled by equilibrating the sample in a controlled atmosphere at 1173 K with subsequent quenching. Due to the reversible generation and annihilation of oxygen vacancies at elevated temperatures, the electronic properties can be tuned to some extent. A maximum oxygen deficiency of δ = 0.3 was determined for Nb8W9O47−δ. Each oxygen vacancy acts as a donor of two electrons to the system, so that the maximum oxygen deficiency of 0.3 corresponds to a change in substitution degree of x = 0.6.
Thermoelectric transport measurements were conducted up to 973 K under controlled atmosphere to prevent a change of the oxygen stoichiometry during measurements. All samples exhibited a relatively high electric resistivity above 20 mΩ cm and high negative Seebeck coefficients ranging from −280 to −65 μV K−1, indicating n-type conduction. With increasing degree of substitution, charge carriers are introduced to the system. This leads to an increase of the electric conductivity and a decrease of the (negative) Seebeck coefficients. The complex structure composed of nano-sized grains, and a high concentration of Wadsley defects and crystallographic shear planes results in glass-like, temperature independent thermal conductivities ranging from 1.6 to 2.0 W K−1 m−1. The highest power factor and zT were obtained for the highest substituted sample (Nb6W11O47) with values of 0.8 μW cm−1 K−2 and 0.043, respectively, at 973 K. Although the obtained figure of merit is lower than in other optimized n-type oxides, the high Seebeck coefficient paired with the low, glass-like thermal conductivity demonstrates the potential of tetragonal tungsten bronzes for thermoelectric applications.
Both optimization strategies, (i) cation substitution and (ii) controlled oxygen deficiency proved suitable to tailor the thermoelectric properties. With increasing degree of substitution, significant further improvement of the figure of merit may be expected, which makes the initial zT value of 0.043 at 973 K promising.
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft through the priority program 1386 “Nanostructured Thermoelectrics”. Financial support through the Excellence Initiative (DFG/GSC 266) is acknowledged by C.P.H, while M.S., H.F., T.G.F., and T.N. acknowledge funding from the Norwegian Research Council via the THERMEL (NFR 200022) and THELMA (NFR 228854) projects.Notes and references
- M. S. Dresselhaus, G. Chen, M. Y. Tang, R. G. Yang, H. Lee, D. Z. Wang, Z. F. Ren, J. P. Fleurial and P. Gogna, Adv. Mater., 2007, 19, 1043–1053 CrossRef CAS
.
- T. Claudio, G. Schierning, R. Theissmann, H. Wiggers, H. Schober, M. M. Koza and R. P. Hermann, J. Mater. Sci., 2013, 48, 2836–2845 CrossRef CAS
.
- K. Koumoto, Y. Wang, R. Zhang, A. Kosuga and R. Funahashi, Annu. Rev. Mater. Res., 2010, 40, 363–394 CrossRef CAS
.
- J. He, Y. Liu and R. Funahashi, J. Mater. Res., 2011, 26, 1762–1772 CrossRef CAS
.
- I. Terasaki, Y. Sasago and K. Uchinokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, R12685–R12687 CrossRef CAS
.
- P. Limelette, V. Hardy, P. Auban-Senzier, D. Jérome, D. Flahaut, S. Hébert, R. Frésard, C. Simon, J. Noudem and A. Maignan, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 233108 CrossRef
.
- H. Yamauchi, L. Karvonen, T. Egashira, Y. Tanaka and M. Karppinen, J. Solid State Chem., 2011, 184, 64–69 CrossRef CAS
.
- F. Li, T.-R. Wei, F. Kang and J.-F. Li, J. Mater. Chem. A, 2013, 1, 11942–11949 CAS
.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, J. Mater. Chem., 1997, 7, 85–90 RSC
.
- H. Ohta, W. S. Seo and K. Koumoto, J. Am. Ceram. Soc., 1996, 79, 2193–2196 CrossRef CAS
.
- T. Tani, S. Isobe, W. S. Seo and K. Koumoto, J. Mater. Chem., 2001, 11, 2324–2328 RSC
.
- K. H. Lee, S. W. Kim, H. Ohta and K. Koumoto, J. Appl. Phys., 2006, 100, 063717 CrossRef
.
- E. Guilmeau, D. Bérardan, C. Simon, A. Maignan, B. Raveau, D. O. Ovono and F. Delorme, J. Appl. Phys., 2009, 106, 053715 CrossRef
.
- L. D. Hicks and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 16631 CrossRef CAS
.
- R. Venkatasubramanian, E. Siivola, T. Colpitts and B. O'Quinn, Nature, 2001, 413, 597–602 CrossRef CAS PubMed
.
- T. C. Harman, P. J. Taylor, M. P. Walsh and B. E. LaForge, Science, 2004, 303, 818–821 CrossRef PubMed
.
- A. I. Hochbaum, R. Chen, R. D. Delgado, W. Liang, E. C. Garnett, M. Najarian, A. Majumdar and P. D. Yang, Nature, 2008, 451, 163–167 CrossRef CAS PubMed
.
- A. I. Boukai, Y. Bunimovich, J. Tahir-Kheli, J. Yu, W. A. Goddard and J. R. Heath, Nature, 2008, 451, 168–171 CrossRef CAS PubMed
.
- B. Poudel, Q. Hao, Y. Ma, Y. C. Lan, A. Minnich, B. Yu, X. Yan, D. Z. Wang, A. Muto, D. Vashaee, X. Chen, J. M. Liu, M. S. Dresselhaus, G. Chen and Z. F. Ren, Science, 2008, 320, 634–638 CrossRef CAS PubMed
.
- Y. Ma, Q. Hao, B. Poudel, Y. C. Lan, B. Yu, D. Z. Wang, G. Chen and Z. F. Ren, Nano Lett., 2008, 8, 2580–2584 CrossRef CAS PubMed
.
- R. G. Yang and G. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 195316 CrossRef
.
- P. A. Sharma and J. D. Sugar, Front. Chem., 2014, 2, 111 Search PubMed
.
- Z. Qu, T. D. Sparks, R. Wu, C. Wan, D. R. Clarke and W. Pan, Acta Mater., 2011, 59, 3841–3850 CrossRef CAS
.
- A. V. Kovalevsky, A. A. Yaremchenko, S. Populoh, P. Thiel, D. P. Fagg, A. Weidenkaff and J. R. Frade, Phys. Chem. Chem. Phys., 2014, 16, 26946–26954 RSC
.
- C. P. Heinrich, T. W. Day, W. G. Zeier, M. Panthöfer, G. J. Snyder and W. Tremel, J. Am. Chem. Soc., 2014, 136, 442–448 CrossRef CAS PubMed
.
- J. Rouxel, A. Meerschaut and G. A. Wiegers, J. Alloys Compd., 1995, 229, 144–157 CrossRef CAS
.
- C. Wan, Y. Wang, N. Wang, W. Norimatsu, M. Kusunoki and K. Koumoto, J. Electron. Mater., 2011, 40, 1271–1280 CrossRef CAS
.
- J. H. Kim, Y. J. Song, J.-S. Rhyee, B.-S. Kim, S.-D. Park, H. J. Lee and J.-W. Shin, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 224305 CrossRef
.
- F. R. Harris, S. Standridge, C. Feik and D. C. Johnson, Science, 2007, 315, 351–353 CrossRef PubMed
.
- A. Mavrokefalos, Q. Lin, M. Beekman, J. H. Seol, Y. J. Lee, H. Kong, M. T. Pettes, D. C. Johnson and L. Shi, Appl. Phys. Lett., 2010, 96, 181908 CrossRef
.
- D. B. Moore, M. Beekman, S. Disch and D. C. Johnson, Angew. Chem., Int. Ed., 2014, 53, 5672–5675 CrossRef CAS PubMed
.
- R. D. Westover, R. Atkins, J. Ditto and D. C. Johnson, Chem. Mater., 2014, 26, 3443–3449 CrossRef CAS
.
- G. Kieslich, I. Veremchuk, I. Antonyshyn, W. G. Zeier, K. Weldert, C. S. Birkel, C. P. Heinrich, E. Visnow, M. Panthöfer, U. Burkhardt, Y. Grin and W. Tremel, Phys. Chem. Chem. Phys., 2013, 15, 15399–15403 RSC
.
- W. R. Thurber and A. J. Mante, Phys. Rev., 1965, A139, 1655–1665 CrossRef
.
- Y. Lu, M. Hirohashi and K. Sato, Mater. Trans., JIM, 2006, 47, 1449–1452 CrossRef CAS
.
- S. Harada, K. Tanaka and H. Inui, J. Appl. Phys., 2010, 108, 083703 CrossRef
.
- G. Kieslich, C. S. Birkel, J. E. Douglas, M. Gaultois, R. Seshadri, Y. Grin, G. D. Stucky and W. Tremel, J. Mater. Chem. A, 2013, 1, 13050–13054 CAS
.
- G. Kieslich, U. Burkhardt, C. S. Birkel, I. Veremchuk, J. E. Douglas, M. Gaultois, I. Lieberwirth, R. Seshadri, G. D. Stucky, Y. Grin and W. Tremel, J. Mater. Chem. A, 2014, 2, 13492–13497 CAS
.
- J. S. Anderson, Dalton Trans., 1973, 1107–1115 RSC
.
- S. Lee, R. H. T. Wilke, S. Trolier-McKinstry, S. Zhang and C. A. Randall, Appl. Phys. Lett., 2010, 96, 031910 CrossRef
.
- M. W. Gaultois and T. D. Sparks, Appl. Phys. Lett., 2014, 104, 113906 CrossRef
.
- F. Krumeich, A. Hussain, C. Bartsch and R. Gruehn, Z. Anorg. Allg. Chem., 1995, 621, 799–806 CrossRef CAS
.
- F. Krumeich, C. Bartsch and R. Gruehn, J. Solid State Chem., 1995, 427, 268–274 CrossRef
.
- F. Krumeich, Z. Kristallogr., 1997, 212, 708–711 CAS
.
- F. Krumeich, Acta Crystallogr., 1998, B54, 240–249 CAS
.
- R. Roth and J. Waring, J. Res. NBS, 1966, 70A, 281–303 CrossRef
.
- A. W. Sleight, E. Sletten, J. Sletten, E. Kulonen, J. Brunvoll, E. Bunnenberg, C. Djerassi and R. Records, Acta Chem. Scand., 1966, 20, 1102–1112 CrossRef CAS
.
- D. C. Craig and N. C. Stephenson, Acta Crystallogr., 1969, B25, 2071–2083 CrossRef CAS
.
-
Solid State Physics, ed. F. Seitz and D. Turnbull, Academic Press, 1956, vol. 3, pp. 307–435 Search PubMed
.
- P. England, J. Booth, R. Tilley and T. Ekström, J. Solid State Chem., 1982, 44, 60–74 CrossRef CAS
.
- G. Heurung and R. Gruehn, J. Less-Common Met., 1980, 76, 17–32 CrossRef CAS
.
-
A. Coelho, Topas Academic Version 4.1. Computer Software, Topas Academic, Coelho Software, Brisbane, 2007 Search PubMed
.
- T. Norby, Solid State Ionics, 1988, 28–30, 1586–1591 CrossRef
.
- M. Schrade, H. Fjeld, T. G. Finstad and T. Norby, J. Phys. Chem. C, 2014, 118, 2908–2918 CAS
.
- M. Schrade, H. Fjeld, T. Norby and T. G. Finstad, Rev. Sci. Instrum., 2014, 85, 103906 CrossRef PubMed
.
- D. R. Salmon and R. P. Tye, Int. J. Thermophys., 2010, 31, 338–354 CrossRef CAS
.
- F. Krumeich, J. Solid State Chem., 1995, 119, 420–427 CrossRef CAS
.
- M. Schrade, R. Kabir, S. Li, T. Norby and T. G. Finstad, J. Appl. Phys., 2014, 115, 103705 CrossRef
.
- I. Henning, M. Mertig, R. Plath, G. Pomple, E. Hegenbarth and R. Schalge, Phys. Status Solidi A, 1982, 73, K105–K109 CrossRef CAS
.
- A. Simon, Angew. Chem., Int. Ed., 1983, 22, 95–113 CrossRef
.
- H. Wang, W. D. Porter, H. Böttner, J. König, L. Chen, S. Bai, T. M. Tritt, A. Mayolet, J. Senawiratne, C. Smith, F. Harris, P. Gilbert, J. W. Sharp, J. Lo, H. Kleinke and L. Kiss, J. Electron. Mater., 2013, 42, 654–664 CrossRef CAS
.
- H. Wang, W. D. Porter, H. Böttner, J. König, L. Chen, S. Bai, T. M. Tritt, A. Mayolet, J. Senawiratne, C. Smith, F. Harris, P. Gilbert, J. W. Sharp, J. Lo, H. Kleinke and L. Kiss, J. Electron. Mater., 2013, 42, 1073–1084 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Scanning electron microscopy images of the as-synthesized powder and the consolidated pellet (Fig. S1). A zoomed-in image of a consolidated pellet of the TTB Nb8W9O47 (Fig. S2), relative weight of Nb8W9O47 as a function of time under different oxygen partial pressures (Fig. S3). See DOI: 10.1039/c5mh00033e |
| ‡ Authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |