Self-assembled perylene bisimide J-aggregates as promising cathode modifiers for highly efficient inverted polymer solar cells†
Zengqi
Xie‡
a,
Biao
Xiao‡
a,
Zhicai
He‡
a,
Wenqiang
Zhang
 a,
Xiaoyan
Wu
a,
Hongbin
Wu
*a,
Frank
Würthner
*b,
Chao
Wang
c,
Fangyan
Xie
c,
Linlin
Liu
a,
Yuguang
Ma
*a,
Wai-Yeung
Wong
d and
Yong
Cao
a
a,
Xiaoyan
Wu
a,
Hongbin
Wu
*a,
Frank
Würthner
*b,
Chao
Wang
c,
Fangyan
Xie
c,
Linlin
Liu
a,
Yuguang
Ma
*a,
Wai-Yeung
Wong
d and
Yong
Cao
a
aInstitute of Polymer Optoelectronic Materials and Devices, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: hbwu@scut.edu.cn; ygma@scut.edu.cn
bUniversität Würzburg, Institut für Organische Chemie & Center for Nanosystems Chemistry, Am Hubland, 97074 Würzburg, Germany. E-mail: wuerthner@chemie.uni-wuerzburg.de
cInstrumental Analysis and Research Center, Sun Yat-Sen University, Guangzhou 510275, P. R. China
dInstitute of Molecular Functional Materials, Department of Chemistry and Institute of Advanced Materials, Hong Kong Baptist University, Waterloo Road, Kowloon Tong, Hong Kong, P. R. China
First published on 9th July 2015
Abstract
Solvent-dependent self-assemblies of perylene bisimide (PBI) are applied as cathode modification layers in inverted polymer solar cells (i-PSCs) to afford a power conversion efficiency (PCE) as high as 9.11%. The very high device performance clearly indicates that n-type perylene bisimide based supramolecular assemblies are most promising interfacial materials for highly efficient i-PSCs.
Conceptual insightsSupramolecular assembly of functional π-conjugated molecules into J-aggregates has been shown to be highly important in nature for light harvesting as well as solar energy conversion (photosynthesis). The ordered molecular assemblies often show enhanced physical properties like high charge mobility and long distance exciton migration. In addition, the formation and stability of a supramolecular material are usually strongly dependent on the solvent, e.g. intermolecular hydrogen bonds are very strong in aprotic solvents but will be cleaved by protic solvents, which just meets the requirement of a low-cost solution processible interlayer material. That is the material is soluble enough in one solvent for being casted as an interfacial layer by a directional self-assembly process but insoluble in a different solvent that is suitable for the processing of the subsequent active layer. Herein, we demonstrate the application of perylene bisimide based J-aggregates as cathode modifiers to give highly efficient inverted polymer solar cells. The remarkable device performance indicates that perylene bisimide based supramolecular assemblies can compete with the best known conventional inorganic or polymeric cathode modification materials. |
Polymer solar cells (PSCs) have attracted intense research interest due to their unique advantages such as low-cost fabrication, good flexibility, light weight and compatibility with cheap roll-to-roll processing techniques.1 Recently, inverted polymer solar cells (i-PSCs) have been introduced and shown to provide long-term ambient stability as well as enhanced device performance.2 To achieve an inverted device configuration, the work function of the indium tin oxide (ITO) electrode (∼4.8 eV) needs to be substantially reduced thus facilitating charge extraction and collection. Previously this was usually achieved through modification of the ITO surface by n-type metal oxides or metal carbonates such as titanium oxide, zinc oxide or caesium carbonate,3 polyelectrolytes such as PFN or PEIE (for the full names and chemical structures, see ref. 4), or chemically or electrochemically cross-linked or self-assembled films.5 In addition, in the past several years, transition metal chelates have been demonstrated as promising ITO surface modifiers for highly efficient i-PSCs.6 Most recently, a photoconductive cathode interlayer was developed by us by doping a very small amount of light sensitizer into the interlayer.7 These interface materials play indeed a very important role in the device performance by improving the energy level matching between the electrode and the active layer and thus enhancing the electron collection and transport. For example, some of us reported i-PSCs with a PCE of over 9% by using PFN and its derivatives as cathode modification materials in the devices, which is much higher than the PCE of devices with conventional device configuration.2b,8 However, most of the current interface materials are processed under weak acidic conditions and usually form amorphous thin films, which either possibly cause the ITO electrode being corroded slowly or induce low conductivity of the interlayers. Therefore, it is important to develop novel cathode interface materials that can tune the electronic structure of the electrodes as well as can improve the electron collection/extraction ability in order to further improve the device performance of PSCs.
As an alternative to hitherto applied inorganic or polymeric cathode interlayers, π-conjugated small molecules can also be considered, in particular if they can be processed by self-assembly approaches into regular π-stacks that support n-type transport characteristics.9 For perylene bisimides (PBIs), one of the archetype n-type organic semiconducting materials, a plethora of highly ordered self-assembled nanostructures has been accomplished by means of directional intermolecular interactions.10 For the ordered molecular assemblies of these dyes, new properties often emerge that differ from the properties of the single molecules or the amorphous solid state. For instance, for hydrogen-bond directed slipped PBI stacks electron mobility as high as 1 cm2 V−1 s−1 and exciton migration distances as long as 70 nm have been demonstrated.11 Accordingly, it is not surprising that this class of dyes has been intensively investigated in organic photovoltaics,5e,12 organic field effect transistors,13 and sensors.14 The formation and stability of a supramolecular material are usually strongly dependent on the solvent, e.g. intermolecular hydrogen bonds are very strong in aprotic solvents but will be cleaved by protic solvents.15 The concomitant solvent-dependent solubility just meets the requirement of a low-cost solution processible interlayer material. That is the material is soluble enough in one solvent for being casted as an interfacial layer by a directional self-assembly process but insoluble in a different solvent that is suitable for the processing of the subsequent active layer. In this contribution, we demonstrate that PBI based self-assembled nanofibers can be applied as cathode interlayers in an i-PSC to afford a power conversion efficiency (PCE) as high as 9.11%.
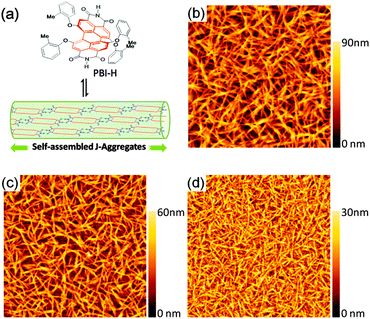
The chosen PBI molecule, i.e, PBI-H, for utilization as a cathode interlayer is shown in Fig. 1a.16 PBI-H bears two N–H groups at the imide positions and four bay-area phenoxy substituents. The bay-area substituents endow the perylene core with a twisted structure, which prohibits the sandwich-type face-to-face π-stacking and supports the hydrogen-bond driven self-assembly of these dyes into a slipped J-aggregate stacking arrangement (Fig. 1a).17 Whilst in earlier work a large number of, a total of twelve, solubilizing dodecyl chains were attached at the phenoxy groups to endow these dyes and their self-assembled J-aggregates with high solubility,17 some of us recently discovered that it is an effective method to tune the solubility of PBI by introducing much smaller methyl groups at the key ortho-positions of the bay-substituents and a self-assembly of PBI-H into nanofibers with the spectroscopic characteristics of J-aggregates can be achieved (Fig. S1, ESI†).16 The tight intermolecular stacking of PBI-H, thanks to the absence of long solubilizing alkyl chains, is a benefit for the electron transportation properties of devices.
The solubility of PBI-H is governed by its non-covalent interactions with the solvent molecules. Accordingly, in apolar solvents such as toluene and chlorobenzene the hydrogen-bond-directed growth of J-aggregate nanofibers is already observed for dilute solutions leading to a low solubility (<10−2 mg mL−1). In contrast, for the dipolar solvent tetrahydrofuran (THF) that has good solvation properties for π-surfaces as well as hydrogen bond acceptor qualities, the solubility of PBI-H becomes very high, i.e. >10 mg mL−1 at room temperature. Such a large solubility difference in different solvents enables the fabrication of multiple organic layers through the step-by-step solution procession method. For the application of PBI-H as an interfacial layer as discussed below, we investigated the morphology evolution with the change of PBI-H concentrations in THF. As shown in Fig. 1b–d, nanorod aggregates are generated during the spin-coating process from anhydrous THF solutions, and with the decreased PBI-H concentrations, the size of the aggregates became smaller. The substrate was covered uniformly by the nanorods spin-coated with 1 mg mL−1 THF solution (Fig. 1d). UV/Vis absorption spectra of similarly prepared PBI-H nanorods on the glass substrate reveal the typical J-aggregate absorption spectra, i.e. a sharp band at 650 nm that is bathochromically shifted by 80 nm compared to the monomeric dyes in solution (Fig. S2, ESI†). Upon rinsing of this substrate using chlorobenzene (which will be used to deposit the conjugated polymer/fullerene active layer) only a minor part of these aggregates dissolved whereas the main part remained on the substrate (Fig. S2, ESI†).
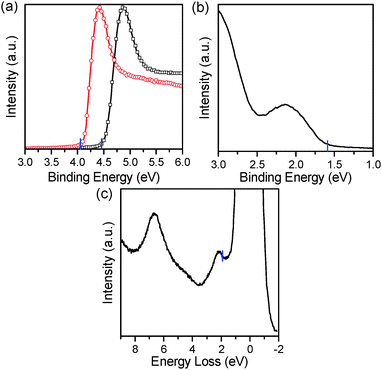
Based on these morphological investigations, we next modified the ITO electrode by spin-coating THF solution of PBI-H (1 mg mL−1) atop it. The electronic structure of the PBI-H modified ITO was then investigated using ultraviolet photoelectron spectroscopy (UPS)18 and the reflection electron energy loss spectrum (REELS), which have been successfully used to investigate ultrathin gate oxide materials in CMOS and polymers recently.19,20 The work function of the PBI-H modified ITO derived from the secondary electron cut-off shown in Fig. 2a was determined to be 4.04 eV which is 0.4 eV lower than that of bare ITO (4.45 eV). Various possible origins for the change of surface work function have been discussed, including the surface dipole due to the redistribution of the electron cloud upon molecular adsorption, the intrinsic molecular dipole moment that is normal to the surface and the chemical dipole due to the charge transfer between the adsorbate and the substrate.21 The origin in the given case may be related to the imide groups in the molecular structure and will be investigated in the future. Meanwhile, from Fig. 2b we observed that the HOMO level of PBI-H is located 1.60 eV lower than that of the Fermi level of the composite electrode of ITO/PBI-H. In addition, the band gap Eg of PBI-H that is estimated from the onset of the energy loss in the REELS spectra was determined to be 1.88 eV (Fig. 2c), which is in excellent agreement with the absorption edge of the J-aggregate (∼680 nm, see Fig. S1 and S2, ESI†). Therefore, the LUMO of PBI-H is located at 0.28 eV above the Fermi level of the composite electrode, while the ionization potential (IP) and electron affinity (EA) energy values of PBI-H were 5.64 eV and 3.76 eV, respectively. Indeed, these results imply that the ITO/PBI-H composite electrode can form an ohmic contact with PC71BM and accordingly facilitate electron extraction.
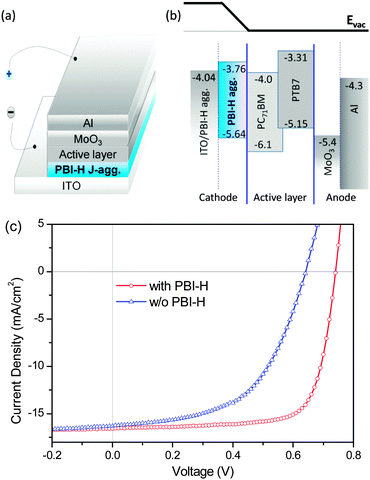
Motivated by this favorable decrease of the ITO work function by the modification with PBI-H J-aggregates, we next tried to use this composite electrode as a cathode to construct an i-PSC with device structure of ITO/PBI-H (10 nm)/PTB7:PC71BM (90 nm)/MoO3 (10 nm)/Al (100 nm) (Fig. 3a and b), where PTB7 is the electron donating polymer, namely poly[4,8-bis(2-ethylhexyloxyl)benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl-alt-ethylhexyl-3-uorothithieno[3,4-b] thiophene-2-carboxylate-4,6-diyl], PC71BM is the electron accepting material, and MoO3/Al acts as the anode. The current density versus voltage characteristics (J–V) of the PBI-H based i-PSCs in dark and under 1000 W m−2 air mass 1.5 global (AM 1.5 G) illumination are shown in Fig. 3c. The J–V curves of a control device without the PBI-H interlayer of ITO/PTB7:PC71BM (90 nm)/MoO3 (10 nm)/Al are also given for comparison (Fig. 3 and Table 1). As compared with the control device, the introduction of the PBI-H interlayer clearly improves the device performance, for instance, open circuit voltage (VOC) increased from 0.64 V to 0.74 V, short circuit current (JSC) increased from 16.30 mA cm−2 to 16.57 mA cm−2 and the fill factor (FF) improved from 54.7% to 74.4%. As a result, a high power conversion efficiency (PCE) of 9.11% was achieved in the PBI-H based inverted device. The increased device performance can be mainly attributed to the matched energy level between the composite cathode and the active layer for electron transport and collection. It is worth mentioning that the device PCE of the PBI-based i-PSC reported here is better than that of the conventional device22 and is very close to that of our best i-PSCs,2b for both using PFN as interlayers as shown in Table 1. Although PBI-based i-PSCs exhibited a slightly lower JSC compared with that of PFN based i-PSCs (16.57 mA cm−2vs. 17.46 mA cm−2), they showed an obviously higher FF (74.4% vs. 69.9%), which will be discussed in detail below. We also note that the too thick PBI-H layer (>20 nm) can be detrimental to performance (Table 1), especially in terms of JSC and FF, which can be mainly attributed to the relative low conductivity of the interlayer. Nevertheless, the incorporation of the thicker PBI-H layer can maintain a similar VOC to that of the optimized devices, indicating that the PBI-H layer indeed functions well as an ITO surface modifier.
| Device structure | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| ITO/PBI-H (10 nm)/PTB7:PC71BM/MoO3/Al | 0.74 | 16.57 | 74.4 | 9.11 |
| ITO/PBI-H (20 nm)/PTB7:PC71BM/MoO3/Al | 0.73 | 14.30 | 54.6 | 5.70 |
| ITO/PBI-H(30 nm)/PTB7:PC71BM/MoO3/Al | 0.73 | 11.04 | 42.1 | 3.39 |
| ITO/PTB7:PC71BM/MoO3/Al | 0.64 | 16.30 | 54.7 | 5.71 |
| ITO/PEDOT:PSS/PTB7:PC71BM/PFN/Al | 0.756 | 15.75 | 70.15 | 8.3722 |
| ITO/PFN/PTB7:PC71BM/MoO3/Al | 0.754 | 17.46 | 69.99 | 9.2142b |
We also investigate the applicability of PBI-H as an ITO surface modifier for other promising donor polymer systems, such as poly[N-9′′-hepta-decanyl-2,7-carbazole-alt-5,5-(4′,7′- di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT).23 Similarly, the inverted devices based on PBI-H showed superior device performance as compared with the control devices (Fig. S4 and Table S1, ESI†).
Such remarkable improvements in device performance are for sure related to the improved electron extraction efficiency by the pinned work function of the composite ITO electrode nearby the LUMO level of PC71BM forming an ohmic contact between them, and the hole blocking capability of the PBI-H layer due to its very low HOMO compared to that of PTB7, as shown in Fig. 3b. Some other beneficial effects might be, however, operative as well. Firstly, PBI-H J-aggregates possess relatively high electron transportation properties compared to the conventional interface material like PFN, which was revealed by the J–V characteristics of electron-dominated devices with device configuration of ITO/Al/interlayer/PC61BM/Ca/Al (see ESI,† Fig. S5). Such enhanced transportation properties may reduce the electron barrier between the active layer and the electrode, thus increasing the electron collection efficiency and also VOC of the devices. Secondly, the nanostructure of the PBI-H nanorods (Fig. 1) may increase the contact between the composite cathode and PC71BM and make it easier for the collection of electrons through the electron tunneling effect. A previous study showed that when being spin-coated with the polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM blend solution, the less soluble PCBM accumulated at the bottom while the polymer is accumulated at the top leading to the formation of vertical phase segregation.24 In an inverted device configuration, the photo-generated electron and hole transport through an increasing gradient of the acceptor and donor material is indeed highly favorable for charge collection as indicated by the energy level depicted above. The application of PBI based pigments as interlayer materials warrant further investigations and is ongoing in our lab.
PCBM blend solution, the less soluble PCBM accumulated at the bottom while the polymer is accumulated at the top leading to the formation of vertical phase segregation.24 In an inverted device configuration, the photo-generated electron and hole transport through an increasing gradient of the acceptor and donor material is indeed highly favorable for charge collection as indicated by the energy level depicted above. The application of PBI based pigments as interlayer materials warrant further investigations and is ongoing in our lab.
Conclusions
In conclusion, we introduced an example of small molecule based supramolecular cathode buffer layer materials based on solution-processible n-type PBI J-aggregates which afforded highly efficient inverted-type PSCs. Nanosized rod-like aggregates of PBI-H with J-type excitonic coupling were fabricated on ITO via self-assembly by the spin-coating method from anhydrous THF to afford a composite electrode on which a PTB7:PC71BM layer could be directly deposited from the usual chlorobenzene solvent. Compared to the device with a conventional device configuration, PSCs with PBI-H buffer layers show an enhanced power conversion efficiency of >9%, as a result of enhancements in the short current density, fill factor and open circuit voltage. The results reported here clearly indicate that n-type solution-processible PBIs are most promising interfacial materials for highly efficient PSCs and that future research is warranted to elucidate the impact of the supramolecular arrangement, optical features (J-aggregation), nanoporosity, PBI HOMO/LUMO energy levels and the origin of ITO work function pinning.Acknowledgements
We thank the support from the National Natural Science Foundation of China (No. 51225301, 51373054, 51403066, 51473052, 91333206), the National Basic Research Program of China (973 Program) (2013CB834705, 2014CB643504), the Guangdong Natural Science Foundation (S2012030006232), the Guangdong Innovative Research Team (No. 201101C0105067115), and the Bavarian State Ministry of Science, Research, and the Arts (Collaborative Research Network) ‘‘Solar Technologies go Hybrid’’.Notes and references
- (a) G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS; (b) S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef PubMed.
- (a) G. Li, C.-W. Chu, V. Shrotriya, J. Huang and Y. Yang, Appl. Phys. Lett., 2006, 88, 253503 CrossRef PubMed; (b) Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed; (c) M. Jørgensen, K. Norrman, S. A. Gevorgyan, T. Tromholt, B. Andreasen and F. C. Krebs, Adv. Mater., 2012, 24, 580 CrossRef PubMed; (d) A. Savva, F. Petraki, P. Elefteriou, L. Sygellou, M. Voigt, M. Giannouli, S. Kennou, J. Nelson, D. D. C. Bradley, C. J. Brabec and S. A. Choulis, Adv. Energy Mater., 2012, 2, 391 Search PubMed.
- (a) C. Waldauf, M. Morana, P. Denk, P. Schilinsky, K. Coakley, S. A. Choulis and C. J. Brabec, Appl. Phys. Lett., 2006, 89, 233517 CrossRef PubMed; (b) S. Chen, C. E. Small, C. M. Amb, J. Subbiah, T.-H. Lai, S.-W. Tsang, J. R. Manders, J. R. Reynolds and F. So, Adv. Energy Mater., 2012, 2, 1333 CrossRef CAS PubMed.
- (a) F. Huang, H. B. Wu and Y. Cao, Chem. Soc. Rev., 2010, 39, 2500 RSC; (b) Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun, J. Kim, M. Fenoll, A. Dindar, W. Haske, E. Najafabadi, T. M. Khan, H. Sojoudi, S. Barlow, S. Graham, J.-L. Brédas, S. R. Marder, A. Kahn and B. Kippelen, Science, 2012, 336, 327 CrossRef CAS PubMed.
- (a) C.-H. Hsieh, Y.-J. Cheng, P.-J. Li, C.-H. Chen, M. Dubosc, R.-M. Liang and C.-S. Hsu, J. Am. Chem. Soc., 2010, 132, 4887 CrossRef CAS PubMed; (b) N. Cho, H.-L. Yip, J. A. Davies, P. D. Kazarinoff, D. F. Zeigler, M. M. Durban, Y. Segawa, K. M. O'Malley, C. K. Luscombe and A. K.-Y. Jen, Adv. Energy Mater., 2011, 1, 1148 CrossRef CAS PubMed; (c) T. Stubhan, M. Salinas, A. Ebel, F. C. Krebs, A. Hirsch, M. Halik and C. J. Brabec, Adv. Energy Mater., 2012, 2, 532 CrossRef CAS PubMed; (d) T. Feng, B. Xiao, Y. Lv, Z. Q. Xie, H. B. Wu and Y. G. Ma, Chem. Commun., 2013, 49, 6283 RSC; (e) Z. G. Zhang, B. Y. Qi, Z. W. Jin, D. Chi, Z. Qi, Y. F. Li and J. Z. Wang, Energy Environ. Sci., 2014, 7, 1966 RSC.
- (a) Z. Tan, W. Zhang, Z. Zhang, D. Qian, Y. Huang, J. Hou and Y. Li, Adv. Mater., 2012, 24, 1476 CrossRef CAS PubMed; (b) F. Wang, Z. Tan and Y. Li, Energy Environ. Sci., 2015, 8, 1059 RSC.
- L. Nian, W. Zhang, N. Zhu, L. Liu, Z. Xie, H. Wu, F. Würthner and Y. Ma, J. Am. Chem. Soc., 2015, 137, 6995 CrossRef CAS PubMed.
- S. Liu, K. Zhang, J. Lu, J. Zhang, H.-L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2013, 135, 15326 CrossRef CAS PubMed.
- P. H. J. Schenning and E. W. Meijer, Chem. Commun., 2005, 3245 RSC.
- (a) F. Würthner, Chem. Commun., 2004, 1564 RSC; (b) M. R. Wasielewski, Acc. Chem. Res., 2009, 42, 1910 CrossRef CAS PubMed; (c) T. Seki, X. Lin and S. Yagai, Asian J. Org. Chem., 2013, 2, 708 CrossRef CAS PubMed.
- (a) M. Gsänger, J. H. Oh, M. Könemann, H. W. Höffken, A.-M. Krause, Z. Bao and F. Würthner, Angew. Chem., Int. Ed., 2010, 49, 740 CrossRef PubMed; (b) H. Lin, R. Camacho, Y. Tian, T. E. Kaiser, F. Würthner and I. G. Scheblykin, Nano Lett., 2010, 10, 620 CrossRef CAS PubMed; (c) T. E. Kaiser, H. Wang, V. Stepanenko and F. Würthner, Angew. Chem., Int. Ed., 2007, 46, 5541 CrossRef CAS PubMed.
- (a) H. C. Hesse, J. Weickert, C. Hundschell, X. Feng, K. Müllen, B. Nickel, A. J. Mozer and L. Schmidt-Mende, Adv. Energy Mater., 2011, 1, 861 CrossRef CAS PubMed; (b) C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613 CrossRef CAS PubMed; (c) X. Zhang, Z. Lu, L. Ye, C. Zhan, J. Hou, S. Zhang, B. Jiang, Y. Zhao, J. Huang, S. Zhang, Y. Liu, Q. Shi, Y. Liu and J. Yao, Adv. Mater., 2013, 25, 5791 CrossRef CAS PubMed; (d) X. Zhang, C. L. Zhan and J. N. Yao, Chem. Mater., 2015, 27, 166 CrossRef CAS; (e) Y. Z. Lin and X. W. Zhan, Mater. Horiz., 2014, 1, 470 RSC.
- (a) X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed; (b) R. Schmidt, J. H. Oh, Y.-S. Sun, M. Deppisch, A.-M. Krause, K. Radacki, H. Braunschweig, M. Könemann, P. Erk, Z. Bao and F. Würthner, J. Am. Chem. Soc., 2009, 131, 6215 CrossRef CAS PubMed.
- L. Zang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596 CrossRef CAS PubMed.
- G. Armstrong and M. Buggy, J. Mater. Sci., 2005, 40, 547 CrossRef CAS.
- Z. Xie, V. Stepanenko, B. Fimmel and F. Würthner, Mater. Horiz., 2014, 1, 355 RSC.
- (a) F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed; (b) Z. Xie, V. Stepanenko, K. Radacki and F. Würthner, Chem. – Eur. J., 2012, 18, 7060 CrossRef CAS PubMed.
- J. Meyer, S. Hamwi, M. Kröger, W. Kowalsky, T. Riedl and A. Kahn, Adv. Mater., 2012, 24, 5408 CrossRef CAS PubMed.
- H. C. Shin, D. Tahir, S. Seo, Y. R. Denny, S. K. Oh, H. J. Kang, S. Heo, J. G. Chung, J. C. Lee and S. Tougaard, Surf. Interface Anal., 2012, 44, 623 CrossRef CAS PubMed.
- D. Tahir and S. Tougaard, J. Appl. Phys., 2012, 111, 054101 CrossRef PubMed.
- (a) P. Amsalem, J. Niederhausen, A. Wilke, G. Heimel, R. Schlesinger, S. Winkler, A. Vollmer, J. P. Rabe and N. Koch, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 035440 CrossRef; (b) W. Osikowicz, X. Crispin, C. Tengstedt, L. Lindell, T. Kugler and W. R. Salaneck, Appl. Phys. Lett., 2004, 85, 1616 CrossRef CAS PubMed.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS PubMed.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. N. Plesu, M. Bellette, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS PubMed.
- M. Campoy-Quiles, T. Ferenczi, T. Agostinelli, P. G. Etchegoin, Y. Kim, T. D. Anthopoulos, P. N. Stavrinou, D. D. C. Bradley and J. Nelson, Nat. Mater., 2008, 7, 158 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, aggregation and absorption of PBI-H, XPS characterization, electron mobility, and additional device performance. See DOI: 10.1039/c5mh00056d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |