Probing cytochrome P450-mediated activation with a truncated azinomycin analogue†
Victoria Vinader a, Maria Sadiq a, Mark Sutherland a, Mengying Huang b, Paul M. Loadman a, Lina Elsalem a, Steven D. Shnyder a, Hongjuan Cui b, Kamyar Afarinkia a, Mark Searcey *c, Laurence H. Patterson a and Klaus Pors *a
aInstitute of Cancer Therapeutics, University of Bradford, West Yorkshire BD7 1DP, UK. E-mail: k.pors1@bradford.ac.uk
bState Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing, 400715, China
cSchool of Pharmacy, University of East Anglia, Norwich Research Park, Norwich NR4 7TJ, UK. E-mail: M.Searcey@uea.ac.uk
First published on 22nd October 2014
Abstract
A deactivated alkene precursor (IC50 = 81 μM) to the azinomycin epoxide natural product can be bioactivated by several cytochromes P450 (CYP) to generate antiproliferative metabolites with increased potency (IC50 = 1–30 μM) in CHOwt cells. CYP1A1 and 3A4 were shown to generate exclusively the unnatural and the natural-configured azinomycin epoxide diastereoisomer respectively, while CYP1B1 produced both epoxides in a 3 : 1 mixture. The antiproliferative activity is linked to DNA damage as demonstrated using the comet assay.
The azinomycins A (1), B (2) and the azinomycin epoxide (3) are natural products isolated from Streptomyces griseofuscus (strain S42227) that have been extensively studied due to their potent antitumour activity, their novel structures and their mode of action.1–4 The complete azinomycin A and B structures 1 and 2 (Fig. 1) bind to the major groove of DNA and form interstrand crosslinks through alkylation by the epoxide and aziridine moieties.5,6 The truncated epoxide structure 3 has been shown to alkylate at the N7 of guanine and promote a depurination reaction that leads to single strand breaks.7 The naphthalene moiety is important in 3 due to an ability to intercalate into the DNA duplex following alkylation.8
Although the azinomycins possess potent cytotoxicity in vitro, they have variable in vivo activity, most likely as a consequence of the metabolic instability of the epoxide and aziridine moieties. In studies to define the biosynthetic pathways to the azinomycins, Watanabe and co-workers have demonstrated that valine is the precursor to the epoxide.9 This amino acid undergoes a series of transformations to generate 3-methyl-2-oxobutenoic acid, which is predicted to be reduced to the alcohol and incorporated into the azinomycin structure. Enzymatic epoxidation of the alkene amide also occurs in this sequence of events. This incorporation of an alkene is similar to the synthetic sequence described previously and adapted by us to generate all four diastereoisomers of 3.7 We hypothesised that the alkene derivative has utility as a bioprecursor that could increase the therapeutic utility of the azinomycins in malignant tissues that (over) express enzymes capable of epoxidising xenobiotics notably the cytochrome P450 (CYP) monooxygenases. Several drug metabolising CYPs have been shown to be expressed at high frequency in cancer tissues10 and we have focused our attention on several CYP1 and 2 family members as tumour selective prodrug activating enzymes. We developed AQ4N, a Phase II clinical trial candidate, to undergo bioreductive activation by CYP1A1 and 3A4 selectively in hypoxic tumours.11,12 More recently we demonstrated that bioprecursors of the duocarmycins can be activated selectively to ultrapotent anti-proliferative metabolites by tumours expressing CYP1A1 (ref. 13) and 2W1 (ref. 14) and we are proposing this approach to address the failure of the duocarmycins in the clinic due to unacceptable normal tissue toxicity. Similarly, despite the promise of the azinomycins as cancer chemotherapeutics, they have thus far failed to progress clinically.
Towards the aim of developing a class of chemotherapeutics based on the azinomycins we describe the synthesis and biological evaluation of a library of azinomycin epoxide bioprecursors with potential for CYP bioactivation.
We previously7 adopted the stereoselective synthesis of the epoxide 4 using Shipman's methodology (i.e. a Sharpless dihydroxylation) in order to set the stereochemistry of the subsequent alkene (in this case, compound 6). In this study, we reasoned that as a proof-of-concept, synthesis of the racemic alkene is appropriate, as it is not predictable as to whether the stereochemistry of the naphthoate ester amide fragment has an influence on enzymic oxidation of the alkene by selected CYPs. The benzyl ester of commercially available 3,3-dimethylacrylic acid was epoxidised directly using m-CPBA to give an excellent yield (93%) of the racemic compound, a reaction which can be carried out on a multigram scale. Acid catalysed ring opening to give the alkene was followed by direct treatment with ammonia to give the amide 6.
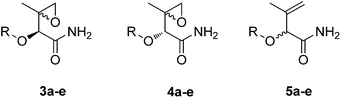
It has previously been shown that changes in substitution on the naphthalene ring maintain antitumour activity.15 In this study we wanted to establish whether the substitution pattern influenced CYP mediated epoxidation of the alkene. To facilitate this structure activity relationship (SAR) for CYP-catalysed epoxidation, we introduced a number of different analogues of the naphthalene moiety alongside the natural product structure. The aromatic substituents were introduced via DCC mediated coupling with fragment 6 (Scheme 1). Subsequent chemical oxidation of the alkenes gave a mixture of diastereoisomers that were separable into the racemic mixtures. The relative stereochemistry was assigned through reference to the H2 proton (-proton) in the 1H NMR which was shifted upfield (5.32 from 5.21 ppm) in the configuration opposite to that found in the natural azinomycin analogue.
 | ||
| Scheme 1 Synthesis of compounds 3–6. | ||
To determine the cytotoxicity of the truncated azinomycin precursors, chemosensitivity studies were carried out using a Chinese Hamster Ovary (CHO) cell line +/− transfected with CYP1A1. The anti-proliferative activity of the various naphthalene derivatives against this isogenic pair of CHO cell lines was assessed using the MTT cell viability assay (Table 1). The substitution pattern of the naphthalene moiety in the epoxyamide compounds 3 and 4 has little effect on the biological activity, with IC50 values between 2 and 10 μM for all compounds tested. However, incubation of the alkenes 5a–e with the cell lines demonstrated that only the natural product naphthalene structure (5a) was bioactivated in the CYP1A1 expressing cell line, indicating that the substitution pattern on the naphthalene moiety affects the binding mode with CYP1A1.
Since the naphthoate substitution pattern of compound 5a is that of the natural azinomycin we explored whether other CYPs important in drug metabolism could influence its cytotoxicity. Bioactivation by CYP1A1 was compared to CYP1B1, known to be overexpressed in many tumour types16 and three hepatic CYPs (1A2, 2D6 and 3A4). NADPH dependent CYP/CYP reductase expressing bactosomes were incubated for 1 h with 5a. Subsequently the incubation extract was added to CHO wild type cells which were subjected to the MTT antiproliferative assay. This revealed that all CYPs could significantly (3–24 fold) potentiate 5a activity. Potentiation by CYP3A4 (24.4-fold) was much greater than CYP1B1 (7-fold) or CYP1A1 (5.8-fold), Table 2. The bioactivation of the CYP bactosomes brings the potency of the inactive azinomycin precursor to the low micromolar region, which is similar to what we have observed for the four diastereoisomers of compound 3 in the NCI 60 cell line panel.7 The CYP-mediated potentiation observed here suggests the possible formation of an azinomycin epoxide metabolite capable of causing DNA damage. We therefore explored the nature of this activation further.
| 5a | 5a + CYP1A1 | 5a + CYP1A2 | 5a + CYP1B1 | 5a + CYP2D6 | 5a + CYP3A4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50a | IC50 | PFb | IC50 | PFb | IC50 | PFb | IC50 | PFb | IC50 | PFb |
| a IC50 = μM. b Potentiation factor (IC50 of 5a in CHO cells/IC50 of 5a + CYP isoform metabolites). | ||||||||||
| 81.61 ± 4.23 | 14.15 ± 3.07 | 5.8 | 20.30 ± 1.46 | 4.0 | 11.75 ± 2.36 | 7.0 | 28.31 ± 2.06 | 2.9 | 3.34 ± 0.92 | 24.4 |
To investigate if the antiproliferative activity could be correlated with DNA damage, CHO cells were treated with bioprecursor 5a or the metabolite fraction generated from 5a incubation with the CYP3A4 bactosomes. After 1 h exposure to cells, DNA damage was assessed using the comet assay. EO9, camptothecin and melphalan were used as positive controls for DNA single strand breaks (SSBs), DNA double strand breaks (DSBs) and DNA interstrand cross-links (ICLs), respectively. The results showed that CYP3A4-generated metabolites were capable of producing DNA damage primarily through SSBs (Fig. 2) with no evidence of DSBs as measured by increase in mean tail moment (MTM) (Fig. S3†).
 | ||
| Fig. 2 DNA damage caused by 5a metabolite generated from incubation with CYP3A4. DSBs and SSBs were measured by increase in MTM (A) and ICLs by a decrease in MTM (B). Typical comet images show SSBs with no drug (C), 20 μM E09 (D), 50 μM 5a (E) and 50 μM CYP3A4-metabolised 5a (F). DNA ICLs with no drug (G), 20 μM melphalan (H), 50 μM 5a (I) and 50 μM CYP3A4-metabolised 5a (J). The results are the mean ± SD of at least three independent assays. | ||
The results also indicated that a small amount of ICLs were generated. This is intriguing because it would require two sites of metabolic activation to generate the requisite proximate crosslinking alkylation sites. Furthermore, 5a alone produced no DNA damage confirming that CYP-activation is required for the generation of DNA damage.
CYP2W1 has been reported to be expressed in both primary17 and metastatic18 colon cancer tissue but not healthy tissues and we are engaged with discovering new pharmacophores that are bioactivated by CYP2W1. In this context, we explored the potential for 5a to be bioactivated by CYP2W1 using our isogenic SW480 +/− CYP2W1 cell line pair, but we did not observe any significant antiproliferative activity in the CYP2W1-transfected cells (Fig. S4†). However, 5a displayed a dose-dependent growth inhibition against two human gastric cancer cell lines, SGC-7901 and MKN-45, which expressed CYP1A1 and 3A4 at both gene and protein level (Fig. 3).
 | ||
| Fig. 3 CYP1A1 and 3A4 expression and antiproliferative activity in gastric cell lines SGC-7901 and MKN-45. CYP1A1 and 3A4 expression was measured by qRT-PCR (A) and Western blot (B). The growth inhibition was measured using the MTT assay (C). | ||
 | ||
| Fig. 4 Metabolism profile of 5a incubated with CYP1A1, 1B1 or 3A4. | ||
To identify the active metabolites likely to be responsible for the DNA damage and CYP-associated antiproliferative activity, metabolite fractions from CYP1A1, 1B1 and 3A4 were analysed by LC/MS/MS. All three CYPs oxidised the alkene to the epoxide, the anticipated alkylating species of these truncated azinomycins. However the epoxide stereochemistry generated by different CYP isoforms was not the same. CYP1A1 and 3A4 were shown to generate exclusively the ‘unnatural’ diastereoisomer epoxide 3a and the naturally-configured epoxide 4a respectively, while CYP1B1 produced both epoxides in a 3 : 1 mixture as identified by co-elution with the respective authentic truncated azinomycins (Fig. 4). The IC50 data and our CYP-metabolism data from this study are consistent with the high potency of the naturally-configured diastereoisomer epoxides previously observed.7
The identification of a m/z 330 metabolite, consistent with epoxide formation (Scheme 2), and DNA SSBs following CYP-oxidation indicates the potential for bioactivation of the precursor azinomycins prepared in this study.
 | ||
| Scheme 2 Generation of metabolites by CYP1A1, 1B1 and 3A4. | ||
Conclusions
We have demonstrated that the putative bioprecursor 5a to the natural azinomycin product 3 can be activated in cells expressing a number of CYPs known to be responsible for drug metabolism. Certain isoforms, notably CYP1A1, 1B1 and 3A4 are identified as highly expressed in certain tumours10 indicating the value of truncated azinomycin analogues as potential bioprecursors with chemotherapeutic efficacy. We have demonstrated proof of principle with bioprecursors of the duocarmycin natural products targeting CYP1A1 (ref.13 and 19) and 2W1 (ref. 14 and 20) enzymes. Uniquely, we have demonstrated that CYP selective bioactivation in vivo can be obtained with no overt toxicity to normal tissues including the liver.13,14 Further development of such a bioprecursor approach could provide a strategy to exploit the azinomycins for therapeutic use. While CYP1A1 and 1B1 provide selective cancer drug targets, the hepatic CYP3A4 enzyme could be exploited for liver specific treatment of hepatitis B, hepatitis C and hepatocellular carcinoma21 or with the reemergence of the oncolytic virus, a ‘suicide’ GDEPT approach also exists as a therapeutic strategy.22,23Acknowledgements
The authors wish to gratefully acknowledge financial support from Yorkshire Cancer Research for Medicines Discovery Programme grant number B209 and EPSRC Science Bridges China for grant number EP/G042365/1. We thank Miss Gemma Marston and Miss Komal Nadeem for their contribution to generation of experimental data and the EPSRC NMSSC for HRMS data.References
- G. T. Kelly, C. Liu, R. Smith 3rd, R. S. Coleman and C. M. Watanabe, Chem. Biol., 2006, 13, 485–492 CrossRef CAS PubMed.
- S. Ishizeki, M. Ohtsuka, K. Irinoda, K. Kukita, K. Nagaoka and T. Nakashima, J. Antibiot., 1987, 40, 60–65 CrossRef CAS.
- K. Nagaoka, M. Matsumoto, J. Oono, K. Yokoi, S. Ishizeki and T. Nakashima, J. Antibiot., 1986, 39, 1527–1532 CrossRef CAS.
- K. Yokoi, K. Nagaoka and T. Nakashima, Chem. Pharm. Bull., 1986, 34, 4554–4561 CrossRef CAS.
- R. S. Coleman, R. J. Perez, C. H. Burk and A. Navarro, J. Am. Chem. Soc., 2002, 124, 13008–13017 CrossRef CAS PubMed.
- R. S. Coleman, R. L. Woodward, A. M. Hayes, E. A. Crane, A. Artese, F. Ortuso and S. Alcaro, Org. Lett., 2007, 9, 1891–1894 CrossRef CAS PubMed.
- M. H. David-Cordonnier, M. Casely-Hayford, M. Kouach, G. Briand, L. H. Patterson, C. Bailly and M. Searcey, ChemBioChem, 2006, 7, 1658–1661 CrossRef CAS PubMed.
- M. A. Casely-Hayford, K. Pors, L. H. Patterson, C. Gerner, S. Neidle and M. Searcey, Bioorg. Med. Chem. Lett., 2005, 15, 653–656 CrossRef CAS PubMed.
- V. Sharma, G. T. Kelly and C. M. Watanabe, Org. Lett., 2008, 10, 4815–4818 CrossRef CAS PubMed.
- C. Rodriguez-Antona and M. Ingelman-Sundberg, Oncogene, 2006, 25, 1679–1691 CrossRef CAS PubMed.
- A. Yakkundi, V. McErlane, M. Murray, H. O. McCarthy, C. Ward, C. M. Hughes, L. H. Patterson, D. G. Hirst, S. R. McKeown and T. Robson, Cancer Gene Ther., 2006, 13, 598–605 CrossRef CAS PubMed.
- L. H. Patterson, S. R. McKeown, T. Robson, R. Gallagher, S. M. Raleigh and S. Orr, Anti-Cancer Drug Des., 1999, 14, 473–486 CAS.
- M. Sutherland, J. H. Gill, P. M. Loadman, J. P. Laye, H. M. Sheldrake, N. A. Illingworth, M. N. Alandas, P. A. Cooper, M. Searcey, K. Pors, S. D. Shnyder and L. H. Patterson, Mol. Cancer Ther., 2013, 12, 27–37 CrossRef CAS PubMed.
- S. Travica, K. Pors, P. M. Loadman, S. D. Shnyder, I. Johansson, M. N. Alandas, H. M. Sheldrake, S. Mkrtchian, L. H. Patterson and M. Ingelman-Sundberg, Clin. Cancer Res., 2013, 19, 2952–2961 CrossRef CAS PubMed.
- T. J. Hodgkinson, L. R. Kelland, M. Shipman and F. Suzenet, Bioorg. Med. Chem. Lett., 2000, 10, 239–241 CrossRef CAS.
- M. C. McFadyen and G. I. Murray, Future Oncol., 2005, 1, 259–263 CrossRef CAS PubMed.
- K. Stenstedt, M. Hallstrom, I. Johansson, M. Ingelman-Sundberg, P. Ragnhammar and D. Edler, Anticancer Res., 2012, 32, 3869–3874 Search PubMed.
- K. Stenstedt, M. Hallstrom, F. Ledel, P. Ragnhammar, M. Ingelman-Sundberg, I. Johansson and D. Edler, Acta Oncol., 2014, 53, 885–891 CrossRef CAS PubMed.
- K. Pors, P. M. Loadman, S. D. Shnyder, M. Sutherland, H. M. Sheldrake, M. Guino, K. Kiakos, J. A. Hartley, M. Searcey and L. H. Patterson, Chem. Commun., 2011, 47, 12062–12064 RSC.
- H. M. Sheldrake, S. Travica, I. Johansson, P. M. Loadman, M. Sutherland, L. Elsalem, N. Illingworth, A. J. Cresswell, T. Reuillon, S. D. Shnyder, S. Mkrtchian, M. Searcey, M. Ingelman-Sundberg, L. H. Patterson and K. Pors, J. Med. Chem., 2013, 56, 6273–6277 CrossRef CAS PubMed.
- M. D. Erion, K. R. Reddy, S. H. Boyer, M. C. Matelich, J. Gomez-Galeno, R. H. Lemus, B. G. Ugarkar, T. J. Colby, J. Schanzer and P. D. Van Poelje, J. Am. Chem. Soc., 2004, 126, 5154–5163 CrossRef CAS PubMed.
- H. Lu, C. S. Chen and D. J. Waxman, Cancer Gene Ther., 2009, 16, 393–404 CrossRef CAS PubMed.
- H. O. McCarthy, A. Yakkundi, V. McErlane, C. M. Hughes, G. Keilty, M. Murray, L. H. Patterson, D. G. Hirst, S. R. McKeown and T. Robson, Cancer Gene Ther., 2003, 10, 40–48 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for representative compounds synthesised and description of biological assays. See DOI: 10.1039/c4md00411f |
| This journal is © The Royal Society of Chemistry 2015 |