CCK8 peptide-labeled Pluronic® F127 micelles as a targeted vehicle of gold-based anticancer chemotherapeutics
Chiara Nardon† a, Giulia Boscutti† a, Lisa Dalla Via b, Paola Ringhieri c, Vito Di Noto a, Giancarlo Morelli c, Antonella Accardo *c and Dolores Fregona *a
aUniversity of Padova, Department of Chemical Sciences, Via Marzolo 1, 35131, Padova, Italy
bUniversity of Padova, Department of Pharmaceutical and Pharmacological Sciences, Via Marzolo 5, 35131, Padova, Italy
cUniversity of Naples Federico II, Department of Pharmacy, CIRPeB & IBB CNR, Via Mezzocannone 16, 80134 Naples, Italy. E-mail: antonella.accardo@unina.it; Fax: +39 0812536642; Tel: +39 0812532045
First published on 30th September 2014
Abstract
The bioavailability and target selectivity of chemotherapeutics are significant issues in drug development. Here, we report the loading of the antiproliferative gold(III) complex, dibromo[ethyl-N-(dithiocarboxy-kS,kS′)-N-methylglycinato] gold(III) (AuL12), into the lipophilic core of micelles produced from the surfactant Pluronic® F127 (PF127). When AuL12 is encapsulated in PF127-based micelles it remains stable in saline solution up to 72 h with the gold center in the +3 oxidation state. PF127-based aggregates are efficient carriers as they enhance the water solubility of the gold complex. In vitro studies indicate that after micelle encapsulation, AuL12 gold complex preserves its antiproliferative efficacy. Moreover, by labeling the hydrophilic shell of micelles with the bioactive CCK8 peptide, the aggregates act as target-selective vehicles. In fact, cytotoxic activity towards the A431 cells overexpressing the CCK2 receptors is 10-fold higher than that towards the control cells.
1. Introduction
In the last decade, from the biological point of view, we have designed, synthesized, widely characterized and studied many metal-based anticancer agents with the drug, cisplatin, being their archetype (Fig. 1A, compound 1).1–5 Among metal-based anticancer agents, the gold(III) dithiocarbamato complexes have proved to be very promising because of their biological activity, despite their low water solubility. Many metal-based anticancer agents dissolve in isotonic saline solutions to a lesser extent than cisplatin, with the latter intravenously administered in the presence of sugars such as mannitol and dextrose.6–8 However, in contrast to the reference drugs, which cause severe side effects, including nausea, alopecia and nephrotoxicity, the toxicological profile of our potential anticancer drugs is to date encouraging in animal models, likely resulting from an enhanced selectivity toward cancerous cells.1,2,9,10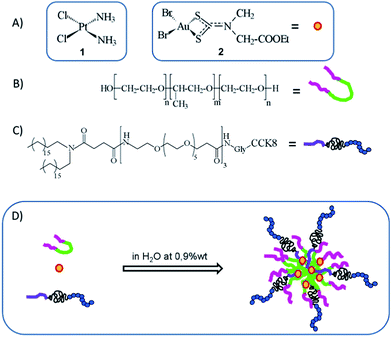 | ||
| Fig. 1 Chemical representation of cis-diaminodichloroplatinum(II) (cisplatin) (A-1), dibromo[ethyl N-(dithiocarboxy-kS,kS′)-N-methylglycinato] gold(III) (AuL12) (A-2), non-ionic surfactant Pluronic® F127 with n = 98 and m = 57 (B), (C18)2-PEG1000-G-CCK8 (C) and schematic representation of AuL12 loaded into PF127/(C18)2-PEG1000-G-CCK8-targeted micelles (D). | ||
In this work, we studied the incorporation of an active gold(III) dithiocarbamate complex into supramolecular aggregates in order to increase its bioavailability. Moreover, the functionalization of the aggregate with a targeting moiety (the cholecystokinin octapeptide CCK8) was carried out to obtain tumor-selective delivery, thus further improving the chemotherapeutic index of our compounds.
The gold(III)–dithiocarbamato complex, AuL12 (Fig. 1A, compound 2), was chosen as a model compound because of its good antiproliferative rates, which were recorded both in vitro and in vivo.11,12 The gold(III) derivative was loaded into two types of self-assembling supramolecular aggregates, consisting of either the tri-block copolymer Pluronic® F127 (PF127, Fig. 1B) or its combination with the amphiphilic peptide (C18)2-PEG1000-G-CCK8 (Fig. 1C), thus yielding non-targeted (hereafter referred as PF127) and targeted micelles (referred as PF127/CCK8), respectively. In fact, after undergoing self-assembly, the second type of drug carrier is characterized by the presence of the CCK8 octapeptide bound to the hydrophilic corona of the resulting micelles (Fig. 1D).
Among the nanocarriers that are studied for biomedical applications, the non-ionic surfactant Pluronic® F127 is the one that has been most frequently used.13,14 Regarding its biocompatibility, it is worth emphasizing that this class of organic carriers remains metabolically intact until renal clearance and has been proven to exhibit little or no toxic effects.14,15 The polymer is characterized by the amphiphilic architecture of the A–B–A type, comprising a central hydrophobic region (poly-propyleneoxide, PPO) and two terminal hydrophilic portions (poly-ethyleneoxide, PEO) (Fig. 1B), which above the critical micelle concentration (2.8 × 10−6 M at 37 °C, pH 7.4)16 undergoes self-assembly and yields spherical micelles.
With regards to the mixed micelles PF127/CCK8, it is worth highlighting that the targeting peptide CCK8 belongs to the family of cholecystokinin (CCK) peptides whose receptors are normally present on the cell surface but are known to be upregulated after malignant transformation.17,18 Two different receptor types, namely, CCK1-R and CCK2-R, recognize CCK and mediate its action.18 Both the receptors are predominantly located in the gastrointestinal tract and in the central nervous system.19 Moreover, CCK1-R has been found to be overexpressed in a number of pancreatic adenocarcinomas, as well as in gastroenteropancreatic neuroendocrine tumors (38%), meningiomas (30%), and in certain neuroblastomas (19%); however, CCK2-R has been found to be overexpressed in stromal ovarian cancers (100%) and has been shown to be upregulated in a large percentage of medullary thyroid cancers (92%), astrocytomas (62%) and small-cell lung cancers (57%).17,20–23 The octapeptide amide CCK8 (amino acid sequence: Asp26-Tyr27-Met28-Gly29-Trp30-Met31-Asp32-Phe33-amide) binds both the receptor subtypes with nanomolar affinity, even after being modified on its N-terminus with chelating agents24 or alkyl chains.25,26 In fact, it has been successfully used to deliver, in a selective way, supramolecular aggregates carrying contrast agents for nuclear medicine applications27 and/or anticancer drugs.28
2. Experimental
2.1 Materials
Protected Nα-Fmoc-amino acid derivatives, coupling reagents and Rink amide p-methylbenzhydrylamine (MBHA) resin were purchased from Calbiochem-Novabiochem (Lafelfingen, Switzerland). Fmoc-21-amino-4,7,10,13,16,19-hexaoxaheneicosanoic acid (Fmoc-Ahoh-OH) was purchased from Neosystem (Strasbourg, France). N,N-Dioctadecylsuccinamic acid was synthesized according to the previously published methods.29 A tri-block copolymer, containing ethylene oxide (EO) and propylene oxide (PO) groups, with the trade name Pluronic® F127 (PF127) and an approximate formula of EO98PO57EO98 (average molecular weight of 12 600 g mol−1), was obtained from BASF Svenska AB (Helsingborg, Sweden). Nitric acid (70%) and hydrochloric acid (37%), both for trace analysis, were obtained from Sigma Aldrich. Gold standard solution (1000 ppm in 5 M HCl) was supplied by ROMIL – Pure Chemists. The snake skin dialysis tubing (3.5 kDa MWCO) was purchased from Pierce Protein (USA). All other chemicals were commercially available from Sigma-Aldrich (Bucks, Switzerland) or LabScan (Stillorgan, Dublin, Ireland) and were used as received unless otherwise stated. All the solutions were prepared by weight using double distilled water. UV-Vis measurements were performed on a UV-Vis Jasco V-5505 spectrophotometer (Easton, MD) equipped with a Jasco ETC-505T Peltier temperature controller with a 1 cm quartz cuvette (Hellma). ICP-AES measurements were carried out using an inductively coupled plasma atomic emission spectrometer (ICP SPECTRO Arcos with EndOnPlasma torch; Spectro Analytical, Kleve, Germany) equipped with a capillary cross-flow nebulizer.2.2 Peptide amphiphile synthesis
(C18)2-PEG1000-G-CCK8 peptide amphiphile was synthesized using standard solid-phase 9-fluorenylmethoxycarbonyl (Fmoc) procedures. The Rink amide MBHA resin (substitution 0.65 mmol g−1) was used to be the solid-phase support, and the synthesis was performed at a scale of 0.1 mmol. The elongation of the peptide Gly-CCK8 was achieved by the sequential addition of Fmoc-AA-OH to PyBOP/HOBt/DIPEA (1/1/2) as coupling reagents in DMF in the pre-activation mode. All the couplings were performed twice for 1 hour by an excess of 4 equivalents for a single amino acid. Fmoc deprotection was carried out with a 30% solution of piperidine in DMF after the coupling of each amino acidic residue. When the Gly-CCK8 synthesis was complete, the Fmoc N-terminal protecting group was removed and three residues of the Fmoc-Ahoh-OH hexaoxoethylene linker and the N,N-dioctadecylsuccinamic acid were sequentially condensed as previously reported.30 Then, the peptide amphiphile was fully deprotected and cleaved from the resin with TFA and 2.5% (v/v) water and 2.5% (v/v) TIS as scavengers, at room temperature, and then precipitated with ice-cold water. The precipitate was then filtered, dissolved in H2O–CH3CN mixture and lyophilized. The crude peptide derivative was purified by RP-HPLC. The purity and identity were assessed with analytical LC-MS analysis.(C18)2-PEG1000-G-CCK8: Rt =17.26 min; MS (ESI): m/z: 2724 calcd. for C136H226N15O37S2; [M + 2H+]/2= 1363.0.
2.3 Aggregate formulation and DLS characterization
Pure PF127 micelles were prepared by dissolving the copolymer at 2% w/w in an aqueous solution. Mixed PF127/(C18)2-PEG1000-G-CCK8 micelles were prepared by adding 4% w/w of the CCK8 peptide amphiphile to PF127. Briefly, the two amphiphiles were dissolved in a small amount of MeOH/CHCl3 (50 : 50) and a thin film of amphiphiles was obtained by evaporating the solvent by slowly rotating the tube containing the solution under a steady stream of nitrogen. The lipid film was hydrated (1 mL) for 5 min by vortexing, and it was used without further treatment. The concentrations of CCK8-containing solutions were determined by absorbance with a UV-Vis Jasco (Easton, MD) Model 440 spectrophotometer with a path length of 1 cm and a molar absorptivity (ε280) of 6845 M−1 cm−1 for CCK8. This value was calculated according to the Edelhoch method,31 taking into account the contributions from the tyrosine and tryptophan residues present in the primary structure, which amount to 1215 and 5630 M−1 cm−1, respectively.32 The mean diameter was measured using a ZetasizerNano ZS (Malvern Instruments, Westborough, MA) that employs a 173° backscatter detector. The other instrumental settings were as follows: measurement position (mm): 4.65; attenuator: 8; temperature 25 °C; cell: disposable sizing cuvette. The concentration of the DLS samples was 2.0 × 10−4 M, and it was centrifuged at room temperature at 13 000 rpm for 5 min. For each batch, the hydrodynamic radius and size distributions were the means of three measurements and the final values were calculated as the means of three different batches.2.4 AuL12 synthesis and micelle loading
AuL12 was synthesized, purified (purity->96%, analyzed by elemental analysis and chromatography) and fully characterized according to our previously reported procedure.33 Loading of the AuL12 cytotoxic agent in the PF127 pure micelles and in PF127/(C18)2-PEG1000-G-CCK8 mixed micelles was performed via the thin film hydration method.34 Briefly, following the previously described lipid film preparation, AuL12 (0.35 mg in 20 mg of PF127) was added to a stock solution (1 mg mL−1) prepared in a chloroform–methanol (50/50) mixture. The organic solvent was freshly evaporated under a nitrogen flow and an aqueous solution containing 10 mM PBS or 0.9% w/w NaCl was added as previously described. Subsequently, the unloaded AuL12 was removed using a pre-equilibrated Sephadex G50 column. The drug loading coefficient (DL%), defined as the weight percentage between the encapsulated AuL12 complex and the micelle components plus the gold complex; the encapsulation ratio (ER%), defined as the weight percentage of gold complex encapsulated in the micelles in the total compound previously added during preparation; and the AuL12 concentrations were all quantified using ICP-AES analysis (inductively coupled plasma atomic emission spectroscopy) after the subtraction of the amount of AuL12 removed from the column from the total amount of AuL12 loaded earlier.2.5 Stability studies by UV-Vis spectrophotometry
Stability in the saline solution of the compound AuL12 alone (pre-dissolved in DMSO) and of AuL12 loaded in either targeted or non-targeted PF127-based micelles (final complex concentration of 100 μM) was studied by recording various electronic spectra over 24 h (or either 72 h) at 25 °C between 240 and 500 nm. The other experimental settings were as follows: scan speed-300 nm min−1, spectral bandwidth-2.0 nm, average time-0.100 s and data pitch-0.5 nm.2.6 Fluorescence spectroscopy
The fluorescence emission spectra were recorded using a Jasco Model FP-750 spectrofluorometer (Easton, MD) equipped with a Peltier temperature controller in a 1.0 cm path length quartz cell at 25 °C. The emission spectra of the CCK8 peptide-containing micelles were recorded at room temperature between 290 and 450 nm at an excitation wavelength of 280 nm at a CCK8 peptide concentration of 1.0 × 10−5 M in saline solution. Equal excitation and emission bandwidths were used throughout the experiments with a recording speed of 125 nm min−1 and the automatic selection of the time constant.2.7 AuL12 release
Previously prepared targeted and untargeted AuL12-containing micelles were transferred into a dialysis bag (MW cut-off = 3500 Da), which was immediately placed into 15 mL (hereinafter, V0) of water solution containing 0.9% wt NaCl and incubated with stirring for 72 h at 37 °C. 2 mL (VD) of the dialyzed solution was withdrawn at different time points to evaluate the released drug amount over time; this volume was immediately replenished with the same amount of a fresh saline solution. We assumed that the crossing of gold-containing species through the dialysis membrane quickly occurred, and that the overall release of AuL12 from the micelle vehicle to the dialysis bag medium corresponded to the rate determining step.The extent of gold complex release was evaluated by ICP-AES analysis (AuI λem= 242.795 nm) as the ratio of the concentration of released metal (CJ) to the total metal that was previously loaded into the micelles. In particular, CJ is the released concentration immediately after drawing of each sample (labeled as J) and refilling of the removed volume, VD. Eqn (1) defines CJ (wherein the subscript “a” stands for “analysed”) and the first term takes into account the effects of both drawing (system resulting in a volume of 13 mL = VR) and dilution (system volume = V0). The second term considers all the 2 mL aliquots related to the jth drawing and the ith previous aliquots, α and β being VR/V0 and VD/V0, respectively. The second term could be neglected if VD ≪ V0, which is not true in our case.
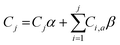 | (1) |
Then, we analyzed the overall release kinetics from the micelles via experimental data fitting assuming a first-order dissociation process.35Eqn (2) describes that AuL12 is released from the supramolecular aggregates because of two parallel dissociation events. The parameters a1 and a2 are the maximum amount of gold compound released during the first or the second event, respectively. The parameters k (s−1) are the overall experimental rate constants, which correspond to the combination of single microscopic rate constants associated with the different types of AuL12 unloading processes from the micelle hydrophobic core.
| CJ = a1(1 − e−k2t)+ a2(1 − e−k2t) | (2) |
By fitting the CJvs. t profile with eqn (2), the following parameters were obtained:
a 1 = 3470 ± 26 ppb; a2 = 1730 ± 57 ppb; k1 = (6.7 ± 0.3) × 10−4 s−1; k2 = (8.7 ± 0.8) × 10−6 s−1.
A standard solution of gold(III) (1000 ppm) was used to prepare nine solutions by sequential dilution in 5% (v/v) aqua regia (HCl–HNO3 3 : 1 v/v), to yield the calibration curves in the range 2–150 ppb. Each sample was mineralized with aqua regia, using nitric acid and hydrochloric acid in double distilled water, to a final aqua regia content of 5% v/v. Analytical determinations were performed using a plasma power of 1.4 kW, a radiofrequency generator of 27.12 MHz and an argon gas flow with nebulizer, auxiliary, and coolant set at 0.8, 0.8 and 13 L min−1, respectively.
2.8 Cell lines and cultures
A431 cells (epidermoid carcinoma) were obtained from the American type culture collection (ATCC). Cells were grown under a humidified atmosphere containing 5% CO2 at 37 °C in Dulbecco's modified Eagle's medium (DMEM, Sigma Aldrich) supplemented with D-glucose (final concentration 4.5 g L−1, Sigma), 10% heat-inactivated fetal bovine serum (Invitrogen), 100 U mL−1 penicillin (Sigma), 100 μg mL−1 streptomycin (Sigma) and 0.25 μg mL−1 amphotericin B (Sigma). Human epidermoid carcinoma cell line A431 overexpressing the CCK2-R by stable transfection,36 which was kindly provided by Dr L. Aloj (Instituto Nazionale Tumori, Fondazione G. Pascale, Napoli, Italy), was cultured to be an exponentially growing subconfluent monolayer on 100 mm plates in DMEM supplemented with 10% FCS, 2 mM L-glutamine, and 250 g mL−1 neomycin analog G418 (GibcoBRL) under a humidified atmosphere containing 5% CO2 at 37 °C.2.9 In vitro cytotoxicity assays
A431 and A431-transfected cells (3 × 104 cells per mL−1) were seeded into each well of 24-well cell culture plates. After 24 h incubation, the medium was discarded from the plates and replaced with an equal volume of fresh medium containing the test complexes at different concentrations. Then, the cells were incubated for 72 h. A DMSO solution of AuL12 was added for the tests in amounts not exceeding the 0.4% v/v of organic solvent per well. Cisplatin (Sigma-Aldrich) was stocked in a saline solution (0.9% w/w NaCl in water) at a concentration up to 4 mM. The formulations of AuL12-PF127 and AuL12-PF127/(C18)2-PEG1000-G-CCK8 were equally hydrated in a saline solution, at a final concentration of 5.4 × 10−4 M. A Trypan blue dye exclusion assay was performed to determine cell viability, and the cytotoxicity data were expressed as GI50 values, i.e. the concentration of the test compound inducing 50% reduction in cell number as compared to that in control cultures. Empty PF127 non-targeted micelles and their CCK8-labeled counterparts were similarly tested to evaluate any influence on cell growth.3. Results and discussion
3.1 Aggregate formulation and characterization
(C18)2-PEG1000-G-CCK8 peptide amphiphile was synthesized using solid-phase methods. The CCK8 peptide was elongated on the amide resin using a standard Fmoc procedure. The glycine residue, the PEG spacer and the hydrophobic chains were added on the peptide N-terminus by Fmoc-Gly-OH, three residues of Fmoc-Ahoh-OH hexaoxoethylene linkers and the N,N-dioctadecylsuccinamic acid, which were sequentially condensed as previously reported.30 The amphiphilic peptide derivative was purified by RP-HLPC methods on a C4 column. The desired compound was obtained in a good yield with high purity, as confirmed by analytical HPLC and mass spectrometry measurements. The pure micelles of PF127 were prepared according to the thin film hydration method34 by dissolving copolymer at 2% w/w in 10 mM of PBS or aqueous solution at 0.9% w/w NaCl. The same procedure was used to prepare mixed PF127/(C18)2-PEG1000-G-CCK8 (PF127/CCK8) micelles, in which 4% w/w of the CCK8 peptide amphiphile was added to PF127.AuL12 loading was performed, for both kind of micelles, by adding the gold complex to the micelle components during lipid film preparation. The encapsulation ratio (ER%) for both PF127 and PF127/CCK8 micelles was around 85% with drug loading coefficients (DL%) of 1.47 and 1.42, respectively. Although the loading of metal complexes in Pluronic based micelles has not been studied before, there are several reports on the loading of organic based drugs (paclitaxel, ibuprofen, aspirin, and erythromycin).13,37 Very recently, the influence of several parameters (such as drug dimension, its hydrophobicity and pKa) on the drug loading was also reported.13 Our data indicate that AuL12 encapsulation is efficiently achieved, with loading parameters comparable to those reported for organically synthesized drugs. For comparison purposes, the encapsulation assays of cisplatin in non-targeted and targeted PF127 micelles via the film method were also performed. Results highlighted the very low cisplatin encapsulation degree in micelles based on Pluronic® F127 (1 μg for 20 mg of PF127, about 350-fold lower than AuL12 in the same amount of PF127), thus ruling out the possibility to carry out comparative biological tests, under the same experimental conditions, between platinum- and gold-based cytotoxic drugs.
Dynamic Light Scattering (DLS) measurements were performed to assess the size of the two micelle systems (data reported in Table 1). In agreement with the previously reported data,13,38 the mean hydrodynamic diameter of PF127 at 2% w/w and 25 °C is 25 nm (Table 1). A slight increase in the size was observed for PF127/CCK8 mixed targeted micelles (mean diameter values calculated from DLS was 34 nm).
| Systems | Diameter nm ± SD | P.I. ± SD | D [m2 s−1] × 10−12 |
|---|---|---|---|
| PF127 | 25 ± 14 | 0.23 ± 0.01 | 20 ± 11 |
| AuL12-PF127 | 26 ± 13 | 0.23 ± 0.01 | 19 ± 10 |
| PF127/CCK8 | 34 ± 11 | 0.43 ± 0.02 | 15 ± 5 |
| AuL12-F127/CCK8 | 32 ± 12 | 0.48 ± 0.01 | 15 ± 6 |
DLS measurements on the AuL12-loaded micelles indicate that there is no significant size differences between the empty and loaded micelles. In addition, an increase in the polydispersity index is found for both empty and loaded mixed micelles probably due to the presence of the CCK8 bioactive peptide on the external aggregate surface.
3.2 Fluorescence of tryptophan
It is well-known that the last four residues (WMDF) at C-terminus of the CCK8 are determinant for binding to both cholecystokinin receptors. The exposure of this bioactive portion on the outer shell of mixed micelles PF127/CCK8 was evaluated by the fluorescence spectroscopy of the tryptophan residue. Emission peak around 350–360 nm indicates that the indole group is surrounded by a polar environment, whereas around 330 nm indicates the presence of a hydrophobic solvent (blue-shift effect). The fluorescent emission spectrum of targeted micelles PF127/CCK8 was recorded at 25 °C and at a peptide concentration of 1.0× 10−5 M after excitation at 280 nm. The spectrum showed a maximum at ∼360 nm, indicating that the peptide is well exposed on the aggregate surface and bioavailable for the receptor binding (data not shown).3.3 Spectroscopic studies
UV-Vis spectroscopic studies have been performed for AuL12-encapsulating micelles prepared in 10 mM PBS (or in aqueous solution at 0.9% w/w NaCl) and compared to AuL12 in water–DMSO 95/5 mixture. The UV-Vis spectrum of AuL12-PF127 in PBS solution shows no significant change in λmax (258.0 and 311.5 nm) with respect to AuL12 in water/DMSO.37 However, a hypochromic effect and a bathochromic shift are detected over a period of time for both bands (Fig. 2A) along with the appearance of a new band at 380.0 nm (band III), thus highlighting the reactivity of the compound in this medium, which is in agreement with the literature data.39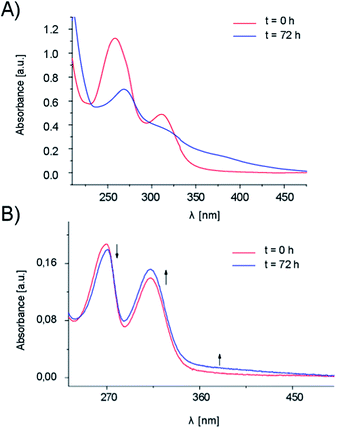 | ||
| Fig. 2 UV-Vis spectra collected over a period of time for AuL12-PF127 micelles prepared in (A) 10 mM phosphate buffer and (B) saline solution (0.9% NaCl). | ||
On the contrary, no notable change, in terms of spectral features (e.g., intensity, λmax), were observed in the kinetic trend recorded for the AuL12-loaded micelles prepared in saline solution (Fig. 2B). This effect suggests a higher stability in this type of aqueous medium. On the whole, the position of the bands (269.7 and 312.5 nm, respectively) is basically unchanged with time, indicating that the gold center remains in the +3 oxidation state because of the stabilization effects of chelating dithiocarbamato ligand.40,41 The slight changes in spectral intensity (hypochromic and hyperchromic effect for band I and II, respectively) have been ascribed to the progressive hydrolysis of gold(III)-bound bromide ions, leading to the formation of the hydroxo derivative.41–43 In fact, because the gold(III) center is quite acidic, the pKa of the coordinated water molecules is lowered, accounting for the formation of the hydroxo complex [AuIII(dtc–ES)(OH)2] (wherein, dtc–ES = ethylsarcosine–dithiocarbamate) in agreement with literature data.42,43
Similar hypochromic and hyperchromic effects, observed respectively for band I and II (261.5 and 311.2 nm, respectively), were detected in the electronic spectrum of AuL12 (DMSO-predissolved) in saline solution (Fig. 3). Furthermore, in the spectra recorded at a higher concentration (not shown in Fig. 3), an increased intensity for band III (M → L charge transfer involving the M nd orbitals and the dithiocarbamato π* system44,45) is detected at about 385 nm, and it could be explained by considering the effect of the Br−/OH− substitution on the transition dipole moment, and therefore on the transition probability. Based on the higher stability of the formulation in 0.9% w/w NaCl saline solution compared to PBS, all the subsequent studies were carried out in the first medium.
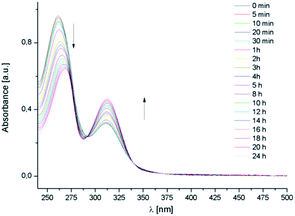 | ||
| Fig. 3 UV-Vis spectra of free AuL12 (pre-dissolved in DMSO) recorded in saline solution over 24 h at 25 °C. | ||
3.4 Drug release
The drug release from micelle carriers was evaluated using a dialysis membrane (MWcut-off = 3500). Fig. 4 shows the release profile of AuL12 from the untargeted micelles obtained at 37 °C up to 72 h. The release profile observed for the targeted micelles was the same (data not shown), thus indicating that the small amount of peptide amphiphile in the micelle composition does not influence the release rate. The amount of released complex was evaluated by ICP-AES analysis as percentage of released gold(III) complex to the total compound previously encapsulated in the aggregates. It is worth noting that most of the drug (around 73%) is released after 72 h and the process takes place considerably quickly, which is evident from the fact that 35% of the compound is released from the micelles after 30 min, and more 56% is released after 12 h. The gold(III) complex release could be probably ascribed to its low affinity toward the micelle core. The low water solubility of AuL12 favors the encapsulation process, but probably the inorganic nature of the gold complex causes a rapid release from the PPO micelle compartment.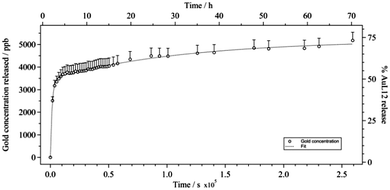 | ||
| Fig. 4 Gold concentration (CJ, according to eqn (1)) evaluated over 72 h in saline solution at 37 °C, viz. extent of AuL12 release from non-targeted micelles (%, with respect to total compound previously encapsulated in the aggregates). Error bars were determined by calculating the means from the error propagation equation. | ||
3.5 Cytotoxicity assays
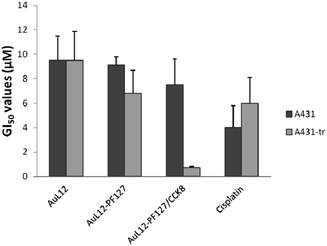
The antiproliferative activity of the AuL12-loaded targeted or untargeted micelles (Table 2 and Fig. 5) was evaluated with the help of two tumor cell lines, namely A431 (epidermoid carcinoma) (A431-tr) and A431 modified by stable transfection to overexpress the CCK2 receptor at 37 °C (CCK2-R). Before starting with the cytotoxicity studies the overexpression of CCK2-R in A431 transfected cells was checked by nuclear medicine assays, as previously reported elsewhere.36| Compound | A431 cells | CCK2-R-transfected A431 cells |
|---|---|---|
| AuL12-DMSO | 9.5 ± 2.0 | 9.5 ± 2.4 |
| AuL12-PF127 | 9.1 ± 0.7 | 6.8 ± 1.9 |
| AuL12-PF127/CCK8 | 7.5 ± 2.1 | 0.75 ± 0.08 |
| Cisplatin | 4.0 ± 1.8 | 6.0 ± 2.1 |
Briefly, A431-CCK2R transfected cells (A431-tr) and A431 control cells were incubated with 111In-DTPAGlu-G-CCK8 radiolabeled peptide for different times at 37 °C. After incubation, cells were washed with PBS, and then recovered by trypsinization. Cell-associated radioactivity and total radioactivity were determined with a wizard γ-counter (Wallac Oy). A431-CCKBR cells exhibited a saturating binding with Kds of the magnitude of 20 nmol L−1, and they present a large number of receptors (approximately 4.7 × 106 sites per cell), while a negligible presence of receptors was detected on A431 control cells.36 AuL12 dissolved in H2O/DMSO 95/5 w/w (AuL12-DMSO) and cisplatin in saline solution were also studied for comparison. AuL12 encapsulated in non-targeted micelles (AuL12-PF127) displayed similar GI50 values against both the tumour cell lines. However, cisplatin continues to be the most potent compound against A431 non-transfected cells, showing a cytotoxic effect about 2-fold higher than AuL12-DMSO and AuL12-PF127, and a slightly higher activity towards A431 CCK2-R-transfected cells.
We tested AuL12 loaded into the hydrophobic core of self-assembling peptide-conjugated micelles (AuL12-PF127/CCK8) against A431 CCK2-R-transfected cells. We found that the GI50 value decreases by approximately one order of magnitude with respect to those of cisplatin, AuL12-DMSO and AuL12-PF127 (Table 2). Thus, in agreement with the release data, it is possible to assume that the endocytic process occurs faster (in the order of minutes46) than the AuL12 release from the micelles, thus allowing tumor cell targeting and cell internalization before the active gold(III) complex leaves the supramolecular aggregates. Additional control experiments were carried out to assess the effects on cell growth by both empty non-targeted (PF127) and CCK8-receptor targeting (PF127/CCK8) micelles.
Notably, Pluronic® F127 micelles showed no cytotoxic effect (maximum 2% viability reduction) at the highest concentration used for the cytotoxicity tests employing AuL12-loaded micelles, against both cell lines. Similarly, the CCK8-functionalized micelles basically proved non-toxic at the highest concentration used for the tests, showing the signs of cytotoxicity (30% of cell viability reduction) only towards CCK8-transfected A431 cells when using concentrations 2.6-fold higher than the highest concentration used.
4. Conclusions
The sparingly water-soluble gold(III) complex dibromo[ethyl-N-(dithiocarboxy-kS,kS′)-N-methylglycinato] gold(III) (AuL12), which is already known for its in vitro and in vivo anti-proliferative effect, has been loaded into the lipophilic core of PF127-based micelles. Pluronic® F127 is a non-ionic, highly biocompatible, and nontoxic surfactant, and it is considered to be one of the most promising nanocarriers for biomedical applications.We have demonstrated that when AuL12 is encapsulated in PF127 based micelles in saline solution it remains stable up to 72 h with the gold center in the +3 oxidation state, indicating that some of the studied PF127-based supramolecular aggregates serve to be efficient carriers, by enhancing the water solubility along with maintaining or increasing the anti-proliferative activity of the gold-based drug with respect to the same compound in the DMSO vehicle. Even if the release from the micelle systems to the aqueous medium was shown to rapidly occur, reaching 50% after 2 h; if a targeting moiety is present on the hydrophilic shell of micelles, the designed supramolecular scaffold, in addition of being a vehicle, acts to be a targeted bullet as well.
In fact, when we compare A431 cells to their CCK2-R-transfected counterparts, the cytotoxicity of AuL12 encapsulated in PF127/CCK8 increases 10-fold with respect to that of the AuL12 encapsulated into PF127.
In conclusion, the loading of metal-based anti-proliferative compounds in peptide-labeled micelles of Pluronic® appears to be a significant approach for increasing its water solubility as well as for enhancing the tumor selectivity using a single strategy. Based on our promising preliminary studies, we have been able to load some gold(III) peptidomimetics into Pluronic® aggregates to enhance their bioavailability for subsequent use in future phase I clinical trials.47,48
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge the Ministry of the Education, University and Research (type of grant: PRIN-2009) and A.R.TE.M.O. association (Italy) for financial support.Notes and references
- E. M. Nagy, L. Ronconi, C. Nardon and D. Fregona, Noble metal-dithiocarbamates precious allies in the fight against cancer, Mini-Rev. Med. Chem., 2012, 12, 1216–1229 CrossRef CAS.
- C. Nardon, G. Boscutti and D. Fregona, Beyond platinums: gold complexes as anticancer agents, Anticancer Res., 2014, 34, 487–492 CAS.
- L. Dalla Via, C. Nardon and D. Fregona, Targeting the ubiquitin-proteasome pathway with inorganic compounds to fight cancer. A challenge for the future, Future Med. Chem., 2012, 4, 525–543 CrossRef CAS PubMed.
- E. M. Nagy, C. Nardon, L. Giovagnini, L. Marchiò, A. Trevisan and D. Fregona, Promising anticancer mono- and dinuclear ruthenium(III) dithiocarbamato complexes: Systematic solution studies, Dalton Trans., 2011, 40, 11885–11895 RSC.
- L. Ronconi, C. Nardon, G. Boscutti and D. Fregona, Perspective gold(III)-dithiocarbamato anticancer therapeutics: learning from the past, moving to the future, e-book series Advances in Anti-Cancer Agents in Medicinal Chemistry, 2013, 2, 130–172 CAS.
- A. A. Hincal, D. F. Long and A. J. Repta, Cis-platin stability in aqueous parenteral vehicles, J. Parenter. Drug Assoc., 1979, 33, 107–116 CAS.
- J. M. LaFollette, M. H. Arbus and R. D. Lauper, Stability of cisplatin admixtures in polyvinyl chloride bags, Am. J. Hosp. Pharm., 1985, 42, 2652 CAS.
- C. M. Riley and L. A. Sternson, Cisplatin, Anal. Profiles Drug Subst. Excipients, 1985, 14, 77–105 CAS.
- A. Casini, G. Kelter, C. Gabbiani, M. A. Cinellu, G. Minghetti, D. Fregona, H. Fiebig and L. Messori, Chemistry, antiproliferative properties, tumor selectivity, and molecular mechanisms of novel gold(III) compounds for cancer treatment: A systematic study, JBIC, J. Biol. Inorg. Chem., 2009, 14, 1139–1149 CrossRef CAS PubMed.
- C. Marzano, L. Ronconi, F. Chiara, M. C. Giron, I. Faustinelli, P. Cristofori, A. Trevisan and D. Fregona, Gold(III)-dithiocarbamato anticancer agents: activity, toxicology and histopathological studies in rodents, Int. J. Cancer, 2011, 129, 487–496 CrossRef CAS PubMed.
- L. Cattaruzza, D. Fregona, M. Mongiat, L. Ronconi, A. Fassina, A. Colombatti and D. Aldinucci, Antitumor activity of gold(III)-dithiocarbamato derivatives on prostate cancer cells and xenografts, Int. J. Cancer, 2011, 128, 206–215 CrossRef CAS PubMed.
- X. Zhang, M. Frezza, V. Milacic, L. Ronconi, Y. Fan, C. Bi, D. Fregona and Q. P. Dou, Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redox-dependent and -independent processes, J. Cell. Biochem., 2010, 109, 162–172 CAS.
- R. Basak and R. Bandyopadhyay, Encapsulation of hydrophobic drugs in pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH, Langmuir, 2013, 29, 4350–4356 CrossRef CAS PubMed.
- D. A. Chiappetta and A. Sosnik, Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs, Eur. J. Pharm. Biopharm., 2007, 66, 303–317 CrossRef CAS PubMed.
- J. M. Grindel, T. Jaworski, O. Piraner, R. M. Emanuele and M. Balasubramanian, Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans, J. Pharm. Sci., 2002, 91, 1936–1947 CrossRef CAS PubMed.
- E. Batrakova, S. Lee, S. Li, A. Venne, V. Alakhov and A. Kabanov, Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells, Pharm. Res., 1999, 16, 1373–1379 CrossRef CAS.
- J. C. Reubi, J. Schaer and B. Waser, Cholecystokinin (CCK)-A and CCK-B/gastrin receptors in human tumors, Cancer Res., 1997, 57, 1377–1386 CAS.
- S. A. Wank, J. R. Pisegna and A. De Weerth, Brain and gastrointestinal cholecystokinin receptor family: Structure and functional expression, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 8691–8695 CrossRef CAS.
- S. A. Wank, G protein-coupled receptors in gastrointestinal physiology I. CCK receptors: An exemplary family, Am. J. Physiol., 1998, 274, G607–G613 CAS.
- J. C. Reubi, Peptide receptors as molecular targets for cancer diagnosis and therapy, Endocr. Rev., 2003, 24, 389–427 CrossRef CAS PubMed.
- J. C. Reubi and B. Waser, Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting, Eur. J. Nucl. Med. Mol. Imaging, 2003, 30, 781–793 CrossRef CAS PubMed.
- J. C. Reubi and B. Waser, Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas, Int. J. Cancer, 1996, 67, 644–647 CrossRef CAS.
- A. Imdahl, T. Mantamadiotis, S. Eggstein, E. H. Farthmann and G. S. Baldwin, Expression of gastrin, gastrin/CCK-B and gastrin/CCK-C receptors in human colorectal carcinomas, J. Cancer Res. Clin. Oncol., 1995, 121, 661–666 CrossRef CAS.
- L. Aloj, M. Panico, C. Caracò, S. Del Vecchio, C. Arra, A. Affuso, A. Accardo, R. Mansi, D. Tesauro, S. De Luca, C. Pedone, R. Visentin, U. Mazzi, G. Morelli and M. Salvatore, In Vitro and In Vivo characterization of Indium-111 and Technetium-99m labeled CCK-8 derivatives for CCK-B receptor imaging, Cancer Biother.Radiopharm., 2004, 19, 93–98 CrossRef CAS PubMed.
- E. Benedetti, G. Morelli, A. Accardo, R. Mansi, D. Tesauro and L. Aloj, Criteria for the design and biological characterization of radiolabeled peptide-based pharmaceuticals, BioDrugs, 2004, 18, 279–295 CrossRef CAS PubMed.
- A. Accardo, A. Morisco, P. Palladino, R. Palumbo, D. Tesauro and G. Morelli, Amphiphilic CCK peptides assembled in supramolecular aggregates: structural investigations and in vitro studies, Mol. BioSyst., 2011, 7, 862–870 RSC.
- M. Vaccaro, G. Mangiapia, L. Paduano, E. Gianolio, A. Accardo, D. Tesauro and G. Morelli, Structural and relaxometric characterization of peptide aggregates containing gadolinium complexes as potential selective contrast agents in MRI, ChemPhysChem, 2007, 8, 2526–2538 CrossRef CAS PubMed.
- A. Accardo, D. Tesauro and G. Morelli, Peptide-based targeting strategies for simultaneous imaging and therapy with nanovectors, Polym. J., 2013, 45, 481–493 CrossRef CAS.
- L. Schmitt, C. Dietric and R. Tampé, Synthesis and characterization of chelator-lipids for reversible immobilization of engineered proteins at self-assembled lipid interfaces, J. Am. Chem. Soc., 1994, 116, 8485–8491 CrossRef CAS.
- A. Morisco, A. Accardo, E. Gianolio, D. Tesauro, E. Benedetti and G. Morelli, Micelles derivatized with octreotide as potential target-selective contrast agents in MRI, J. Pept. Sci., 2009, 15, 242–250 CrossRef CAS PubMed.
- H. Edelhoch, Spectroscopic determination of tryptophan and tyrosine in proteins, Biochemistry, 1967, 6, 1948–1954 CrossRef CAS.
- C. N. Pace, F. Vajdos, L. Fee, G. Grimsley and T. Gray, How to measure and predict the molar absorption coefficient of a protein, Protein Sci., 1995, 4, 2411–2423 CrossRef CAS PubMed.
- L. Ronconi, L. Giovagnini, C. Marzano, F. Bettìo, R. Graziani, G. Pilloni and D. Fregona, Gold dithiocarbamate derivatives as potential antineoplastic agents: Design, spectroscopic properties, and in vitro antitumor activity, Inorg. Chem., 2005, 44, 1867–1881 CrossRef CAS PubMed.
- L. Chen, X. Sha, X. Jiang, Y. Chen, Q. Ren and X. Fang, Pluronic P105/F127 mixed micelles for the delivery of docetaxel against Taxol-resistant non-small cell lung cancer: Optimization and in vitro, in vivo evaluation, Int. J. Nanomed., 2013, 8, 73–84 Search PubMed.
- L. Dalla Via, V. Di Noto, O. Gia and S. Marciani Magno, Photoaddition of thienocoumarin derivatives to DNA: stoichiometry and kinetics of binding, J. Photochem. Photobiol., B, 2005, 79, 59–65 CrossRef CAS PubMed.
- L. Aloj, C. Caracò, M. Panico, A. Zannetti, S. Del Vecchio, D. Tesauro, S. De Luca, C. Arra, C. Pedone, G. Morelli and M. Salvatore, In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-b receptor imaging, J. Nucl. Med., 2004, 45, 485–494 CAS.
- Z. Wei, S. Yuan, Y. Chen, S. Yu, J. Hao, J. Luo, X. Sha and X. Fang, Enhanced antitumor efficacy by paclitaxel-loaded Pluronic P123/F127 mixed micelles against non-small cell lung cancer based on passive tumor targeting and modulation of drug resistance, Eur. J. Pharm. Biopharm., 2010, 75, 341–353 CrossRef PubMed.
- P. K. Sharma and S. R. Bhatia, Effect of anti-inflammatories on Pluronic® F127: Micellar assembly, gelation and partitioning, Int. J. Pharm., 2004, 278, 361–377 CrossRef CAS PubMed.
- L. Ronconi, C. Marzano, P. Zanello, M. Corsini, G. Miolo, C. Maccà, A. Trevisan and D. Fregona, Gold(III) dithiocarbamate derivatives for the treatment of cancer: Solution chemistry, DNA binding, and hemolytic properties, J. Med. Chem., 2006, 49, 1648–1657 CrossRef CAS PubMed.
- L. Ronconi and D. Fregona, The Midas touch in cancer chemotherapy: From platinum- to gold-dithiocarbamato complexes, Dalton Trans., 2009, 10670–10680 RSC.
- L. Messori, F. Abbate, G. Marcon, P. Orioli, M. Fontani, E. Mini, T. Mazzei, S. Carotti, T. O'Connell and P. Zanello, Gold(III) complexes as potential antitumor agents: Solution chemistry and cytotoxic properties of some selected gold(III) compounds, J. Med. Chem., 2000, 43, 3541–3548 CrossRef CAS PubMed.
- G. Marcon, S. Carotti, M. Coronnello, L. Messori, E. Mini, L. Orioli, T. Mazzei, M. A. Cinellu and G. Minghetti, Gold(III) complexes with bipyridyl ligands: Solution chemistry, cytotoxicity, and DNA binding properties, J. Med. Chem., 2002, 45, 1672–1677 CrossRef CAS PubMed.
- G. C. Franchini, A. Giusti, C. Preti, L. Tassi and P. Zannini, Coordinating ability of methylpiperidine dithiocarbamates towards platinum group metals, Polyhedron, 1985, 4, 1553–1558 CrossRef CAS.
- C. C. Hadjikostas, G. A. Katsoulos and S. K. Shakhatreh, Synthesis and spectral studies of some new palladium(II) and platinum(II) dithiocarbimato complexes. Reactions of bases with the corresponding N-alkyldithiocarbamates, Inorg. Chim. Acta, 1987, 133, 129–132 CrossRef CAS.
- B. F. Roettger, R. U. Rentsch, D. Pinon, E. Holicky, E. Hadac, J. M. Larkin and L. J. Miller, Dual pathways of internalization of the cholecystokinin receptor, J. Cell Biol., 1995, 128, 1029–1041 CrossRef CAS.
- M. N. Kouodom, L. Ronconi, M. Celegato, C. Nardon, L. Marchiò, Q. P. Dou, D. Aldinucci, F. Formaggio and D. Fregona, Toward the selective delivery of chemotherapeutics into tumor cells by targeting peptide transporters: tailored gold-based anticancer peptidomimetics, J. Med. Chem., 2012, 55, 2212–2226 CrossRef PubMed.
- C. Nardon, S. M. Schmitt, H. Yang, J. Zuo, D. Fregona and Q. P. Dou, Gold(III)-Dithiocarbamato Peptidomimetics in the Forefront of the Targeted Anticancer Therapy: Preclinical Studies against Human Breast Neoplasia, PLoS One, 2014, 9(1), e84248 Search PubMed.
- D. Fregona, L. Ronconi, F. Formaggio, Q. P. Dou and D. Aldinucci, Gold(III) complexes with oligopeptides functionalized with sulfur donors and use thereof as antitumor agents, PCT Int. Appl., 2009 Search PubMed, 2009-EP53296; 2009-EP53296 (WO2010105691): 34.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |