Discovery of mitochondria-targeting berberine derivatives as the inhibitors of proliferation, invasion and migration against rat C6 and human U87 glioma cells†
Shengnan Fu‡ ac, Yanqi Xie‡ a, Jue Tuo a, Yalong Wang a, Wenbo Zhu b, Sihan Wu b, Guangmei Yan b and Haiyan Hu *a
aSchool of Pharmaceutical Sciences, Sun Yat-sen University, Waihuan East Road 132, Guangzhou 510006, China. E-mail: lsshhy@mail.sysu.edu.cn; Fax: +86-020-39343118; Tel: +86-020-39343118
bDepartment of Pharmacology, Zhongshan School of Medicine, Sun Yat-sen University, Zhongshan II Road 74, Guangzhou 510080, China
cDepartment of Pharmacy, The People's Hospital of ZhongShan, Sunwen East Road 2, Zhongshan 528403, China
First published on 30th September 2014
Abstract
This research aims to synthesize lipophilic berberine derivatives and evaluate their antiglioma effects on C6 and U87 cells. The introduction of substituents with various carbon chain lengths on C-13- or C-9-O-position of the berberine scaffold led to the discovery of several potent inhibitors against glioblastoma cells. Derivatives substituted with the carbon chains of moderate length (twelve carbons) displayed improved lipophilicity and the strongest inhibitory effects. Several compounds presented dose-dependent repression against proliferation (IC50, 1.12–6.12 μM) and blocked migration and invasion by over 60% at lower dose levels. Furthermore, preliminary research about the underlying mechanism for the enhanced antiglioma ability indicated that these analogues preferentially localized into mitochondria, inducing the up-regulation of ROS production. Overall, these compounds represent promising candidates to combat glioblastoma and highlight new insight into the antiglioma therapy through interaction with mitochondria.
Introduction
Glioblastoma multiforme (GBM), a grade IV astrocytoma, is the most common and malignant primary brain tumor.1 Despite multiple therapies, patients suffering from GBM lives typically less than 14 months after diagnosis.2 One subtle characteristic of GBM is the highly invasive behavior, conferring glioma cells with the propensity of rapidly infiltrating neighboring normal tissue and making the complete surgical resection impossible.3 Actually, after surgical removal of a glioma, 96% of the recurrent tumors derived from the residual invasive glioma cells arise within 2 cm of the resection cavity4 or immediately adjacent to the resection rim.5 Moreover, radiotherapy also appears to have reached its practical limit for at least the partly inherent and/or acquired radio-resistance of high-grade glioma.6 Moreover, traditional non-selective chemotherapeutics, such as temozolomide (an alkylating agent) and irinotecan (a topoisomerase 1 inhibitor), have been reported. However, the survival advantage brought by these chemicals is accompanied by certain observed severe side effects.7 In addition, some low molecular inhibitors of matrix metalloproteases (MMPs) and anti-vascular pharmaceuticals have been investigated to suppress cancer invasion, but they achieved only limited clinical results.8Conclusively, the invasion and migration ability of glioma cells remains the main reason for current unsatisfactory therapies. Indeed, accumulated data substantiate that GBM invasion is regulated and influenced by extremely diversified and overlapping signal pathways instead of a single one.9 Therefore, it is extensively suggested that the future successful therapies in the treatment of GBM will target multiple signal transduction cascades, either by one drug that is aiming at the pivotal intersection of several pathways or by a group of agents aiming at multiple signaling pathways.3
Coincidentally, dozens of lethal signaling pathways appear to be a promising therapeutic target, the mitochondrion organelle.10 Recent studies have described the crucial role that mitochondria, at least partially, play in tumor invasion. The high in vivo expression feature of Bcl-W (a mitochondrial outer membrane anti-apoptotic protein) has been revealed in invasive glioma cells,11 and the depletion of Bcl-W or Bcl-XL mediated by siRNA renders invasive glioma cells susceptible to cytotoxic-therapy-induced apoptosis.12 Moreover, research have shown that the active Akt controlling the balance between cell survival and apoptosis13 predominantly localizes at the leading edge of migrating cells,14 suggesting the relationship between tumor invasion and mitochondria. Hurd et al. have efficiently validated the dual effects of intracellular reactive oxygen species (ROS) mainly produced by mitochondria on triggering signal pathways for cell migration and invasion.15 In addition, the inhibition of heat-shock protein 90 (HSP90), which preferentially localizes in malignant cell mitochondria but not in their normal cell counterparts,10 will reduce cell migration and invasion by decreasing MMP-9 expression in GBM.9 Therefore, it may be possible to target mitochondria to be the junction of multiple signal cascades to achieve improved therapeutic efficacy for GBM patients.
Berberine (1), an isoquinoline alkaloid, possesses many striking pharmacological effects, which have been elaborated in the available literature.16 Interestingly, berberine can selectively accumulate in tumor cell mitochondria,17 which could be attributed to its structural amphiphilicity and delocalized positive charge.18 Moreover, mitochondria-based cancer cell apoptosis induced by berberine has been confirmed in several non-CNS malignant cell lines, with the underlying mechanisms interpreted as decreasing ATP levels and activating apoptotic cascades via lowering the mitochondrial membrane potential, inhibiting complex I of the respiration chain, induction of mitochondrial permeability transition (MPT) and release of pro-apoptotic proteins into cytosol.19 Furthermore, the recent investigations have started to uncover the potential of berberine in suppressing migration and invasion in non-CNS cancer cell lines. For example, berberine attenuates human gastric tumor cell invasion and inhibits the metastatic potential of breast cancer cells through the down-regulation of MMPs and Akt pathway modulation, respectively.20 However, current literature about whether berberine derivatives could selectively interact with mitochondria and repress the proliferation, migration and invasion of malignant glioma cells is rare. In addition, a significant obstacle that limits the clinical application of berberine is its innate poor solubility and lipophilicity.16 A structure-activity relationship study on berberrubine derivatives indicated that the presence of the lipophilic alkyl substituents of moderate length presents an optimal antibacterial activity,21 prompting us that the lipophilic alteration of berberine may favor its performance in cytotoxicity and anti-migration activity.
Previously, we had evaluated the antiglioma ability of four berberine derivatives varying the alkyl chain length in the C-9-O-position of berberine (3b–e), and found that they showed inhibitory effects on C6 glioma cells in proliferation, migration and invasion.22 In this study, five 13-O-substitued berberine analogs with varying alkyl chain length (5f–j) were synthesized and characterized. However, to comprehensively compare the antiglioma effects of ten berberine derivatives and achieve more reliable results, in other words to avoid time interference on experimental results, the MTT, migration and invasion assays previously used to evaluate the four 9-O-substitued berberine derivatives (3b–e) on C6 cells were performed again. Simultaneously, the same assays of another 9-O-substitued berberine derivative (3a) and the five newly synthesized 13-O-substitued berberine derivatives (5f–j) on rat C6 glioma cells were carried out. In addition, human U87 glioma cells were selected to assess the total ten berberine derivatives in this study. The underlying mechanisms for the improved antiglioma activity were preliminarily explored by studying the subcellular localization of these compounds and measuring ROS production of the C6 and U87 glioma cells with or without berberine derivatives. The BBB penetration ability was also predicted because these compounds have to function inside the brain.
Results and discussion
Chemistry
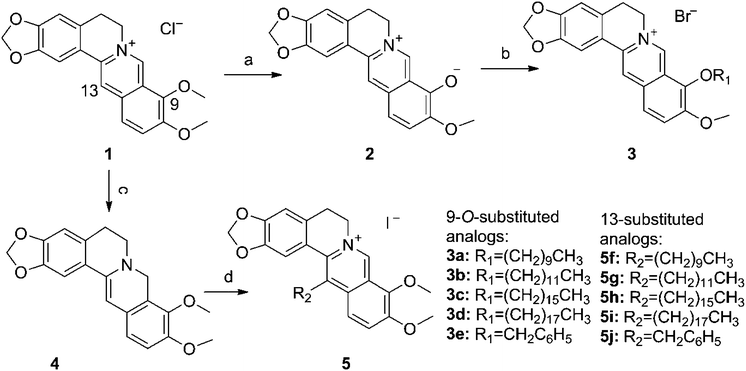 | ||
| Scheme 1 Synthesis of 3a–e and 5f–j. Reagents and conditions: (a) decalin, N2, 190 °C. (b) R1Br, DMF, N2, stirring, 80 °C. (c) NaBH4, pyridine, stirring, rt. (d) R2Br, anhydrous acetonitrile, NaI, N2, stirring, 80 °C. | ||
In addition to the above approach for synthesizing 5f–j, we attempted another method involving the reduction of 1 to an intermediate 8-acetonyldihydroberberine, and then alkylation of this intermediate with bromides.24 However, the yield of 8-acetonyldihydroberberine was lower than that of the intermediate 4 because of the former's easy rearrangement to 1 during the heating process. In addition, during the preparation of 4, we compared two reaction solvents, pyridine and the methanol solution containing potassium carbonate, in terms of reaction temperatures, time and yields. Results manifested that the yield of 4 from the reaction with pyridine as the solvent at room temperature for 20 min exceeded that of the reaction with the latter as the solvent at 0 °C for 8 h.
The first obstacle in the synthesis of 5f–i was the considerably low yields that could be ascribed to the following reasons: approximate 30–40% of 4 was converted back into 1 25 in the enamine alkylation process, which per se possesses the drawbacks of low yields and numerous by-products. The second reason is that alkyl bromides with long carbon chains present poor reaction activity. To improve the yield, we tried to optimize the reaction solvent, pressure, temperature, catalyst, feeding sequence and feeding ratio for enamine alkylation. Firstly, using ESI-MS monitoring the reaction process, we finally selected acetonitrile to dissolve 4 instead of dichloromethane or DMF, both of which exhibited excellent solubility to 4, though the molecular ion peaks of target compounds failed to appear. Secondly, an elevated yield was achieved under high pressure using an airtight thick-walled flask as the reaction apparatus rather than the usual reflux method.23 Thirdly, we selected 80 °C to be the reaction temperature because no significant improvement of the yield was attained at 100 °C and 130 °C. Fourthly, sodium iodide exhibited higher catalytic activity than potassium iodide and the possible explanation could be the higher solubility of sodium iodide in acetonitrile. Finally, different feeding sequences and ratios were tried and the present sequence and ratio, which are described in the experimental section, were the relatively advantageous combination in the favor of the final yield.
Another obstruction was the purification of 5f–i. Highly pure intermediate 4 was difficult to obtain from either recrystallization or chromatography because 4, as mentioned earlier, easily oxidized back to 1 under aerobic conditions. Consequently, adding impure 4 to the following enamine alkylation produced six by-products per reaction, whose Rf values were very close to those of target products in silica gel-based TLC (Developing solvent: dichloromethane/methanol, 20 : 1, v/v). Neither recrystallization nor silica gel-based column or alumina column chromatography could separate the total resulting mixture.26 After extensive attempts, we developed a three-step approach to remove impurities. Firstly, most of the byproducts were separated by neutral alumina column chromatography (petroleum ether/n-butanol). Secondly, column chromatography using silica gel (n-butanol/water/acetic acid) was harnessed to remove a byproduct whose polarity was closer to that of target product per reaction. Finally, an unidentified impurity peak in 1H NMR spectra was successfully eliminated after the purification by Sephadex™ LH-20 column chromatography (methanol).
| Compounds | Purity (%) | Log P | IC50 (μM) | |
|---|---|---|---|---|
| C6 | U87 | |||
| a Log P means the logarithm of the n-octanol/water partition coefficients; purity of these compounds was determined by high-performance liquid chromatography. b IC50: inhibitory concentration causing a 50% reduction in cell proliferation in μM. Data were expressed as mean ± SD. | ||||
| 1 | 98.89 | −1.68 ± 0.24 | >20 | >20 |
| 3a | 99.12 | 2.26 ± 0.34 | 2.95 ± 0.22 | 5.73 ± 0.51 |
| 3b | 99.08 | 3.43 ± 0.21 | 1.24 ± 0.13 | 4.55 ± 0.28 |
| 3c | 96.23 | 3.41 ± 0.40 | 6.12 ± 0.64 | 15.2 ± 2.02 |
| 3d | 99.16 | 3.01 ± 0.40 | 4.45 ± 0.57 | 22.49 ±3.38 |
| 3e | 96.80 | −0.86 ± 0.16 | 11.48 ± 2.05 | >20 |
| 5f | 98.17 | 2.37 ± 0.27 | 1.12 ± 0.35 | 3.65 ± 0.61 |
| 5g | 96.75 | 3.66 ± 0.30 | 1.65 ± 0.38 | 3.09 ± 0.54 |
| 5h | 95.62 | 3.24 ± 0.22 | 1.67 ± 0.46 | 3.25 ± 0.27 |
| 5i | 95.49 | 3.22 ± 0.31 | 2.37 ± 0.29 | 5.75 ± 0.68 |
| 5j | 99.46 | −0.73 ± 0.14 | 14.4 ± 1.48 | >20 |
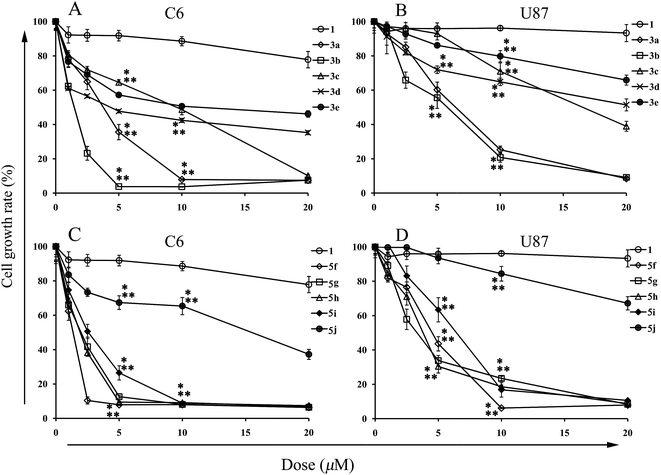
Based on the MTT tests, IC50 values of the assayed compounds were calculated, which are shown in Table 1. As can be seen, 3a–c and 5f–i are superior to the clinically commonly used chemotherapeutics for glioblastoma in terms of cytotoxicity. Indeed, the IC50 values of temozolomide and irinotecan on U87 glioma cells are 19.38 μM and higher than 250 μM, respectively.27 Recently, 3b was synthesized and also proved to be a more robust inhibitor on human cancer HepG2 and HT29 cell lines.28 Moreover, data trends in Fig. 1 and Table 1 demonstrate that the variation of cytotoxic activity of the synthetic analogues correlates with the alternation of alkyl chain length. Namely, the presence of alkyl chains with moderate length (12 carbons) at C-9-O-position or C-13-position on the berberine scaffold guarantees the most appreciable antiglioma activity. However, the introduction of the benzyl group at aforementioned positions inconspicuously contributes to activity elevation. These highly similar varying patterns between the lipophilicity and cytotoxicity of compounds 1, 3a–e and 5f–j led us to postulate that the elevated lipophilicity might promote the transmembrane permeability of these compounds, thus enhancing the intracellular concentrations and cytotoxicity.
As shown in Fig. 2–5, all compounds exhibit robust or moderate inhibitory effects against invasion and migration in both the cancer cell lines, except 13-benzyl-subtituted berberine analog 5j. A modest anti-migration and anti-invasion activity is observed in the presence of berberine, which agrees with the previous evidence that berberine could attenuate migration and invasion in other tumor cell lines.20 Furthermore, 3d, 3e, 5h and 5i display greater than 30% suppression and three other potent derivatives (3b, 3c and 5g) block migration or invasion by over 60%. Overall, these experimental results substantiate that the tested compounds are effective migration and invasion inhibitors.
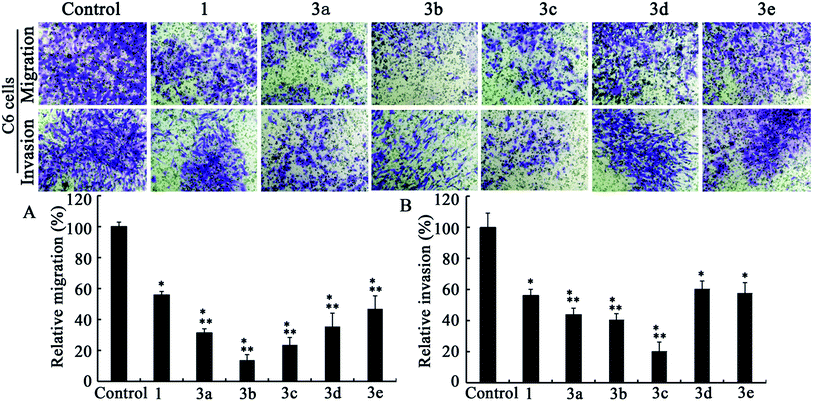 | ||
| Fig. 2 Transwell migration (A) and invasion (B) assays in C6 cell lines (n = 3). Dose levels for 1 and 3a–e are 5, 0.5, 0.5, 0.5, 0.5 and 0.5 μM, respectively. Data are presented as mean ± standard deviation. P < 0.05; * versus control; ** versus berberine. | ||
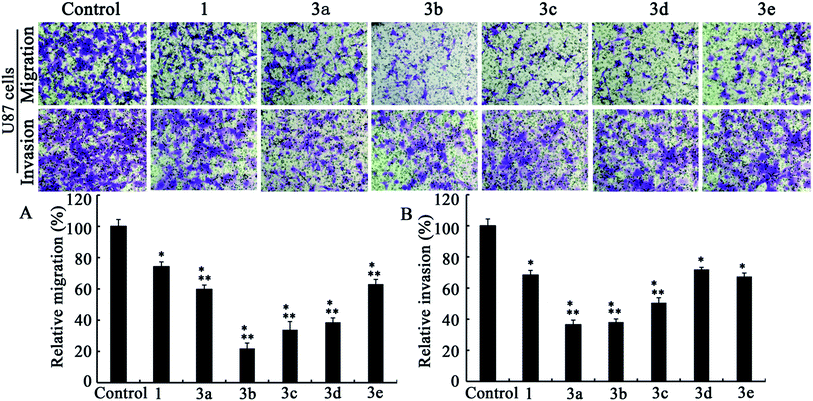 | ||
| Fig. 3 Transwell migration (A) and invasion (B) assays in U87 cell lines (n = 3). Dose levels for 1 and 3a–e are 5, 2.5, 2.0, 2.5, 2.5 and 2.5 μM, respectively. Data are presented as mean ± standard deviation. P < 0.05; * versus control; ** versus berberine. | ||
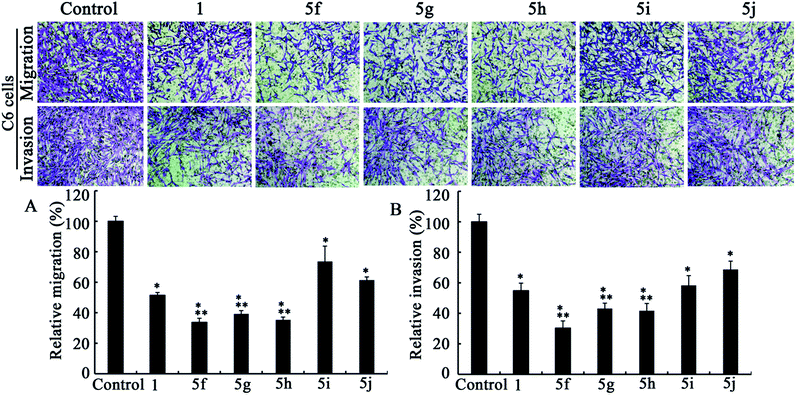 | ||
| Fig. 4 Transwell migration (A) and invasion (B) assays in C6 cell lines (n = 3). Dose levels for 1 and 5f–j are 5, 0.5, 0.5, 0.5, 0.5 and 1 μM, respectively. Data are presented as mean ± standard deviation. P < 0.05; * versus control; ** versus berberine. | ||
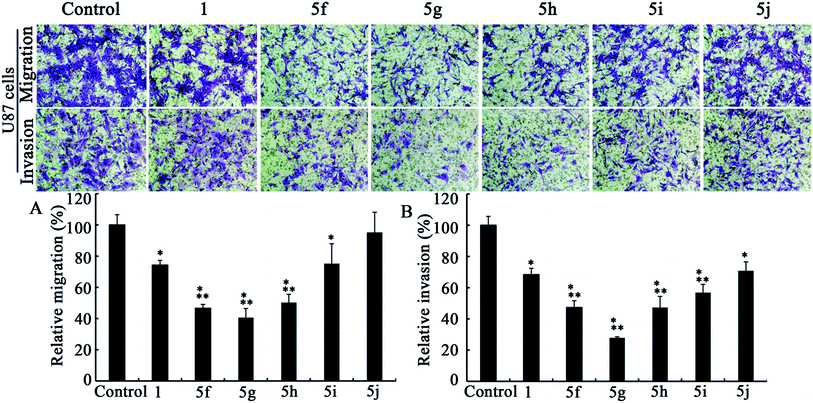 | ||
| Fig. 5 Transwell migration (A) and invasion (B) assays in U87 cell lines (n = 3). Dose levels for 1 and 5f–j are 5, 0.5, 0.5, 0.5, 2.5 and 2.5 μM, respectively. Data are presented as mean ± standard deviation. P < 0.05; * versus control; ** versus berberine. | ||
After comparing Fig. 2A and 3A, it could be concluded that only those analogs with moderate alkyl chain length (3b and 3c) could significantly lower tumor migration tendency. Lengthening the carbon chain (3d) or shortening the substituent (3a and 3e) will compromise the anti-migration potency. Likewise, the similar phenomenon is noted for 5f–j in transwell migration assays (Fig. 4A and 5A). Moreover, there is no cancer type-specific mode of action revealed in migration inhibition evaluation. On the contrary, C6 and U87 cells are differently sensitive to the derivatives regarding invasiveness. For example, the most powerful anti-invasion agents for C6 cells are 3c and 5f, whereas for U87 cells 3a, 3b and 5g are the most remarkable ones (Fig. 2B, 3B, 4B and 5B). Therefore, both the substituents with suitable carbon chain length and tumor cell types influence the activity of synthetic analogues in terms of subduing invasion ability. Intriguingly, agents 1, 3d, 3e and 5j could attenuate cancer cell migration and invasion without exerting acute cytotoxicity. Using these effective but low cytotoxic inhibitors to block tumor cell metastasis will be more advantageous in the long-term treatment of invasive tumor because most of the chemotherapeutics targeting cancer cell proliferation provoke acquired chemoresistance and toxicity to healthy cells after long-time use.29
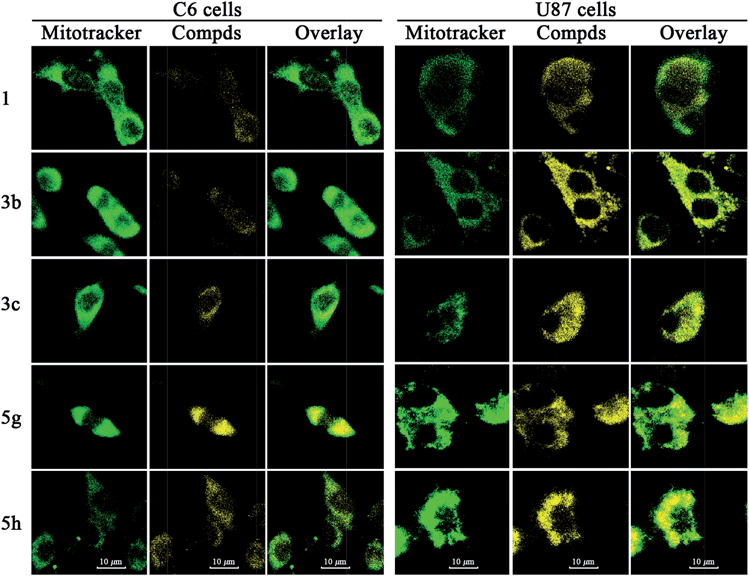 | ||
| Fig. 6 Laser confocal micrographs of double-stained C6 cells (Left panel) and U87 cells (Right panel). Notes: 1. Green channel: 500 nM Mitotracker Green FM stained mitochondria; 2. Yellow channel: 1 μM of respective compounds; 3. Composite images of the former two channels, indicating the co-localization of synthesized derivatives into mitochondria of C6 or U87 cells. | ||
A perfect superposition between Mitotracker Green FM dye images and those of berberine proves the specific subcellular localization of berberine in cell mitochondria, which is in agreement with the previous results.17 The overlapping photographs for 3b, 3c, 5g and 5h unequivocally demonstrate that these berberine derivatives have a preference for mitochondrial organelles. This type of a mitochondrion-targeting feature could be ascribed to two determinants. The primary reason is that the amphiphilic berberine analogs retain the delocalized cationic charge centers that are crucial for selective mitochondrial accumulation.18 Secondly, it has been verified that the driving force for this selective accumulation is the mitochondrial transmembrane potential, which proves to be elevated in cancer cells, further favoring the mitochondrial localization propensity.30 In addition, yellow fluorescence intensities of 3b, 3c, 5g and 5h are stronger than that of berberine in both the tumor cell lines. The possible underlying mechanism for the enhanced fluorescence intensities may be that an increase in the lipophilicity from the alkyl substituents promotes the penetration of the synthetic analogs through the cytomembrane, thus improving mitochondrial accumulation.26b
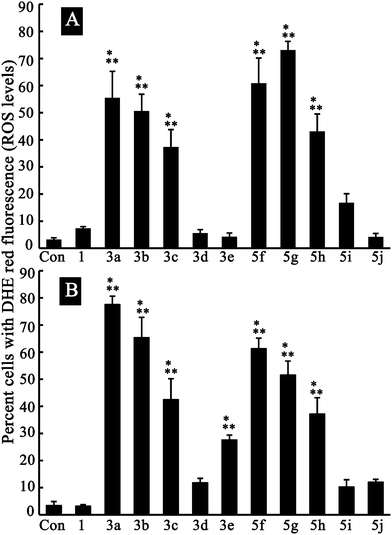 | ||
| Fig. 7 Reactive oxygen species (ROS) generation induced by 2 μM of compounds 1, 3a–e and 5f–j (n = 4). Data are presented as mean ± standard deviation. A: C6 cells; B: U87 cells. ROS levels were determined by the detection of dihydroethidium (DHE) red fluorescence in the nuclei resulting from the oxidation of DHE by intracellular ROS. P < 0.05; *versus control; **versus berberine. Con = Control group. | ||
Because mitochondria are the junction of multiple signal pathways responsible for cancer cell proliferation, migration and invasion, the mitochondrion-specific ability and high ROS production tend to be a tentative underlying mechanism for certain derivatives such as 3b, 3c, 5g and 5h, which spontaneously possess anti-proliferation, anti-migration and anti-invasion activity at different dose levels. To further understand the underlying mechanism by which ROS production, which is induced by berberine derivatives, regulates C6 and U87 glioma cells, studies on the mitochondrial membrane potential (ΔΨm), release of cytochrome C and apoptosis-inducing factor (AIF), and permeabilization of lysosomes are underway.
| Calculationa | MW | c log P | HBA | HBD | PSA | log BBb |
|---|---|---|---|---|---|---|
| a MW: molecular weight; c log P: calculated logarithm of the octanol–water partition coefficient; HBA: hydrogen-bond acceptor atoms; HBD: hydrogen-bond donor atoms; PSA: polar surface area. b compounds with log BB > 0.3 are able to cross the BBB readily, with log BB <−1.0 are only poorly distributed in the brain. | ||||||
| 1 | 371.87 | 0.20 | 4 | 0 | 40.82 | −0.44 |
| 3a | 542.51 | 4.665 | 4 | 0 | 40.82 | 0.23 |
| 3b | 570.56 | 5.675 | 4 | 0 | 40.82 | 0.39 |
| 5f | 603.54 | 5.019 | 4 | 0 | 40.82 | 0.28 |
| 5g | 631.59 | 6.029 | 4 | 0 | 40.82 | 0.44 |
| Lipinski's rules | ≤500 | ≤5.0 | ≤10 | ≤5 | ≤90 | >0.3 (readily cross the BBB); <−1.0 (only poorly distributed to the brain) |
Conclusions
Glioblastoma still poses an urgent challenge to both patients and neuro-oncologists. Unfortunately, the management of glioblastoma is mainly palliative all over the world. In this study, an effort was made to develop therapeutics against malignant gliomas through enhancing the lipophilicity of berberine and retaining the mitochondriotropic activity by potentially influencing the intersection of multiple signaling cascades in cancer pathology. Inspired by our previously evaluated 9-O-substitued berberine derivatives on C6 glioma cells,22 9-O-decyl-berberine (3a) and five cationic C-13-position berberine derivatives (5f–j) varying in the length of substituted alkyl chains were synthesized. The data from biological experiments on C6 and U87 cells suggested that the introduction of the lipophilic substituents with moderate alkyl chain length to the C-9-O-position and C-13-position of the berberine scaffold led to the identification of several potent proliferation, migration and invasion inhibitors in two glioblastoma cell lines. Indeed, the IC50 values for the anti-proliferation of several synthesized compounds reached to low micromolar range (3b, 1.24 μM; 5f, 1.12 μM; C6 cells). Over 60% of invasion and migration was blocked by the derivatives with moderate alkyl chain length (3b and 5g). Furthermore, the simultaneous inhibitory effects against glioma cell survival, migration and invasion should be an integrated result of the improved lipophilicity, the preferential localization into neoplastic mitochondria and the elevated intracellular ROS production. Overall, these compounds provide novel chemotherapeutic candidates and also offer new insight into the antiglioma therapy through the mitochondrial pathway.Conflict of interest
There is no conflict of interest in this work.Abbreviations
| GBM | Glioblastoma multiforme |
| MMP | Matrix metalloprotease |
| Bcl-2 | B-cell CLL/lymphoma 2 |
| TLC | Thin-layer chromatography |
| MTT | (3,4,5-dimethylthiazol-yl)-2,5-diphenyl tetrazolium |
| IC50 | Half maximal inhibitory concentration |
Acknowledgements
This study was financially supported by Guangdong Natural Science Foundation (S2012010009237).Notes and references
- D. N. Louis, H. Ohgaki, O. D. Wiestler, W. K. Cavenee, P. C. Burger, A. Jouvet, B. W. Scheithauer and P. Kleihues, Acta Neuropathol., 2007, 114, 97 CrossRef PubMed.
- E. G. Van Meir, C. G. Hadjipanayis, A. D. Norden, H. K. Shu, P. Y. Wen and J. J. Olson, Ca-Cancer J. Clin., 2010, 60, 166 CrossRef PubMed.
- C. Di, A. K. Mattox, S. Harward and C. Adamson, Anti-Cancer Agents Med. Chem., 2010, 10, 543 CrossRef CAS.
- T. Demuth and M. E. Berens, J. Neuro-Oncol., 2004, 70, 217 CrossRef PubMed.
- M. E. Berens and A. Giese, Neoplasia, 1999, 1, 208 CrossRef CAS.
- A. Deorukhkar and S. Krishnan, Biochem. Pharmacol., 2010, 80, 1904 CrossRef CAS PubMed.
- (a) Y. Su, S. Sohn, S. E. Krown, P. O. Livingston, J. D. Wolchok, C. Quinn, L. Williams, T. Foster, K. A. Sepkowitz and P. B. Chapman, J. Clin. Oncol., 2004, 22, 610 CrossRef CAS PubMed; (b) A. B. Schwarzberg, E. H. Stover, T. Sengupta, A. Michelini, M. Vincitore, L. R. Baden and M. H. Kulke, Cancer Invest., 2007, 25, 249 CrossRef CAS PubMed; (c) H. S. Friedman, T. Kerby and H. Calvert, Clin. Cancer Res., 2000, 6, 2585 CAS; (d) S. M. Chang, K. R. Lamborn, M. Malec, D. Larson, W. Wara, P. Sneed, J. Rabbitt, M. Page, M. K. Nicholas and M. D. Prados, Int. J. Radiat. Oncol., Biol., Phys., 2004, 60, 353 CrossRef CAS PubMed; (e) J. J. Vredenburgh, A. Desjardins, J. E. Herndon, J. M. Dowell, D. A. Reardon, J. A. Quinn, J. N. Rich, S. Sathornsumetee, S. Gururangan and M. Wagner, Clin. Cancer Res., 2007, 13, 1253 CrossRef CAS PubMed; (f) T. N. Kreisl, L. Kim, K. Moore, P. Duic, C. Royce, I. Stroud, N. Garren, M. Mackey, J. A. Butman and K. Camphausen, J. Clin. Oncol., 2009, 27, 740 CrossRef CAS PubMed.
- S. Zheng, Q. Zhong, Q. Jiang, M. Mottamal, Q. Zhang, N. Zhu, M. E. Burow, R. A. Worthylake and G. Wang, ACS Med. Chem. Lett., 2013, 4, 191 CrossRef CAS PubMed.
- J. Drappatz, A. D. Norden and P. Y. Wen, Expert Rev. Neurother., 2009, 9, 519 CrossRef PubMed.
- S. Fulda, L. Galluzzi and G. Kroemer, Nat. Rev. Drug Discovery, 2010, 9, 447 CrossRef CAS PubMed.
- D. B. Hoelzinger, L. Marianiz, J. Weis, T. Woykeb, T. J. Berens, W. S. McDonough, A. Sloan, S. W. Coons and M. E. Berens, Neoplasia, 2005, 7, 7 CrossRef CAS PubMed.
- N. L. Tran, W. S. McDonough, B. A. Savitch, T. F. Sawyer, J. A. Winkles and M. E. Berens, J. Biol. Chem., 2005, 280, 3483 CrossRef CAS PubMed.
- X.-M. Yin, Z. N. Oltvai and S. J. Korsmeyer, Nature, 1994, 369, 321 CrossRef CAS PubMed.
- A. M. Joy, C. E. Beaudry, N. L. Tran, F. A. Ponce, D. R. Holz, T. Demuth and M. E. Berens, J. Cell Sci., 2003, 116, 4409 CrossRef CAS PubMed.
- T. R. Hurd, M. DeGennaro and R. Lehmann, Trends Cell Biol., 2012, 22, 107 CrossRef CAS PubMed.
- P. R. Vuddanda, S. Chakraborty and S. Singh, Expert Opin. Invest. Drugs, 2010, 19, 1297 CrossRef CAS PubMed.
- G. C. Pereira, A. F. Branco, J. A. Matos, S. L. Pereira, D. Parke, E. L. Perkins, T. L. Serafim, V. A. Sardão, M. S. Santos and A. J. Moreno, J. Pharmacol. Exp. Ther., 2007, 323, 636 CrossRef CAS PubMed.
- V. Weissig and V. P. Torchilin, Adv. Drug Delivery Rev., 2001, 49, 127 CrossRef CAS.
- Y. Sun, K. Xun, Y. Wang and X. Chen, Anti-Cancer Drugs, 2009, 20, 757 CrossRef CAS PubMed.
- (a) J. P. Lin, J. S. Yang, C. C. Wu, S. S. Lin, W. T. Hsieh, M. L. Lin, F. S. Yu, C. S. Yu, G. W. Chen, Y. H. Chang and J. G. Chung, In Vivo, 2008, 22, 223 CAS; (b) H.-P. Kuo, T.-C. Chuang, S.-C. Tsai, H.-H. Tseng, S.-C. Hsu, Y.-C. Chen, C.-L. Kuo, Y.-H. Kuo, J.-Y. Liu and M.-C. Kao, J. Agric. Food Chem., 2012, 60, 9649 CrossRef CAS PubMed.
- S. H. Kim, S. J. Lee, J. H. Lee, W. S. Sun and J. H. Kim, Planta Med., 2002, 68, 277 CrossRef CAS PubMed.
- Z. G.-g. FU Sheng-nan, T. U. O. JUe, Y. A. N. G. Xiao-xiao, Z. H. U. Wen-bo, Y. A. N. Guang-mei and H. U. Hai-yan, J. Sun Yat-sen Univ., Med. Sci., 2013, 34, 531 Search PubMed.
- Y. H. Li, P. Yang, W. J. Kong, Y. X. Wang, C. Q. Hu, Z. Y. Zuo, Y. M. Wang, H. Gao, L. M. Gao, Y. C. Feng, N. N. Du, Y. Liu, D. Q. Song and J. D. Jiang, J. Med. Chem., 2009, 52, 492 CrossRef CAS PubMed.
- K. D. Park, J. H. Lee, S. H. Kim, T. H. Kang, J. S. Moon and S. U. Kim, Bioorg. Med. Chem. Lett., 2006, 16, 3913 CrossRef CAS PubMed.
- K. Iwasa, M. Kamigauchi, M. Ueki and M. Taniguchi, European J. Med. Chem., 1996, 31, 469 CrossRef CAS.
- (a) J. B. Bremner and S. Samosorn, Aust. J. Chem., 2003, 56, 871 CrossRef CAS; (b) I. I. Severina, M. S. Muntyan, K. Lewis and V. P. Skulachev, IUBMB Life, 2001, 52, 321 CrossRef CAS PubMed.
- V. Milano, Y. Piao, T. LaFortune and J. de Groot, Mol. Cancer Ther., 2009, 8, 394 CrossRef CAS PubMed.
- C.-Y. LO, L.-C. HSU, M.-S. CHEN, Y.-J. LIN, L.-G. CHEN, C.-D. KUO and J.-Y. WU, Bioorg. Med. Chem. Lett., 2013, 23, 305 CrossRef CAS PubMed.
- M. Viale, C. Cordazzo, B. Cosimelli, D. de Totero, P. Castagnola, C. Aiello, E. Severi, G. Petrillo, M. Cianfriglia and D. Spinelli, J. Med. Chem., 2009, 52, 259 CrossRef CAS PubMed.
- F. Wang, M. A. Ogasawara and P. Huang, Mol. Aspects Med., 2010, 31, 75 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experiments and characterisation of compounds are in the ESI. See DOI: 10.1039/c4md00264d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |