Scavenging of superoxide anions by lecithinized superoxide dismutase in HL-60 cells†
Tsutomu
Ishihara
*a,
Misaki
Shibui
a,
Takaya
Hoshi
a and
Tohru
Mizushima
b
aDepartment of Chemical Biology and Applied Chemistry, College of Engineering, Nihon University, 1 Nakagawara, Tokusada, Tamuramachi, Koriyama, Fukushima 963-8642, Japan. E-mail: ishihara@chem.ce.nihon-u.ac.jp; Fax: +81-24-956-8805; Tel: +81-24-956-8805
bDepartment of Analytical Chemistry, Faculty of Pharmacy, Keio University, Tokyo 105-8512, Japan
First published on 13th November 2015
Abstract
Superoxide dismutase covalently bound to four lecithin molecules (PC-SOD) has been found to have beneficial therapeutic effects in animal models of various diseases. However, the mechanism underlying these improved therapeutic effects has not yet been elucidated. It has previously been shown that PC-SOD localizes on the plasma membrane and in the lysosomes of cells. In this study, we evaluated the superoxide anion-scavenging activity of PC-SOD in HL-60 human promyelocytic leukemia cells. Compared to SOD, PC-SOD had only 17% scavenging activity in cell-free systems. Nevertheless, by analyzing enzyme activities in cell suspensions containing PC-SOD or SOD, PC-SOD and SOD showed almost equal activity for scavenging extracellular superoxide anions produced by HL-60 cells. Furthermore, the activity for scavenging extracellular superoxide anions increased with increased amount of PC-SOD on the plasma membrane. Moreover, PC-SOD exhibited no obvious inhibitory effect on the scavenging of intracellular superoxide anions. These results suggested that the association of PC-SOD with the plasma membrane plays a key role in its beneficial therapeutic effects. Thus, this finding may provide a rationale for selecting target diseases for PC-SOD treatment.
1. Introduction
Reactive oxygen species (ROS), including superoxide anions, are produced in various types of cells such as phagocytes, fibroblasts, endothelial cells, vascular smooth muscle cells, and cardiac myocytes.1–3 ROS influence numerous physiological processes, including host defense, hormone biosynthesis, fertilization, and cellular signaling. However, the overproduction of ROS is implicated in various diseases such as hypertension, atherosclerosis, diabetic complications, cancer, and Alzheimer's disease.1–3 In biological systems, excess ROS are rapidly removed from cells by various native antioxidants. Superoxide dismutase (SOD), which is classified into three types, cytosolic CuZn-SOD, mitochondrial Mn-SOD, and extracellular Ec-SOD, is one of the most important antioxidative enzymes. SOD catalyzes the dismutation of superoxide anions into hydrogen peroxide and oxygen.4 Numerous efforts have been made to develop antioxidants capable of scavenging ROS as therapeutic agents. Genetic overexpression of SOD in animal models of various diseases showed a beneficial effect,5,6 which indicates that SOD is useful as a potential therapeutic antioxidant drug. However, SOD has two major drawbacks in its therapeutic applications: (i) short lifespan in blood circulation due to its rapid excretion from the kidney7,8 and (ii) low cellular uptake.9Chemical modification of proteins may improve their therapeutic characteristics; however, this depends on the type of protein.10,11 Such improvements in therapeutic effect are mainly due to altered biodistribution of the proteins, as their activities are generally decreased by chemical modification. Poly(ethylene glycol) (PEG) has been widely used as a modifying material for proteins.10,11 Pegylated proteins, which tend to remain in blood circulation for a prolonged duration with reduced cellular uptake, are already being used in clinical settings. Several researchers have reported beneficial effects of SOD conjugated with PEG, both in cultured cells and in animal models of disease.12–14 However, the therapeutic applications of these SOD derivatives in humans have not been approved to date, potentially due to the insufficient therapeutic activity of pegylated SOD. Most clinically approved proteins (e.g., interferon, erythropoietin, and granulocyte-colony stimulating factor) exert their activity by binding receptors on plasma membranes; however, SOD functions as an enzyme. Pegylated SOD is capable of scavenging superoxide anions released from cells into circulating blood. However, for more rapid and continuous scavenging of superoxide anions, the accumulation of SOD at locations where superoxide anions are generated may be preferable.
CuZn-SOD covalently bound to an average of four molecules of a lecithin derivative (PC-SOD) has been developed.15–17 As compared to unmodified SOD, PC-SOD exhibits higher affinity for cells in vitro,16 more prolonged residence in blood circulation, and greater accumulation in various tissues in vivo.15,18 In addition, PC-SOD exhibits beneficial effects in animal models of various disease conditions such as ulcerative colitis,19 bleomycin-induced pulmonary fibrosis,20,21 elastase-induced emphysema,22 focal cerebral ischemic injury,23 and spinal cord injury-induced motor dysfunction.24 In our previous study, we attempted to clarify the mechanism underlying the improvement in effect by analyzing the interaction of PC-SOD with biological components such as plasma proteins and cells.17 PC-SOD formed a complex with serum protein(s) such as albumin, while unmodified SOD did not. The PC-SOD content in HeLa cells (human epithelial carcinoma cell line) was markedly higher than that of unmodified SOD, and was mainly distributed to lysosomes.17 The data indicated that the increased hydrophobicity of PC-SOD enhanced its association with both serum protein(s) and the plasma membrane; the former inhibited SOD excretion and promoted long-term retention in blood circulation, while the latter enhanced internalization into cells via endocytosis. In the current study, we further examined the superoxide anion-scavenging activity of PC-SOD in HL-60 cells (human promyelocytic leukemia cell line).
2. Materials and methods
Materials
Recombinant human CuZn-SOD (molecular mass, 32 kDa) was supplied by Asahi Kasei Pharma (Tokyo, Japan). Fluorescein isothiocyanate isomer I (FITC) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). FITC-labeled SOD and FITC-labeled PC-SOD were synthesized according to the literature.17 In the current study, FITC-labeled PC-SOD modified with an average number of 3.7 lecithin molecules was used. The modification of PC-SOD with FITC decreased its enzymatic activity. Thus, FITC-labeled PC-SOD and FITC-SOD were used only for the analysis of cellular distribution. The concentrations of SOD and PC-SOD were determined by the BCA protein assay (Thermo Fisher Scientific, Inc., Waltham, MA). 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1) was purchased from Dojindo Laboratories (Kumamoto, Japan). Pyocyanin was purchased from Cayman Chemical Co. (Ann Arbor, MI).Interaction of FITC-labeled PC-SOD with blood cells
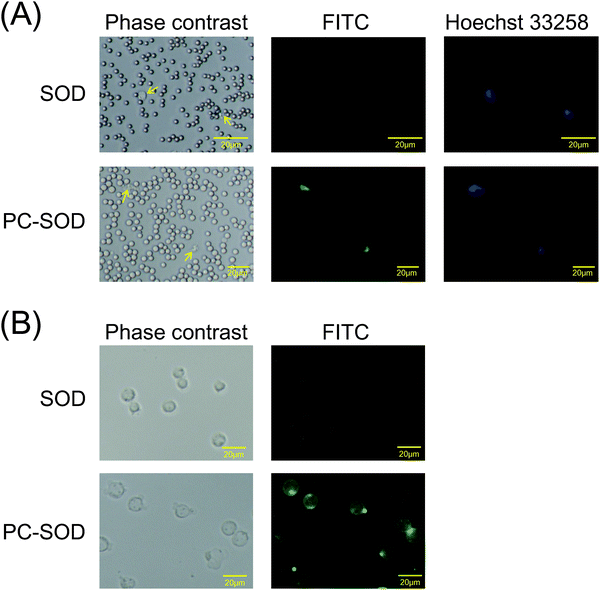
Horse blood to which an equal volume of Alsever's solution (Nippon Bio-Test Laboratories, Tokyo, Japan) had been added was diluted twenty-fold with physiologic saline solution. The diluted blood was incubated with 100 μg mL−1 FITC-labeled SOD or FITC-labeled PC-SOD for 2 h at 37 °C with gentle shaking. The blood cells were collected by centrifugation at 1500g for 5 min, after which the cells were washed with physiologic saline solution. After staining the nuclei of cells with 50 μg mL−1 Hoechst 33258 (Dojindo Laboratories), fluorescence images of cells were captured using a DS-Fi1c digital camera connected to a Nikon DS-L2 controller (Nikon Corp., Tokyo, Japan).Interaction of FITC-labeled PC-SOD with HL-60 cells and HeLa cells
HL-60 cells and HeLa cells were provided by RIKEN BRC through the National Bio-Resource Project of the MEXT Japan, and were cultured in RPMI-1640 medium and Dulbecco's Modified Eagles medium (DMEM), respectively, supplemented with 10% fetal bovine serum, streptomycin, and penicillin. HL-60 cells were precultured in medium with dimethylsulfoxide (final concentration, 1.3%) for 4 days to induce neutrophil-like differentiation before the start of the experiments.25 The HL-60 cells (1.5 × 106 cells per mL) were incubated with 125 μg mL−1 FITC-labeled SOD or FITC-labeled PC-SOD in Opti-MEM (Opti-MEM I Reduced Serum Medium; Life Technologies Corp., Carlsbad, CA) for 2 h at 37 °C. The cells were washed with phosphate-buffered saline (PBS) by centrifugation at 1000 rpm, and the fluorescence images of the cells were captured as mentioned above.In addition, after incubation of HL-60 cells with FITC-labeled PC-SOD and washing with PBS, the cells were further incubated in RPMI-1640 medium for 2 h or 24 h at 37 °C. After incubation, fluorescence images of the cells were captured. Alternatively, the lysosomes of cells were stained with Lysotracker-Red DND-99 (Life Technologies Corp.) in the medium for 2 h, after which the nuclei of the cells were stained with 50 μg mL−1 Hoechst 33258 for 30 min. Fluorescence images of the cells were captured similarly.
HeLa cells were harvested on the 8-well Nunc Lab-Tek II Chambered Coverglass (Thermo Fisher Scientific, Inc., Waltham, MA) at 5 × 104 cells per well and incubated overnight at 37 °C in DMEM. The cells were washed with PBS and incubated with 50 μg mL−1 FITC-labeled PC-SOD in Opti-MEM for 2 h at 37 °C. After the cells were washed with PBS, they were further incubated in DMEM for 24 h at 37 °C. The lysosomes and mitochondria of the cells were costained using Lysotracker-Red DND-99 and Mito-Red (Dojindo Laboratories), respectively, according to the manufacturer's instructions, and the fluorescence images of the cells were captured.
Superoxide anion-scavenging activity of PC-SOD in cell-free system
Superoxide anion-scavenging activities of PC-SOD and SOD were determined using the SOD assay kit-WST (Dojindo Laboratories) according to the manufacturer's instructions. During this analysis, water-soluble tetrazolium salts (WST-1) are converted to chromogenic tetrazolium salt (WST-1 formazan) in the presence of superoxide anions produced by xanthine oxidase.Scavenging activity of PC-SOD for superoxide anions produced by cells
HL-60 cells suspended in Hank's balanced salt solution (HBSS, Life Technologies Corp.) in wells of 96-well plates (1.5 × 106 cells per mL) were incubated with various concentrations of SOD or PC-SOD for 2 h at 37 °C. After incubation, pyocyanin and WST-1 were directly added to the wells at concentrations of 50 μM and 800 μM, respectively. After incubation for 12 h at 37 °C, the absorbance of the wells at 450 nm (reference wavelength, 630 nm) was measured using a microplate reader.Alternatively, HL-60 cells (4.0 × 105 cells per mL) were incubated with various concentrations of SOD or PC-SOD in HBSS for 2 h at 37 °C. After the cells were washed three times with PBS by centrifugation, the cells were incubated for 0, 2, or 4 h in RPMI-1640 medium. After the cells were washed with PBS again, live cells were counted with trypan blue using a Neubauer cell counting chamber. The live cells were harvested in wells of 96-well plates (1.5 × 106 cells per mL), and pyocyanin and WST-1 were added to the wells at concentrations of 50 μM and 800 μM, respectively. After incubation for 12 h at 37 °C, the absorbance of the wells was measured as mentioned above.
Detection of superoxide anions in cells
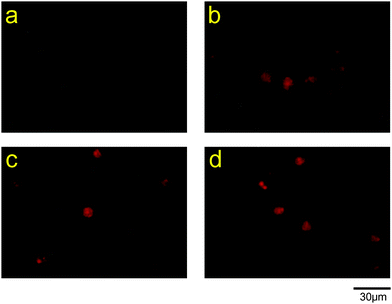
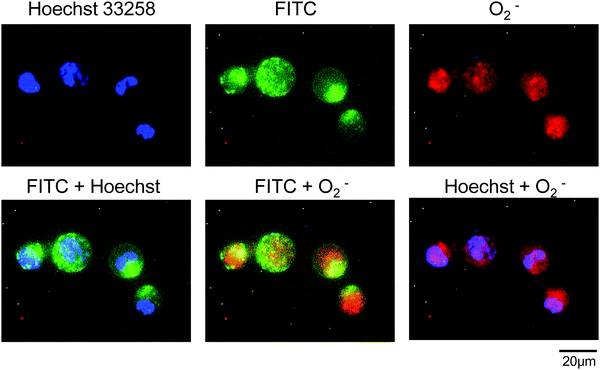
Intracellular superoxide anions were detected using the total ROS/superoxide detection kit (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer's instructions with minor modifications. Briefly, HL-60 cells (4.0 × 105 cells per mL) were incubated with 25 μg mL−1 PC-SOD or SOD in Opti-MEM for 2 h at 37 °C. After washing the cells with the wash buffer provided with the kit by centrifugation, the cells (1.5 × 106 cells per mL) were incubated in RPMI-1640 medium with 500 μM pyocyanin for 30 min at 37 °C. After washing the cells with the wash buffer, the cells were incubated with the superoxide detection reagent included in the kit for 1 h at 37 °C, and the fluorescence images of the cells were captured.The intracellular distributions of PC-SOD and superoxide anions were also investigated. After incubation of HL-60 cells with 125 μg mL−1 FITC-labeled PC-SOD in Opti-MEM for 2 h at 37 °C, the cells were stimulated with pyocyanin as mentioned above. Superoxide anions and the nuclei of the cells were stained using the superoxide detection reagent and Hoechst 33258, respectively, as mentioned above.
3. Results
In our previous study, it was found that PC-SOD was distributed in the plasma but not in blood cells after addition to the blood17 because of complex formation of PC-SOD with serum protein(s) such as albumin. In contrast, PC-SOD was internalized in various types of cells such as HeLa cells, human umbilical vein endothelial cells, and RAW264.7 cells.17 Recently, it was also found that PC-SOD was internalized in Hepa1-6 murine hepatoma cells and Huh-7 human hepatoma cells (ESI,† Fig. S1). However, the interaction of PC-SOD with blood cells has not been investigated yet. Therefore, the interaction of FITC-labeled PC-SOD with the horse blood cells was investigated initially. As shown in Fig. 1A, PC-SOD was internalized into the lymphocytes, while SOD was not. PC-SOD was also internalized in the cells of the human promyelocytic leukemia cell line, HL-60 (Fig. 1B). Taken together with our previous results, these results suggested that a part of PC-SOD was preferentially internalized in lymphocytes, while most of the PC-SOD was distributed in the plasma. This accumulation of PC-SOD in lymphocytes may contribute to the efficient scavenging of superoxide anions in vivo, resulting in improvement in the treatment of diseases associated with excess ROS production of lymphocytes.26In a previous study, when the cells were observed immediately after incubation with PC-SOD for 2 h at 37 °C, it was found that PC-SOD is distributed to the lysosomes of HeLa cells via cholesterol-sensitive endocytosis.17 In the current study, the distribution of PC-SOD in HL-60 cells was also investigated immediately or at 24 h after incubation with PC-SOD (Fig. 2A). The distribution of FITC-labeled PC-SOD almost overlapped with that of Lysotracker-Red in both periods, indicating that most of the PC-SOD localized to the lysosomes of HL-60 cells and remained there even after 24 h. We further examined the intracellular distribution of PC-SOD in HeLa cells at 24 h after internalization. The lysosomes and mitochondria of HeLa cells were costained with Lysotracker-Red and Mito-Red, respectively, (Fig. 2B). The distribution of PC-SOD seems to overlap with that of lysosomes, but not of mitochondria. Thus, it was confirmed that the majority of PC-SOD remained in the lysosomes at 24 h after the internalization in HeLa cells as well as in HL-60 cells.
Superoxide anion-scavenging activities of PC-SOD and SOD were determined using the SOD assay kit. WST-1 is reduced in the presence of superoxide anions produced by xanthine oxidase, resulting in the formation of the chromogenic tetrazolium salt (WST-1 formazan). As the reduction of WST-1 is inhibited by SOD, the superoxide anion-scavenging activity of SOD can be determined by the colorimetric method. The IC50 values (50% inhibition activity) of SOD and PC-SOD were measured to be 0.39 μg mL−1 and 2.3 μg mL−1, respectively, (Fig. 3). Thus, it was determined that PC-SOD has only 17% scavenging activity as compared to that of SOD. In a PC-SOD molecule, approximately four lecithin molecules are non-regiospecifically bound to amino groups on SOD.15 Thus, this decrease in enzymatic activity might be caused by the steric hindrance of surface-bound lecithin and/or changes in the protein conformation by chemical modification.
Extracellular superoxide anions generated by the cells were analyzed according to the literature.27,28 After HL-60 cells were incubated with various amounts of enzymes (SOD or PC-SOD) for 2 h, the cells were stimulated by pyocyanin to produce superoxide anions. Superoxide anions in the cell suspensions were analyzed using WST-1 in the presence of enzymes (Fig. 4A). The amount of superoxide anions decreased with an increase in the enzymes. There was no significant difference between the activities of PC-SOD and SOD, despite the lesser intrinsic activity of PC-SOD, as shown in Fig. 3. Alternatively, the cells incubated with various amounts of enzymes were washed with PBS, after which the superoxide anions in the cell suspensions were similarly detected with WST-1 (Fig. 4B). The amounts of superoxide anions drastically decreased with an increase in PC-SOD, while the amounts of SOD did not decrease. In addition, after incubation of cells with PC-SOD, the cells were washed and further incubated in RPMI-1640 medium for 0, 2, or 4 h. Afterward, the amount of superoxide anions in cell suspensions was detected using WST-1 (Fig. 5A); the amount of superoxide anions at 2 h and 4 h was higher than that at 0 h. Furthermore, the cells incubated with FITC-labeled PC-SOD were similarly observed by fluorescence microscopy (Fig. 5B). The amount of FITC-labeled PC-SOD on the plasma membrane decreased after 2 h of incubation. Taken together, these results show that the association of PC-SOD with the plasma membrane plays an important role in scavenging extracellular superoxide anions.
Intracellular superoxide anions were visualized using a fluorescence dye for staining superoxide anions, after incubation of HL-60 cells with SOD or PC-SOD. The dye, a superoxide detection reagent included in the total ROS/superoxide detection kit, is cell-permeable and reacts specifically with superoxide anions, generating a fluorescent product in live cells. As shown in Fig. 6, the cells treated with pyocyanin showed fluorescence, indicating an increase in superoxide anions in the cells. However, the apparent inhibitory effect of PC-SOD or SOD on the production of superoxide anions was not observed. The intracellular locations of FITC-labeled PC-SOD and superoxide anions in HL-60 cells were also examined. As shown in Fig. 7, the distributions of FITC-labeled PC-SOD, the nucleus, and the superoxide anions did not overlap, suggesting that superoxide anions were distributed to the mitochondria, while FITC-labeled PC-SOD was distributed to the lysosome.
4. Discussion
Superoxide anions are mainly generated by enzymes such as NAD(P)H oxidases (Nox) and xanthine oxidases or nonenzymatically by redox-active compounds of the mitochondrial electron transport chain.1 The Nox protein family generates superoxide anions both intracellularly and extracellularly, because Nox proteins are distributed at various subcellular locations depending on the type.29,30 For example, Nox1 has been identified in the caveolae in the plasma membrane, early endosomes, and nucleus, and Nox2 is found in the plasma membrane, endosomes, and phagosomes. It was reported that Nox plays an important role in the extracellular release of ROS. When phagocytes such as neutrophils are exposed to microbial pathogens, the phagocytes produce large amounts of superoxide anions through activation of Nox.31It is well known that pyocyanin is a redox-active compound and induces ROS generation by cells. Superoxide anions are produced extracellularly by the activated Nox in neutrophils exposed to pyocyanin.32 Because pyocyanin can cross biological membranes, it also induces the production of superoxide anions in mitochondria by direct interaction with the mitochondrial respiratory chain33 and in the cytoplasm by oxidation of intracellularly reduced NADPH.34 The doses of pyocyanin in the experiments of this study were determined preliminarily. The least amount of pyocyanin required to induce sufficient coloring in WST-1 assay or distinguish fluorescence in cells using the superoxide detection reagent was determined. The extracellular superoxide anion-scavenging activity of PC-SOD was investigated using WST-1 (Fig. 4), which is a cell-impermeable tetrazolium salt.27,28 Because negatively charged superoxide anions cannot freely cross biological membranes,35 it was considered that the superoxide anions detected were mainly generated extracellularly by Nox on the pyocyanin-stimulated cells. The production of superoxide anions was inhibited by PC-SOD depending on the amount of PC-SOD present on the plasma membrane (Fig. 4 and 5). Thus, these results indicate that PC-SOD associated with cells efficiently scavenges superoxide anions produced by Nox on the plasma membrane.
It was also reported that the assembly of active Nox and the subsequent ROS production were dependent on lipid rafts.36 Lipid rafts are microdomains of the plasma membrane enriched in cholesterol and sphingolipids, and play many important roles in cellular functions.37 Lipid rafts are related to cell signal transduction for promoting local production of ROS by Nox.36 In addition, lipid rafts are essential for the formation of the primary endocytic vehicle in clathrin-independent endocytic pathways such as caveola-dependent endocytosis.38,39 We previously reported that PC-SOD was internalized in cells via cholesterol-sensitive endocytosis.17 Furthermore, as shown in Fig. 2, most of the PC-SOD was found to be distributed to the lysosomes of the cells in the current study. These results indicated that PC-SOD was initially associated with lipid rafts on the plasma membrane and then internalized into the cells via endocytosis. Taken together, the results suggest that increased superoxide anion-scavenging activity of PC-SOD was caused by the co-localization of PC-SOD and Nox in the lipid raft on the plasma membrane (Fig. 8).
Intracellular superoxide anions were also detected using a fluorescent dye for staining superoxide anions. The subcellular localization of superoxide anions was observed in pyocyanin-stimulated cells (Fig. 7), suggesting the production of superoxide anions in mitochondria. However, the inhibitory effect of PC-SOD on the production of superoxide anions was not detected (Fig. 6). Thus, these results suggested that PC-SOD has low superoxide anion-scavenging activity in mitochondria, because most of the PC-SOD cannot escape from lysosomes to the cytoplasm after internalization and/or are degraded in lysosomes.
It is believed that oxidative stress causes various diseases, although the mechanisms differ depending on the type of disease. In the current study, it was clarified that PC-SOD tends to accumulate in lymphocytes and efficiently scavenges extracellular superoxide anions. Thus, it may be considered that PC-SOD is available for the treatment of diseases caused by excess ROS production by phagocytes such as neutrophils, for example, ischemia-reperfusion injury and autoimmune diseases such as ulcerative colitis and rheumatoid arthritis. In addition, this unique technique of modifying proteins with lecithin may enhance the production of various protein-based therapeutics. The findings of this study will help identify promising proteins and treatment of diseases with them.
5. Conclusions
It has been reported that PC-SOD has a high therapeutic effect in animal disease models, probably because of its high affinity for cells as well as its long circulation time in the blood. In the current study, we evaluated the superoxide anion-scavenging activity of PC-SOD in vitro to clarify the underlying mechanism. PC-SOD showed a decrease in this scavenging activity in cell-free systems; in contrast, it showed high activity against extracellular superoxide anions generated by the cells. These results indicated that the association of PC-SOD with the plasma membrane drastically enhanced the efficacy of scavenging extracellular superoxide anions.Acknowledgements
This work was supported in part by grants from the New Energy and Industrial Technology Development Organization (NEDO) of Japan. The authors thank Shunsuke Nara, Mitsuki Takeda, and Risa Itoh for their technical assistance with the experiments.References
- W. Dröge, Physiol. Rev., 2002, 82, 47–95 CrossRef PubMed.
- C. A. Hamilton, W. H. Miller, S. Al-Benna, M. J. Brosnan, R. D. Drummond, M. W. McBride and A. F. Dominiczak, Clin. Sci., 2004, 106, 219–234 CrossRef CAS PubMed.
- A. F. Chen, D. D. Chen, A. Daiber, F. M. Faraci, H. Li, C. M. Rembold and I. Laher, Clin. Sci., 2012, 123, 73–91 CrossRef CAS PubMed.
- F. Johnson and C. Giulivi, Mol. Aspects Med., 2005, 26, 340–352 CrossRef CAS PubMed.
- Y. Ding, Y. L. Li, M. C. Zimmerman, R. L. Davisson and H. D. Schultz, Cardiovasc. Res., 2009, 81, 678–685 CrossRef CAS PubMed.
- A. S. Davis, H. Zhao, G. H. Sun, R. M. Sapolsky and G. K. Steinberg, Neurosci. Lett., 2007, 411, 32–36 CrossRef CAS PubMed.
- B. Odlind, L. E. Appelgren, A. Bayati and M. Wolgast, Pharmacol. Toxicol., 1988, 62, 95–100 CAS.
- A. Petkau, W. S. Chelack, K. Kelly, C. Barefoot and L. Monasterski, Res. Commun. Chem. Pathol. Pharmacol., 1976, 15, 641–654 CAS.
- A. M. Michelson and K. Puget, Acta Physiol. Scand., Suppl., 1980, 492, 67–80 CAS.
- P. Milla, F. Dosio and L. Cattel, Curr. Drug Metab., 2012, 13, 105–119 CrossRef CAS PubMed.
- S. Jevsevar, M. Kunstelj and V. G. Porekar, Biotechnol. J., 2010, 5, 113–128 CrossRef CAS PubMed.
- V. R. Muzykantov, J. Controlled Release, 2001, 71, 1–21 CrossRef CAS PubMed.
- F. M. Veronese, P. Caliceti, O. Schiavon and M. Sergi, Adv. Drug Delivery Rev., 2002, 54, 587–606 CrossRef CAS PubMed.
- K. Yoshida, G. F. Burton, J. S. McKinney, H. Young and E. F. Ellis, Stroke, 1992, 23, 865–869 CrossRef CAS PubMed.
- R. Igarashi, J. Hoshino, M. Takenaga, S. Kawai, Y. Morizawa, A. Yasuda, M. Otani and Y. Mizushima, J. Pharmacol. Exp. Ther., 1992, 262, 1214–1219 CAS.
- R. Igarashi, J. Hoshino, A. Ochiai, Y. Morizawa and Y. Mizushima, J. Pharmacol. Exp. Ther., 1994, 271, 1672–1677 CAS.
- T. Ishihara, S. Nara and T. Mizushima, J. Pharm. Sci., 2014, 103, 1987–1994 CrossRef CAS PubMed.
- F. J. Broeyer, B. E. van Aken, J. Suzuki, M. J. Kemme, H. C. Schoemaker, A. F. Cohen, Y. Mizushima and J. Burggraaf, Br. J. Clin. Pharmacol., 2008, 65, 22–29 CrossRef CAS PubMed.
- T. Ishihara, K. Tanaka, Y. Tasaka, T. Namba, J. Suzuki, T. Ishihara, S. Okamoto, T. Hibi, M. Takenaga, R. Igarashi, K. Sato, Y. Mizushima and T. Mizushima, J. Pharmacol. Exp. Ther., 2009, 328, 152–164 CrossRef CAS PubMed.
- K. Tanaka, T. Ishihara, A. Azuma, S. Kudoh, M. Ebina, T. Nukiwa, Y. Sugiyama, Y. Tasaka, T. Namba, T. Ishihara, K. Sato, Y. Mizushima and T. Mizushima, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2010, 298, L348–L360 CrossRef CAS PubMed.
- K. Tanaka, A. Azuma, Y. Miyazaki, K. Sato and T. Mizushima, Chest, 2012, 142, 1011–1019 CrossRef CAS PubMed.
- K. Tanaka, K. Sato, K. Aoshiba, A. Azuma and T. Mizushima, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2012, 302, L1250–L1261 CrossRef CAS PubMed.
- T. Tsubokawa, V. Jadhav, I. Solaroglu, Y. Shiokawa, Y. Konishi and J. H. Zhang, Stroke, 2007, 38, 1057–1062 CrossRef CAS PubMed.
- M. Takenaga, Y. Ohta, Y. Tokura, A. Hamaguchi, M. Nakamura, H. Okano and R. Igarashi, J. Controlled Release, 2006, 110, 283–289 CrossRef CAS PubMed.
- S. Bréchard, A. Brunello, J. L. Bueb and E. J. Tschirhart, Biochim. Biophys. Acta, 2006, 1763, 129–136 CrossRef PubMed.
- H. L. Wright, R. J. Moots, R. C. Bucknall and S. W. Edwards, Rheumatology, 2010, 49, 1618–1631 CrossRef CAS PubMed.
- A. S. Tan and M. V. Berridge, J. Immunol. Methods, 2000, 238, 59–68 CrossRef CAS PubMed.
- W. M. Nauseef, Biochim. Biophys. Acta, 2014, 1840, 757–767 CrossRef CAS PubMed.
- B. Lassègue and K. K. Griendling, Arterioscler., Thromb., Vasc. Biol., 2010, 30, 653–661 CrossRef PubMed.
- D. I. Brown and K. K. Griendling, Free Radical Biol. Med., 2009, 47, 1239–1253 CrossRef CAS PubMed.
- T. M. Paravicini and R. M. Touyz, Diabetes Care, 2008, 31, S170–S180 CrossRef CAS PubMed.
- B. Rada, M. A. Jendrysik, L. Pang, C. P. Hayes, D. G. Yoo, J. J. Park, S. M. Moskowitz, H. L. Malech and T. L. Leto, PLoS One, 2013, 8, e54205 CAS.
- A. Managò, K. A. Becker, A. Carpinteiro, B. Wilker, M. Soddemann, A. P. Seitz, M. J. Edwards, H. Grassmé, I. Szabò and E. Gulbins, Antioxid. Redox Signaling, 2015, 22, 1097–1110 CrossRef PubMed.
- B. Rada and T. L. Leto, Trends Microbiol., 2013, 21, 73–81 CrossRef CAS PubMed.
- M. A. Takahashi and K. Asada, Arch. Biochem. Biophys., 1983, 226, 558–566 CrossRef CAS PubMed.
- M. Ushio-Fukai, Antioxid. Redox Signaling, 2009, 11, 1289–1299 CrossRef CAS PubMed.
- K. S. George and S. Wu, Toxicol. Appl. Pharmacol., 2012, 259, 311–319 CrossRef CAS PubMed.
- P. Lajoie and I. R. Nabi, Int. Rev. Cell Mol. Biol., 2010, 282, 135–163 CAS.
- C. Le Roy and J. L. Wrana, Nat. Rev. Mol. Cell Biol., 2005, 6, 112–126 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1. Uptake of FITC-labeled PC-SOD in HeLa cells, Hepa1-6 cells (murine hepatoma cell line), and Huh-7 cells (human hepatoma cell line). The cells were incubated with FITC-labeled PC-SOD (50 μg mL−1) in DMEM supplemented with 10% fetal bovine serum or Opti-MEM for 2 h at 37 °C. The cells were washed with PBS three times, and the fluorescence images of cells were captured using a DS-Fi1c digital camera connected to a Nikon DS-L2 controller. See DOI: 10.1039/c5mb00631g |
| This journal is © The Royal Society of Chemistry 2016 |