Syntheses of novel halogen-free Brønsted–Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate
Xiao-Xiang
Han
*a,
Huan
Du
 a,
Chin-Te
Hung
b,
Li-Li
Liu
b,
Pei-Hao
Wu
b,
Da-Hai
Ren
c,
Shing-Jong
Huang
d and
Shang-Bin
Liu
a,
Chin-Te
Hung
b,
Li-Li
Liu
b,
Pei-Hao
Wu
b,
Da-Hai
Ren
c,
Shing-Jong
Huang
d and
Shang-Bin
Liu
 *be
*be
aDepartment of Applied Chemistry, Zhejiang Gongshang University, Hangzhou, 310035, China. E-mail: hxx74@126.com; Tel: +86-571-28008975
bInstitute of Atomic and Molecular Sciences, Academic Sinica, Taipei 10617, Taiwan. E-mail: sbliu@sinica.edu.tw; Tel: +886-2-23668230
cDepartment of Chemical & Biomolecular Engineering, Clarkson University, Potsdam, New York 13699-5705, USA
dInstrumentation Center, National Taiwan University, Taipei 10617, Taiwan
eDepartment of Chemistry, National Taiwan Normal University, Taipei 11677, Taiwan
First published on 10th September 2014
Abstract
A series of benign halogen-free ionic liquid (IL) catalysts were synthesized by combining the Brønsted acidic ionic liquid [HSO3-pmim]+HSO4− with ZnO in different composition ratios. The IL catalysts, which possess both Brønsted and Lewis acidities, were employed as acidic catalysts for the esterification of n-caprylic acid to methyl caprylate. The [HSO3-pmim]+(1/2Zn2+)SO42−, prepared by cooperating equimolar amounts of Brønsted and Lewis acid sites, was found to exhibit an optimal catalytic performance and excellent durability. This is attributed to a synergy of Brønsted and Lewis acidities manifested by the catalyst. The response surface methodology (RSM) based on the Box–Behnken design (BBD) was utilized to explore the effects of different experimental variables (viz. catalyst amount, methanol to caprylic acid molar ratio, temperature, and reaction time) on the esterification reaction. Analysis of variance (ANOVA) was also employed to study the interactions between variables and their effects on the catalytic process. Accordingly, the deduced optimal reaction conditions led to a high methyl caprylate yield of 95.4%, in good agreement with experimental results and those predicted by the BBD model. Moreover, a kinetic study performed under optimal reaction conditions revealed an apparent reaction order of 1.70 and an active energy of 33.66 kJ mol−1.
Introduction
Esterification of alcohols and carboxylic acids is a fundamental and important reaction in organic synthesis. The corresponding products, carboxylic esters, have been extensively applied in areas such as cosmetics, plastics, food, medicine and intermediates.1 Conventionally, syntheses of carboxylic esters mostly invoke homogeneous catalysts, such as sulfuric, p-toluene sulfonic, and phosphoric acids. However, problems caused by these catalysts are not in conformity with the requirements for sustainable development, e.g., equipment corrosion, environmental pollution, undesirable side reactions, plaguy catalyst recovery and reuse. To avoid these drawbacks, heterogeneous catalysts such as solid superacids,2–5 heteropolyacids,6 zeolites,7–9 resins10 and enzymes11–14 have been employed for esterification. Unfortunately, limited applications were found for these solid catalysts due to their disadvantages such as low activity, easy deactivation, formidable separation and recovery and so on.15 Hence, it is essential to develop environmentally benign catalysts to replace the aforementioned catalysts.Ionic liquids (ILs), which have been considered to be new eco-friendly catalysts, have received considerable attention in various chemical syntheses owing to their unique characteristics such as low melting point, high thermal stability, negligible volatility, easy solubility, adjustable properties, recyclability, and reusability.16,17 Acidic ILs may be classified into Brønsted18–28 and Lewis types29,30 in accordance with their acidic groups. While various Brønsted–Lewis acidic ILs (B–L AILs) have been extensively developed and applied,31,32 the most common types, which invoke the incorporation of metal halides, are moisture-sensitive and hence are inapplicable for aqueous systems. To overcome this problem, efforts have been made to prepare halogen-free B–L AILs using copper oxide as the source of Lewis acidity.33 Herein, we report a series of novel halogen-free B–L AIL catalysts synthesized by combining Brønsted acidic IL [HSO3-pmim]+HSO4− with varied amounts of ZnO as the precursor for Lewis acidity. The acid catalysts so fabricated were characterized by a variety of different analytical and spectroscopic techniques, such as thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR), and 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. Moreover, the acidic properties of these B–L AILs were investigated by solid-state 31P magic-angle spinning (MAS) NMR of adsorbed trimethylphosphine oxide (TMPO) as the probe molecule.34–36 The catalytic activities of these B–L AILs during esterification of caprylic acid with methanol were investigated and the relevant reaction conditions invoked during the synthesis of methyl caprylate were optimized using the response surface methodology (RSM). The effects and correlations of different experimental variables such as catalyst amount, methanol to caprylic acid molar ratio, temperature, and reaction time on the esterification reaction were addressed by the Box–Behnken design (BBD) model, followed by a kinetic study performed under these optimized experimental parameters.
Experimental
Catalyst preparation
The novel B–L AIL catalysts were synthesized by combining the sulfonic acid-functionalized Brønsted acidic ionic liquid [HSO3-pmim]+HSO4− (denoted ILa) with zinc oxide (ZnO). This was carried out by first preparing the [HSO3-pmim]+HSO4− using a known procedure outlined in the literature,37,38 followed by adding varied amounts of ZnO to the aqueous solution. The mixture was stirred continuously till the solid was completely dissolved. The resulting products were evacuated after water removal to obtain the final B–L AIL catalysts with varied L/B ratios, namely [HSO3-pmim]+(1/2H+·1/4Zn2+)SO42−, [HSO3-pmim]+(1/2Zn2+)SO42−, [(1/2H+·1/4Zn2+)SO3-pmim]+(1/2Zn2+)SO42−, and [(1/2Zn2+)SO3-pmim]+(1/2Zn2+)SO42−, denoted ILb, ILc, ILd, and ILe, respectively, as specified in Table 1.| Catalyst | Conv.b (%) | Yieldc (%) | ||
|---|---|---|---|---|
| Name | Formula | L/Ba | ||
| a Molar ratio of Lewis/Brønsted acid sites. b Reaction conditions: catalyst amount = 6 wt%, methanol/caprylic acid molar ratio = 5.0, reaction time = 3.0 h, and temperature = 363 K. c Product yield of methyl caprylate determined by GC. | ||||
| ILa | [HSO3-pmim]+HSO4− | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
92.0 | 91.4 |
| ILb | [HSO3-pmim]+(1/2H+·1/4Zn2+)SO42− | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
93.0 | 92.5 |
| ILc | [HSO3-pmim]+(1/2Zn2+)SO42− | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
94.8 | 94.3 |
| ILd | [(1/2H+·1/4Zn2+)SO3-pmim]+(1/2Zn2+)SO42− | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86.7 | 86.1 |
| ILe | [(1/2Zn2+)SO3-pmim]+(1/2Zn2+)SO42− | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
22.1 | 20.7 |
| Null | — | — | 5.3 | 4.8 |
Catalyst characterization
FT-IR spectra of various AIL catalysts were obtained using KBr wafer on a Bruker IFS-28 spectrometer in the range of 4000–400 cm−1. All thermogravimetric and differential thermogravimetric (TG-DTG) curves were recorded on a TG-209 (NETZSCH) thermal analyzer. Samples were heated from room temperature (RT; 298 K) to 873 K at a heating rate of 10 K min−1 under a dynamic N2 atmosphere. 1H and 13C NMR spectra were acquired on a Bruker AV-500 spectrometer.37,38 Solid-state 31P MAS NMR measurements were performed on a Bruker-Biospin Avance-III 500 spectrometer at a Larmor frequency of 202.46 MHz using a 4 mm double-resonance MAS probehead. 31P spectra were acquired using a single-pulse sequence under a sample spinning rate of 12 kHz using an excitation pulse of 1.5 μs (π/6 pulse) and a recycle delay of 10 s. The 31P chemical shifts were referenced to 85% H3PO4 aqueous solution.Detailed acid features, namely the type, relative concentration, and strength of Brønsted vs. Lewis acidity in the B–L AIL catalysts, were studied by 31P MAS NMR of adsorbed TMPO.34–36 Prior to the adsorption of TMPO probe molecules, each catalyst was first subjected to dehydration treatment at 393 K for 24 h under vacuum (<10−5 Torr). Subsequently, a known amount of TMPO dissolved in anhydrous CH2Cl2 was introduced into a vessel containing the dehydrated sample in a glove box under a N2 environment. The sealed sample vessel was then connected to a vacuum manifold, followed by removal of the CH2Cl2 solvent by evacuation at 323 K. Furthermore, the sample was subjected to thermal treatment at 393 K for at least 24 h to ensure a uniform adsorption of adsorbate probe molecules in the substrate. Finally, the TMPO-loaded sample was transferred into a MAS rotor in a N2 glove box and then sealed with a gas-tight Kel-F cap.
Esterification reaction
n-Caprylic acid (0.1 mol), a desirable amount of methanol, and a known amount of the AIL catalyst were placed into a three-necked flask equipped with a mechanical stirrer, a reflux condenser, and a drop funnel containing microporous 3 Å molecular sieves to absorb the water produced from esterification. The esterification of n-caprylic acid with methanol was first carried out at different temperatures for a desired period of time and then the reaction mixture was cooled to RT. Typically, owing to the self-separation characteristics of the reaction system, the reaction mixture tends to spontaneously separate into two layers. The upper layer was extracted by distillation to obtain the methyl caprylate product, and the lower layer consisting of the AIL catalyst was reused after further treatment. Quantitative analysis of the products was performed by gas chromatography using an Agilent 6890N GC apparatus equipped with a FID detector and an HP-5 capillary column. Reactants and products were identified by comparison with authentic samples using methyl laurate as an internal standard.The conversion of n-caprylic acid was calculated as follows:
| Conversion = (1 − a1/a2) × 100%, | (1) |
Design of experiments and response surface methodology
The response surface methodology (RSM)39,40 was employed to obtain the optimal experimental conditions for esterification of caprylic acid with methanol. A Box–Behnken design (BBD) was applied to study the effects of three process variables, namely amount of catalyst (x1), methanol to caprylic acid molar ratio (x2), and reaction time (x3), as specified in Table 2. A total of 17 experimental sets, which included 12 factorial points and 5 centring points, were adopted. The three experimental variables were designed at three levels coded with a plus sign (+1; high value), zero (0; central value), or a minus sign (−1; low value). The coded values of these factors were obtained using the equation: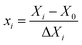 | (2) |
| Variable (unit) | Symbol | Range and level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| a Over the [HSO3-pmim]+(1/2Zn2+)SO42− catalyst at 363 K. | ||||
| Catalyst amount (wt%) | x 1 | 4.0 | 6.0 | 8.0 |
| Methanol/caprylic acid ratio (mol mol−1) | x 2 | 2.5 | 5.0 | 7.5 |
| Reaction time (h) | x 3 | 1.5 | 2.0 | 2.5 |
A model equation based on the quadratic polynomial given by RSM was used to reveal interactive effects between experimental variables, to optimize the reaction process, and to predict the yield of the product (i.e. methyl caprylate). The model equation may be expressed as:
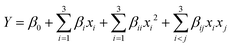 | (3) |
Kinetic study
The reaction rate (r) for esterification of n-caprylic acid to methyl caprylate may be expressed as: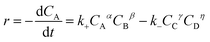 | (4) |
Given that water had been effectively removed by a microporous 3 Å zeolite adsorbent during the esterification reaction, the reaction could be considered as an irreversible process. In this context, the second term associated with the inverse process in eqn (4) may be ignored. Moreover, since the concentration of methanol is always much higher than that of n-caprylic acid, k+CBβ in eqn (4) could be deemed as constant, leading to a simplified rate equation:
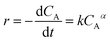 | (5) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r = ln r = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + α k + α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA CA | (6) |
The values of k and α at different temperatures may therefore be obtained easily by linear fitting of the lnr vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA curve using the Origin 8.5 software.
CA curve using the Origin 8.5 software.
It is well-known that the variation of reaction rate with temperature may be expressed by the Arrhenius equation as:
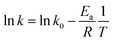 | (7) |
Thus, the activation energy (Ea) and the pre-exponential factor (k0) may be derived from the slope and the intercept of the Arrhenius (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T) plot.
k vs. 1/T) plot.
Results and discussion
Catalyst characterization
Similar 1H and 13C NMR spectra were observed for various B–L AIL catalysts. As an example, the NMR data obtained from the [HSO3-pmim]+(1/2Zn2+)SO42− (i.e. ILc) catalyst having equimolar amounts of Brønsted and Lewis acid sites are depicted below: 1H NMR (500 MHz, D2O): δ 2.31 (m, 2H), 2.91 (t, 2H), 3.89 (s, 3H), 4.36 (t, 2H), 7.44 (s, 1H), 7.51 (s, 1H), 8.74 (s, 1H) ppm; 13C NMR (500 MHz, D2O): δ 27.57, 38.20, 49.69, 50.21, 124.68, 126.26, 138.68 ppm.
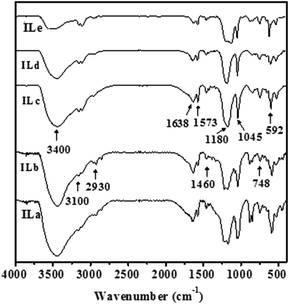
Fig. 1 displays the FT-IR spectra of various IL catalysts specified in Table 1. In spite of the marginal variations in peak intensities and peak positions, distinct characteristic vibrational bands responsible for Brønsted acidic IL [HSO3-pmim]+HSO4− were observed before (ILa) and after substitution of protons (H+) with Zn2+ cations. The absorption peaks at 1638 and 1573 cm−1 may be attributed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds on the imidazole ring, respectively. The peak at 2930 cm−1 could be associated with asymmetric stretching vibrations of –CH2 and the bonds at 1460 and 748 cm−1 may be assigned to bending and rocking vibrations of –CH3, respectively, whereas the strong absorption bonds at 1080 and 1045 cm−1 may be attributed to the asymmetric and symmetric stretching vibrations of S
N bonds on the imidazole ring, respectively. The peak at 2930 cm−1 could be associated with asymmetric stretching vibrations of –CH2 and the bonds at 1460 and 748 cm−1 may be assigned to bending and rocking vibrations of –CH3, respectively, whereas the strong absorption bonds at 1080 and 1045 cm−1 may be attributed to the asymmetric and symmetric stretching vibrations of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. Moreover, a notable decrease in peak intensity of the band responsible for –OH vibrations at 3400 cm−1 with increasing substitution of H+ with Zn2+ ions (i.e. increasing L/B ratio in Table 1) was observed, revealing the progressive incorporation of Lewis acidity into the pristine Brønsted acidic (ILa) catalyst.41,42
O, respectively. Moreover, a notable decrease in peak intensity of the band responsible for –OH vibrations at 3400 cm−1 with increasing substitution of H+ with Zn2+ ions (i.e. increasing L/B ratio in Table 1) was observed, revealing the progressive incorporation of Lewis acidity into the pristine Brønsted acidic (ILa) catalyst.41,42
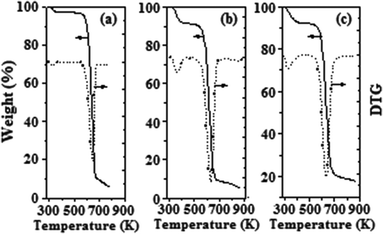
TGA-DTG analyses were performed to investigate the thermal stability of various AIL catalysts. Since similar TGA-DTG profiles were observed for most B–L AIL catalysts over the temperature range from RT to 873 K, only assorted TGA-DTG results obtained from the zwitterion 1-sulfonic acid propyl-3-methylimidazole (MIMPS), Brønsted acidic ILa ([HSO3-pmim]+HSO4−), and B–L AIL with L/B = 1.0 (i.e. ILc) samples are illustrated and discussed. The TGA curves observed for MIMPS and ILa showed similar weight-loss profiles at ca. 300–380 and 575–675 K. For the latter ILa ([HSO3-pmim]+HSO4−) catalyst, an initial weight loss occurred at 356 K (Fig. 2b), which may be attributed to desorption of physisorbed water, while the second weight-loss peak was observed at 625 K, corresponding to the decomposition of the organic MIMPS (Fig. 2a). By comparison, the TGA-DTG profile of [HSO3-pmim]+(1/2Zn2+)SO42− (i.e. ILc) shown in Fig. 2c revealed two major weight-loss peaks at 333 and 636 K. Thus, the acidic ILc catalyst appeared to be thermally more stable than ILa after incorporating Lewis acidity. It is noteworthy that these novel B–L AILs (such as [HSO3-pmim]+(1/2Zn2+)SO42−) retain their structural integrity over the range of reaction temperatures (323–363 K) examined.
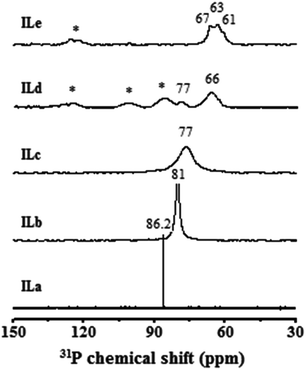
Solid-state 31P MAS NMR of adsorbed TMPO has been proven to be a feasible and powerful approach for the characterization of acid properties of solid acid catalysts.34–36,40,43 The base TMPO probe molecule tends to interact with the acidic proton to form the TMPOH+ complex. It has been demonstrated that a linear correlation between the observed 31P NMR chemical shift (δ31P) and acidic strength of Brønsted acidity may be inferred by means of this 31P-TMPO NMR approach.34–36 A higher observed δ31P value of the adsorbed TMPO therefore indicates stronger acidic strength of the catalyst, and vice versa. Fig. 3 shows 31P NMR spectra of TMPO adsorbed on various AILs. The 31P spectrum of ILa exhibited a sharp singlet at 86.2 ppm, indicating the sole presence of Brønsted acidity whose average strength is in the vicinity of the threshold for superacidity (86 ppm).34–36,40 It is indicative that the parent [HSO3-pmim]+HSO4− catalyst indeed possesses ultra-strong Brønsted acidity. Upon continuing the incorporation of Lewis metallic centers (Zn2+), the 31P resonances become broader due to a consistent increase in viscosity. This is also accompanied by a gradual decrease in δ31P. For example, a single broad resonance peak was observed for TMPO adsorbed on ILb and ILc, corresponding to a δ31P of 81 and 77 ppm, respectively. Clearly, Brønsted acidity became weaker as more H+ are being substituted by Zn2+ ions, creating Lewis acidity. On the other hand, multiple resonances were observed for ILd and ILe; among them, the peak in the vicinity of 63 ppm may be attributed to the presence of Lewis acidity.34,35,43 Thus, we assign the two peaks observed for ILd at 77 and 66 ppm to the TMPO adsorbed on Brønsted and Lewis acid sites, respectively. In this context, the broad multiple resonance around 63 ppm in ILe indicates that the AIL mainly manifests Lewis acidity. In terms of viscosity of these AILs, ILa, ILb, and ILc were found to exhibit a liquid phase with increasing viscosity and ILd appeared to be a mixture of liquid and solid, whereas ILe was a solid powder. These results are in line with the δ31P and corresponding linewidths of the adsorbed TMPO in various catalyst substrates.
Role of Brønsted and Lewis acidities on catalytic activity
To explore the effects and correlations of Lewis and Brønsted acid sites on catalytic performance during the esterification of n-caprylic acid with methanol, catalytic activities of various AIL catalysts were obtained, as summarized in Table 1. Accordingly, it is clear that Brønsted acid sites played a more primary role than Lewis acidity during catalytic esterification reaction. This may be seen from the notable decreases in conversion and product yield with increasing Lewis acidity. Nevertheless, the ILa catalyst, which solely possesses ultra-strong Brønsted acidity, showed inferior catalytic activity as compared to ILb and ILc. This reveals that ultra-strong acidity may be unfavorable for the esterification reaction, likely due to the occurrence of acid-catalysed reverse reactions. Interestingly, optimal conversion (94.8%) and methyl caprylate yield (94.3%) were obtained when equimolar Lewis and Brønsted acid sites were incorporated into the AIL (i.e. ILc). On the basis of these results, it is clear that a synergy of Lewis and Brønsted acid sites manifests catalytic activity during the formation of methyl caprylate over these novel AIL catalysts, particularly when equimolar amounts of both types of acidities were implemented. A possible catalytic mechanism is proposed in Scheme 1. In brief, the Lewis center (Zn2+) may incorporate with the oxygen atom in the carbonyl groups, which possess strong electronegativity, to induce interactions between the oxygen in the alcohol hydroxyl group and the carbon atom in the carbonyl group. On the other hand, coupling of Brønsted acidic H+ with the oxygen atom in the carbonyl group tends to lower the negative charge and hence is favorable for the release of Zn2+.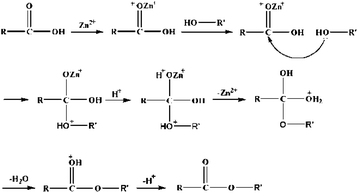 | ||
| Scheme 1 Possible mechanism invoked during the esterification of n-caprylic acid with methanol over the B–L AIL [HSO3-pmim]+(1/2Zn2+)SO42− catalyst. | ||
Effects of experimental variables on esterification
Since the [HSO3-pmim]+(1/2Zn2+)SO42− AIL catalyst having a Lewis/Brønsted molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
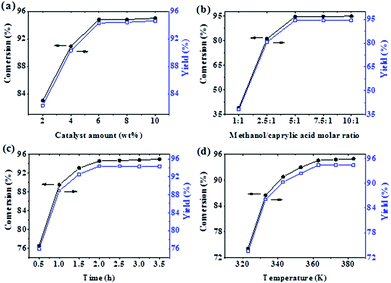
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the best catalytic performance, the ILc catalyst was chosen for further process optimization study. Four important experimental variables, namely the catalyst amount, methanol/n-caprylic acid reactant ratio, reaction time, and temperature, were investigated. Among them, the amount of the catalyst used plays a key role during esterification due to its high importance to the whole reaction process. Fig. 4a shows the effect of [HSO3-pmim]+(1/2Zn2+)SO42− loading on catalytic activity during the esterification of n-caprylic acid with methanol. It is noteworthy that while performing the experiments with varied catalyst loadings, other experimental parameters were kept constant: methanol/caprylic acid ratio = 5.0, reaction time = 3.0 h, and temperature = 363 K. Accordingly, a consistent increase in both conversion and methyl caprylate yield with increasing amount of catalyst dosage was observed, eventually reaching a respective plateau for conversion (94.8%) and product yield (94.3%) at a reaction time of 3 h and a catalyst loading of 6 wt%. This is likely due to the suitable amount of active moieties desirable for the reaction. However, as the catalyst amount exceeded 6 wt%, the conversion of n-caprylic acid and the yield of methyl caprylate remained practically unchanged. This may be attributed to the dissolution of the AIL catalyst in the presence of methanol, but the catalyst is practically unsolvable in methyl caprylate. As such, at a prolonged reaction time, the product ester became the primary component of the reaction mixture, leading to the limited solubility of the catalyst. Thus, in view of the observed high yield of methyl caprylate and the relevant cost effectiveness issue of the process, an optimal catalyst amount of ca. 6 wt% may be inferred.
1 exhibited the best catalytic performance, the ILc catalyst was chosen for further process optimization study. Four important experimental variables, namely the catalyst amount, methanol/n-caprylic acid reactant ratio, reaction time, and temperature, were investigated. Among them, the amount of the catalyst used plays a key role during esterification due to its high importance to the whole reaction process. Fig. 4a shows the effect of [HSO3-pmim]+(1/2Zn2+)SO42− loading on catalytic activity during the esterification of n-caprylic acid with methanol. It is noteworthy that while performing the experiments with varied catalyst loadings, other experimental parameters were kept constant: methanol/caprylic acid ratio = 5.0, reaction time = 3.0 h, and temperature = 363 K. Accordingly, a consistent increase in both conversion and methyl caprylate yield with increasing amount of catalyst dosage was observed, eventually reaching a respective plateau for conversion (94.8%) and product yield (94.3%) at a reaction time of 3 h and a catalyst loading of 6 wt%. This is likely due to the suitable amount of active moieties desirable for the reaction. However, as the catalyst amount exceeded 6 wt%, the conversion of n-caprylic acid and the yield of methyl caprylate remained practically unchanged. This may be attributed to the dissolution of the AIL catalyst in the presence of methanol, but the catalyst is practically unsolvable in methyl caprylate. As such, at a prolonged reaction time, the product ester became the primary component of the reaction mixture, leading to the limited solubility of the catalyst. Thus, in view of the observed high yield of methyl caprylate and the relevant cost effectiveness issue of the process, an optimal catalyst amount of ca. 6 wt% may be inferred.
Regarding the effect of feeds, Fig. 4b displays the variation of the methanol to n-caprylic acid molar ratio on esterification activity. It was found that, as the reactant ratio increased, the initial yield of ester also increased significantly and eventually leveled off at 94.3% when the ratio of methanol to caprylic acid reached 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Since esterification is a reversible reaction, an adequate amount of methanol is required to drive the equilibrium towards formation of methyl caprylate. Meanwhile, the AIL catalyst is highly soluble in methanol and thus resulted in a homogeneous distribution of the catalyst in the reaction mixture to provoke efficient esterification reaction. Nonetheless, as the ratio of methanol to caprylic acid exceeded 5
1. Since esterification is a reversible reaction, an adequate amount of methanol is required to drive the equilibrium towards formation of methyl caprylate. Meanwhile, the AIL catalyst is highly soluble in methanol and thus resulted in a homogeneous distribution of the catalyst in the reaction mixture to provoke efficient esterification reaction. Nonetheless, as the ratio of methanol to caprylic acid exceeded 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the excessive methanol no longer had a decisive impact on the conversion rate and product yield due to dilution of the catalyst in the reaction mixture.
1, the excessive methanol no longer had a decisive impact on the conversion rate and product yield due to dilution of the catalyst in the reaction mixture.
The influence of the reaction time and temperature on the esterification was also investigated, as shown in Fig. 4c and d. It is evident that the methyl caprylate yield reached ca. 88.9% rapidly during the initial stage (1.0 h) and then increased gradually till reaching a maximum of 94.4% at 2.0 h. A marginal decrease in product yield beyond the reaction time of 2.0 h was observed, most likely due to partial hydrolyzation of methyl caprylate. As for the effect of reaction temperature, the yield of methyl caprylate reached a maximum when the temperature reached 363 K at 2.0 h. However, while water removal by methanol evaporation may be more efficient at higher temperatures, no further increase in n-caprylic acid conversion and product yield was observed above 363 K, likely due to the inevitable loss of methanol above its boiling point.
Process and product optimization
The factorial experimental design was applied to minimize the waste of catalyst and to reduce the number of cumbersome experiments without significant loss of information. The design of experiments, experimental results, and predicted responses for the esterification of n-caprylic acid with methanol over the [HSO3-pmim]+(1/2Zn2+)SO42− catalyst are summarized in Table 3. No significant difference between experimental results and predicted data can be found. The methyl caprylate yield (Y) may be correlated with independent experimental variables by a quadratic model, which can be expressed as:| Y = +94.37 + 2.78x1 + 8.80x2 + 3.17x3 − 2.97x12 − 8.23x22 − 1.88x32 − 0.67x1x2 − 1.37x1x3 − 1.69x2x3 | (8) |
| Entry | Variable and levela | Methyl caprylate yield (%) | |||
|---|---|---|---|---|---|
| x 1 | x 2 | x 3 | Experimental | Predicted | |
| a Variables and levels specified in Table 2. | |||||
| 1 | −1 | −1 | 0 | 71.01 | 70.93 |
| 2 | 1 | −1 | 0 | 77.18 | 77.82 |
| 3 | −1 | 1 | 0 | 90.51 | 89.87 |
| 4 | 1 | 1 | 0 | 94.00 | 94.08 |
| 5 | −1 | 0 | −1 | 81.92 | 82.21 |
| 6 | 1 | 0 | −1 | 90.93 | 90.50 |
| 7 | −1 | 0 | 1 | 90.85 | 91.28 |
| 8 | 1 | 0 | 1 | 94.40 | 94.11 |
| 9 | 0 | −1 | −1 | 70.82 | 70.61 |
| 10 | 0 | 1 | −1 | 91.24 | 91.59 |
| 11 | 0 | −1 | 1 | 80.68 | 80.33 |
| 12 | 0 | 1 | 1 | 94.33 | 94.54 |
| 13 | 0 | 0 | 0 | 94.97 | 94.37 |
| 14 | 0 | 0 | 0 | 93.93 | 94.37 |
| 15 | 0 | 0 | 0 | 94.23 | 94.37 |
| 16 | 0 | 0 | 0 | 93.82 | 94.37 |
| 17 | 0 | 0 | 0 | 94.89 | 94.37 |
The fitting quality of the quadratic model (eqn (8)) was verified by the standard analysis of variance (ANOVA) presented in Table 4. The model F-value (309.76) was much greater than the tabular counterpart (F0.01, 9, 7 = 6.71), implying that the model was indeed significant. In addition, the obtained p-value (<0.0001) revealed that the chance (0.01%) that such a large ‘model F-value’ could occur was close to noise level. The observed “Lack of Fit F-value” of 2.00 also implied that the Lack of Fit was not significant relative to pure error. A coefficient of determination (R2 = 0.9975) was attained, indicating that the model was reliable in predicting the response. The “Adeq Precision” (48.755), which measured the signal to noise ratio, was also much greater than the desirable value of 4, as expected. Moreover, the coefficient of variation (C.V. = 0.73%) also demonstrated that the experiments were carried out reliably. On the basis of these statistical values, it is conclusive that the quadratic model was adequate for predicting a reliable methyl caprylate yield within the range of the variables studied. This is supported by the observed F- and p-values, which revealed that all three independent variables were highly significant and that the order of their significance was the methanol/caprylic acid molar ratio > reaction time > amount of catalyst to n-caprylic acid. Moreover, mutual interactions between each pair of these independent variables were also highly significant to the esterification reaction.
| Source | Sum of squares | Degree of freedom | Mean square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| **Represents highly significant. | ||||||
| Model | 1142.19 | 9 | 126.91 | 309.76 | <0.0001 | ** |
| x 1 | 61.72 | 1 | 61.72 | 150.63 | <0.0001 | ** |
| x 2 | 619.34 | 1 | 619.34 | 1511.67 | <0.0001 | ** |
| x 3 | 80.33 | 1 | 80.33 | 196.06 | <0.0001 | ** |
| x 1 2 | 37.08 | 1 | 37.08 | 90.51 | <0.0001 | ** |
| x 2 2 | 284.86 | 1 | 284.86 | 695.28 | <0.0001 | ** |
| x 3 2 | 14.81 | 1 | 14.81 | 36.14 | 0.0005 | ** |
| x 1 x 2 | 1.80 | 1 | 1.80 | 4.38 | 0.0746 | |
| x 1 x 3 | 7.45 | 1 | 7.45 | 18.19 | 0.0037 | ** |
| x 2 x 3 | 11.46 | 1 | 11.46 | 27.97 | 0.0011 | ** |
| Residual | 2.87 | 7 | 0.41 | |||
| Lack of fit | 1.72 | 3 | 0.57 | 2.00 | 0.2559 | |
| Error | 1.15 | 4 | 0.29 | |||
| Cor. total | 1145.06 | 16 | ||||
The three dimensional (3-D) response surface and contour plots obtained from the predicted model are shown in Fig. 5. The correlations between the methanol/n-caprylic acid molar ratio and the catalyst amount at a fixed reaction temperature and time are shown in Fig. 5a and d. It is clear that the yield of methyl caprylate rapidly increased to the optimal value with increasing methanol/acid molar ratio; however, not much improvement in the response for the latter over 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed. Meanwhile, the yield improved gradually as the amount of the catalyst increased but leveled off when the amount exceeded ca. 6 wt%. It is indicative that the methanol/acid molar ratio is a more important variable for the esterification reaction than the catalyst amount; the notion is consistent with the ANOVA result (Table 4). Fig. 5b shows the correlations of the catalyst amount and reaction time with methyl caprylate yield. The yield was found to increase with increasing reaction time, reaching a maximum (94.4%) at 2.0 h and then decreased slightly thereafter. On the other hand, the yield only changed slightly with the catalyst amount. Nonetheless, the contour plot in Fig. 5e revealed that the interaction between the catalyst amount and the reaction time played an important role in esterification, as also indicated by the results shown in Table 4. Fig. 5c and f illustrate the interaction between the reaction time and the methanol/caprylic acid molar ratio with respect to the product yield. The yield increased significantly before the methanol/acid molar ratio reached 5
1 was observed. Meanwhile, the yield improved gradually as the amount of the catalyst increased but leveled off when the amount exceeded ca. 6 wt%. It is indicative that the methanol/acid molar ratio is a more important variable for the esterification reaction than the catalyst amount; the notion is consistent with the ANOVA result (Table 4). Fig. 5b shows the correlations of the catalyst amount and reaction time with methyl caprylate yield. The yield was found to increase with increasing reaction time, reaching a maximum (94.4%) at 2.0 h and then decreased slightly thereafter. On the other hand, the yield only changed slightly with the catalyst amount. Nonetheless, the contour plot in Fig. 5e revealed that the interaction between the catalyst amount and the reaction time played an important role in esterification, as also indicated by the results shown in Table 4. Fig. 5c and f illustrate the interaction between the reaction time and the methanol/caprylic acid molar ratio with respect to the product yield. The yield increased significantly before the methanol/acid molar ratio reached 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but remained practically unchanged beyond this ratio. A slight reduction in ester yield was observed at prolonged reaction time (>2 h). Again, this is attributed to partial hydrolysis of the methyl caprylate product when the reaction time exceeded its maximum value (2 h). Since esterification is a reversible reaction, the hydrolysis rate of ester would increase with reaction time while the yield of methyl caprylate decreased. The effect of interaction of these two variables was also significant, revealing an ellipse mound shape (Fig. 5e), in good agreement with the ANOVA result (Table 4).
1, but remained practically unchanged beyond this ratio. A slight reduction in ester yield was observed at prolonged reaction time (>2 h). Again, this is attributed to partial hydrolysis of the methyl caprylate product when the reaction time exceeded its maximum value (2 h). Since esterification is a reversible reaction, the hydrolysis rate of ester would increase with reaction time while the yield of methyl caprylate decreased. The effect of interaction of these two variables was also significant, revealing an ellipse mound shape (Fig. 5e), in good agreement with the ANOVA result (Table 4).
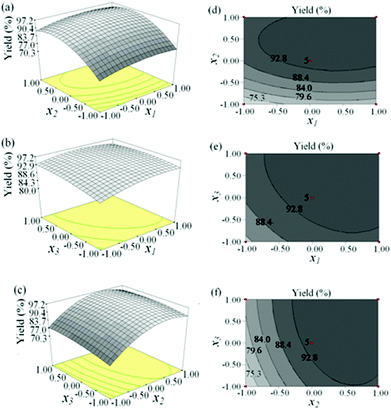 | ||
| Fig. 5 (a–c) 3D response surface and (d–f) contour plots showing variations between a pair of experimental variables (Table 1) on predicted values of methyl caprylate yield while keeping other variables at a constant level of 0: (a) and (d) methanol/n-caprylic acid molar ratio vs. catalyst amount; (b) and (e) reaction time vs. catalyst amount; (c) and (f) reaction time vs. methanol/n-caprylic acid molar ratio. | ||
Optimized responses and model verification
Based on RSM results, the mathematical model predicted an optimal methyl caprylate yield of 96.16% for the esterification of n-caprylic acid with methanol over the [HSO3-pmim]+(1/2Zn2+)SO42− AIL catalyst under the following process conditions: x1 = 6.59 wt% (catalyst amount), x2 = 6.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (methanol/n-caprylic acid molar ratio), and x3 = 2.26 h (reaction time) at a reaction temperature of 363 K. To confirm the validity of the model and optimal process conditions, three additional experiments were conducted in parallel at 363 K with x1 = 7 wt%, x2 = 6
1 (methanol/n-caprylic acid molar ratio), and x3 = 2.26 h (reaction time) at a reaction temperature of 363 K. To confirm the validity of the model and optimal process conditions, three additional experiments were conducted in parallel at 363 K with x1 = 7 wt%, x2 = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, x3 = 2.0 h. Accordingly, methyl caprylate yields of 95.1, 95.5, and 95.7% were obtained, leading to an average experimental yield of 95.4%, in good agreement with the predicted value. Thus, it is conclusive that the model was reliable and that the regression equation could truly reflect the influence of the three variables on the yield of esterification.
1, x3 = 2.0 h. Accordingly, methyl caprylate yields of 95.1, 95.5, and 95.7% were obtained, leading to an average experimental yield of 95.4%, in good agreement with the predicted value. Thus, it is conclusive that the model was reliable and that the regression equation could truly reflect the influence of the three variables on the yield of esterification.
Catalyst recycling
To evaluate the reusability of such a B–L AIL catalyst, cyclic experiments were conducted under the same optimal operating conditions obtained above over the [HSO3-pmim]+(1/2Zn2+)SO42−. For each cycle, the lower layer of the reaction mixture was separated from the system after the esterification reaction, extracted with diethyl ether, and vacuum dried for 5 h at 393 K. The recovered [HSO3-pmim]+(1/2Zn2+)SO42− catalyst was assessed by 1H NMR spectroscopy to ensure that no traces of reactants or products were retained in the catalyst. It was found that the recovered AIL catalyst could be reused for at least five consecutive runs without significant loss in catalytic activity, as summarized in Table 5. The gradual decrease in product yield observed after repeated cycles is ascribed to leaching of Zn. Additional elemental analysis by inductively coupled plasma (ICP) revealed that the concentration of Zn in the spent catalyst decreased from 9.71 wt% (fresh catalyst) to 9.45 wt% after the fifth run.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, x3 = 2 h, and T = 363 K)
1, x3 = 2 h, and T = 363 K)
| Recycle time | Conversion (%) | Methyl caprylate yield (%) |
|---|---|---|
| 1 | 95.9 | 95.4 |
| 2 | 95.6 | 95.1 |
| 3 | 95.2 | 94.6 |
| 4 | 93.8 | 93.2 |
| 5 | 93.0 | 92.6 |
Kinetic model
To establish the kinetic model for the esterification of n-caprylic acid to methyl caprylate over the [HSO3-pmim]+(1/2Zn2+)SO42− B–L AIL catalyst, additional experiments were conducted with x1 = 7 wt%, x2 = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, x3 = 2.0 h at different temperatures. During the reaction, ca. 1 mL sample was withdrawn from the mixture for analysis at different time intervals.44–46 The variations of the instantaneous concentration of n-caprylic acid (CA) versus reaction time at five different temperatures (323, 333, 343, 353, and 363 K) are shown in Fig. 6. Accordingly, the instant reaction rate (r) may be estimated by performing differentiation of the decay curve using a software package (Origin 8.5) to employ in the subsequent linear curve fitting to obtain values of k and α in eqn (6).
1, x3 = 2.0 h at different temperatures. During the reaction, ca. 1 mL sample was withdrawn from the mixture for analysis at different time intervals.44–46 The variations of the instantaneous concentration of n-caprylic acid (CA) versus reaction time at five different temperatures (323, 333, 343, 353, and 363 K) are shown in Fig. 6. Accordingly, the instant reaction rate (r) may be estimated by performing differentiation of the decay curve using a software package (Origin 8.5) to employ in the subsequent linear curve fitting to obtain values of k and α in eqn (6).
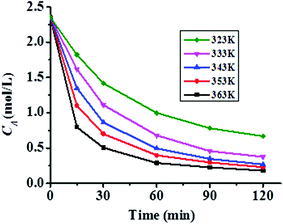 | ||
| Fig. 6 Variations of the instantaneous concentration of n-caprylic acid (CA) with reaction time at different temperatures. | ||
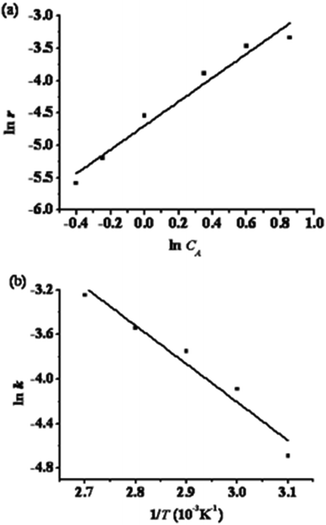
Taking the experiment conducted at 323 K as an example, the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r versus ln
r versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA fitted eqn (6) well with a correlation coefficient (R) of 0.9841, as shown in Fig. 7a. Likewise, satisfactory fits were achieved for plots obtained at other temperatures. Accordingly, the k and α values so deduced from various temperatures are summarized in Table 6, from which an average reaction order α of 1.70 was derived for the esterification reaction. Based on eqn (7), activation energy Ea = 33.66 kJ mol−1 and a pre-exponential factor k0 = 2.86 × 103 may also be deduced from the slope and intercept of the Arrhenius plot shown in Fig. 7b. Therefore, the kinetic equation (eqn (5)) for the esterification of n-caprylic acid to methyl caprylate under the aforementioned optimum conditions may be expressed as:
CA fitted eqn (6) well with a correlation coefficient (R) of 0.9841, as shown in Fig. 7a. Likewise, satisfactory fits were achieved for plots obtained at other temperatures. Accordingly, the k and α values so deduced from various temperatures are summarized in Table 6, from which an average reaction order α of 1.70 was derived for the esterification reaction. Based on eqn (7), activation energy Ea = 33.66 kJ mol−1 and a pre-exponential factor k0 = 2.86 × 103 may also be deduced from the slope and intercept of the Arrhenius plot shown in Fig. 7b. Therefore, the kinetic equation (eqn (5)) for the esterification of n-caprylic acid to methyl caprylate under the aforementioned optimum conditions may be expressed as:
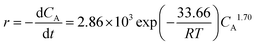 | (9) |
| Temperature (K) | k (mol−0.7 L0.7 min−1) | α |
|---|---|---|
| 323 | 0.92 × 10−2 | 1.8470 |
| 333 | 1.68 × 10−2 | 1.5916 |
| 343 | 2.35 × 10−2 | 1.5907 |
| 353 | 2.90 × 10−2 | 1.6909 |
| 363 | 3.88 × 10−2 | 1.7795 |
The activation energy (Ea) observed herein for the esterification of n-caprylic acid with methanol over the [HSO3-pmim]+(1/2Zn2+)SO42− AIL catalyst is lower than those obtained for the esterification of palmitic acid with methanol over TPA/SnO2 (36.33 kJ mol−1)47 and esterification of acetic acid with methanol over ResinTech SACMP-H (48.2 kJ mol−1).48 This indicates that the [HSO3-pmim]+(1/2Zn2+)SO42− catalyst is indeed a highly effective catalyst for the esterification of n-caprylic acid to methyl caprylate.
Conclusions
A series of new halogen-free Brønsted–Lewis acidic ionic liquids were synthesized and exploited to catalyse the esterification of n-caprylic acid with methanol. The [HSO3-pmim]+(1/2Zn2+)SO42− catalyst, which exhibited the highest catalytic activity, was employed for process optimization to obtain optimal esterification conditions at a reaction temperature of 363 K: a catalyst amount of 7 wt%, a methanol/n-caprylic acid molar ratio of 6, and a reaction time of 2.0 h, leading to a maximum methyl caprylate yield of 95.4%. These optimized experimental values are in good agreement with those predicted by the response surface methodology based on the Box–Behnken design model. A reaction order of 1.7 and an activation energy of 33.66 kJ mol−1 was deduced from the kinetic model developed. In addition to superior catalytic activity, the Brønsted–Lewis acidic ionic liquid catalysts reported herein also have the advantages of facile recovery and recycle use without significant loss in catalytic activity. Our results indicate that the novel eco-friendly [HSO3-pmim]+(1/2Zn2+)SO42− catalyst is a promising substitute for conventional catalysts and is of great value in chemical industry.Acknowledgements
The support of this work by the National Natural Science Foundation of China (no. 21177110), the National Natural Science Foundation of Zhejiang Province, China (no. LY13B070005), and the Ministry of Science and Technology, Taiwan (NSC 101-2113-M-001-020-MY3) is gratefully acknowledged.References
-
J. Otera, Esterification: Methods, Reactions, and Applications, Wiley-VCH, Weinheim, 2003 Search PubMed
.
- K. Saravanan, B. Tyagi and H. C. Bajaj, J. Sol-Gel Sci. Technol., 2012, 62, 13–17 CrossRef CAS
.
- T. Parangi, B. Wani and U. Chudasama, Appl. Catal., A, 2013, 467, 430–438 CrossRef CAS PubMed
.
- K. Mantri, R. Nakamura, Y. Miyata, K. Komura and Y. Sugi, Mater. Sci. Forum, 2007, 539–543, 2317–2322 CrossRef CAS
.
- K. Mantri, K. Komura and Y. Sugi, Green Chem., 2005, 7, 677–682 RSC
.
- Z. Zhao, Z. Li, G. Wang, W. Qiao and L. Cheng, Prog. Chem., 2004, 16, 620–630 CAS
.
- S. R. Kirumakki, N. Nagaraju and K. V. R. Chary, Appl. Catal., A, 2006, 299, 185–192 CrossRef CAS PubMed
.
- K. H. Chung and B. G. Park, J. Ind. Eng. Chem., 2009, 15, 388–392 CrossRef CAS PubMed
.
- N. Gokulakrishnan, A. Pandurangan and P. K. Sinha, J. Mol. Catal. A: Chem., 2007, 263, 55–61 CrossRef CAS PubMed
.
- D. Patel and B. Saha, Ind. Eng. Chem. Res., 2012, 51, 11965–11974 CrossRef CAS
.
- D. P. C. de Barros, P. Fernanders, J. M. S. Cabral and L. P. Fonseca, Catal. Today, 2011, 173, 95–102 CrossRef CAS PubMed
.
- E. H. Ahmed, T. Raghavendra and D. Madamwar, Bioresour. Technol., 2010, 101, 3628–3634 CrossRef CAS PubMed
.
- W. Huang, Y. M. Xia, H. Gao, Y. J. Fang, Y. Wang and Y. Fang, J. Mol. Catal. B: Enzym., 2005, 35, 113–116 CrossRef CAS PubMed
.
- B. Major, I. Kelemen-Horváth, Z. Csanádi, K. Bélafi-Bakó and L. Gubicza, Green Chem., 2009, 11, 614–616 RSC
.
- J. M. Marchetti and A. F. Errazu, Fuel, 2008, 87, 3477–3480 CrossRef CAS PubMed
.
- R. Hagiwara and Y. Ito, J. Fluorine Chem., 2000, 105, 221–227 CrossRef CAS
.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed
.
- R. Juárez, R. Martín, M. Álvaro and H. García, Appl. Catal., A, 2009, 369, 133–137 CrossRef PubMed
.
- H. Shi, W. Zhu, H. Li, H. Liu, M. Zhang, Y. Yan and Z. Wang, Catal. Commun., 2010, 11, 588–591 CrossRef CAS PubMed
.
- H. Du, Y. He, Z. Tan and X. Han, Asian J. Chem., 2013, 25, 7675–7678 CrossRef
.
- H. Zhu, F. Yang, J. Tang and M. He, Green Chem., 2003, 5, 38–39 RSC
.
- C. Imrie, E. R. T. Elago, C. W. McCleland and N. Williams, Green Chem., 2002, 4, 159–160 RSC
.
- C. Zhang, X. Y. Pan, M. J. Yu, L. Jin and G. Wu, Chem. Eng. J., 2012, 209, 464–468 CrossRef CAS PubMed
.
- C. Chiappe, S. Rajamania and F. D'Andreab, Green Chem., 2013, 15, 137–143 RSC
.
- K. Matuszek, A. Chrobok, F. Coleman, K. R. Seddon and M. Swadźba-Kwaśny, Green Chem., 2014, 16, 3463–3471 RSC
.
- H. Wang, C. Wu, X. Bu, W. Tang, L. Li and T. Qiu, Chem. Eng. J., 2014, 246, 366–372 CrossRef CAS PubMed
.
- F. Shirini, A. Yahyazadeh, K. Mohammadi and N. G. Khaligh, C. R. Chim., 2014, 17, 370–376 CrossRef CAS PubMed
.
- B. Aghabarari, N. Dorostkar, M. Ghiaci, S. G. Amini, E. Rahimi and M. V. Martinez-Huerta, J. Taiwan Inst. Chem. Eng., 2014, 45, 431–435 CrossRef CAS PubMed
.
- Y. Yang, W. He, C. Jia, Y. Ma, X. Zhang and B. Feng, J. Mol. Catal. A: Chem., 2012, 357, 39–43 CrossRef CAS PubMed
.
- D. Zhao, J. Ge, J. Zhai, J. Zhang, M. Liu and J. Li, J. Chem. Ind. Eng., 2014, 2561–2569 Search PubMed
.
- S. Liu, C. Xie, S. Yu, M. Xian and F. Liu, Chin. J. Catal., 2009, 30, 401–406 CrossRef CAS
.
- C. Chiappe and S. Rajamani, Eur. J. Org. Chem., 2011, 5517–5539 CrossRef CAS
.
- X. Liang and C. Qi, Catal. Commun., 2011, 12, 808–812 CrossRef CAS PubMed
.
- A. Zheng, F. Deng and S. B. Liu, Ann. Rep. NMR Spectrosc., 2014, 81, 47–108 CrossRef CAS
.
- A. Zheng, S. J. Huang, S. B. Liu and F. Deng, Phys. Chem. Chem. Phys., 2011, 13, 14889–14901 RSC
.
- A. Zheng, S. B. Liu and F. Deng, Solid State Nucl. Magn. Reson., 2013, 55–56, 12–27 CrossRef CAS PubMed
.
- X. Han and L. Zhou, Chem. Eng. J., 2011, 172, 459–466 CrossRef CAS PubMed
.
- X. Wu, X. Han, L. Zhou and A. Li, Indian J. Chem., 2012, 51A, 791–799 CAS
.
- A. H. M. Fauzi and N. A. S. Amin, Energy Convers. Manage., 2013, 76, 818–827 CrossRef PubMed
.
- X. Han, Y. He, C. T. Hung, L. L. Liu, S. J. Huang and S. B. Liu, Chem. Eng. Sci., 2013, 104, 64–72 CrossRef CAS PubMed
.
- Y. Leng, P. Jiang and J. Wang, Catal. Commun., 2012, 25, 41–44 CrossRef CAS PubMed
.
- X. Tong, N. Tian, W. Zhu, Q. Wu, F. Cao and W. Yan, J. Alloys Compd., 2012, 544, 37–41 CrossRef CAS PubMed
.
- Y. Chu, Z. Yu, A. Zheng, H. Fang, H. Zhang, S. J. Huang, S. B. Liu and F. Deng, J. Phys. Chem. C, 2011, 115, 7660–7667 CAS
.
- T. Garcia, A. Coteron, M. Martinez and J. Aracil, Chem. Eng. Sci., 2000, 55, 1411–1423 CrossRef CAS
.
- D. J. Tao, Y. T. Wu, Z. Zhou, J. Geng, X. B. Hu and Z. B. Zhang, Ind. Eng. Chem. Res., 2011, 50, 1989–1996 CrossRef CAS
.
- A. Talebian-Kiakalaieh, N. A. S. Amin, A. Zarei and I. Noshadi, Appl. Energy, 2013, 102, 283–292 CrossRef CAS PubMed
.
- K. Srilatha, C. R. Kumar, B. L. A. P. Devi, R. B. N. Prasad, P. S. S. Prasad and N. Lingaiah, Catal. Sci. Technol., 2011, 1, 662–668 CAS
.
- E. Sert and F. S. Atalay, Prog. React. Kinet. Mech., 2011, 36, 239–251 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |