Increasing the dyeability of polyester fabrics by photochemical treatment at room-temperature using H2O2 in air†
Akihiko
Ouchi
*,
Chuanxiang
Liu
and
Masao
Kunioka
National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Ibaraki 305-8565, Japan. E-mail: ouchi.akihiko@aist.go.jp
First published on 16th September 2014
Abstract
Poly(ethylene terephthalate) (PET) fabrics are currently dyed using disperse dyes at high temperatures and pressures because of their low dyeability, which implies the use of a large amount of energy and pressurized vessels that are unsuitable for safe operation. Simple irradiation with a commercially available germicidal lamp was applied to the PET fabrics using aqueous H2O2 and H2O2 + acrylic acid solutions at room temperature under air. A more than seven-fold increase in dyeability with cationic dyes was obtained at atmospheric pressure at reduced temperatures. For the first time, the increase in dyeability was observed only with H2O2 by a reaction with O2 in air. The increase in dyeability was attributed to the introduction of carboxylic acid groups on the fabric surface, which was confirmed by IR and UV spectroscopy and chemical reactions.
1. Introduction
Poly(ethylene terephthalate) (PET) fabrics have been widely used in the textile industry because of their excellent mechanical and physical properties. However, a significant disadvantage of PET fabrics is their low dyeability towards various dyes because of their high crystallinity and low polar and reactive group content. To obtain sufficient dyeability, disperse dyes are used currently for dyeing PET fabrics. General dyeing conditions used for disperse dyes require high temperatures and pressures, which implies the use of large amounts of energy and pressurized vessels that are unsuitable for safe operation and efficient production. In addition, it is difficult to obtain deep and clear shades with disperse dyes because of their small molar absorption coefficients and high reflection from the fabric surface. Therefore, a demand exists for increasing the dyeability of PET fabrics using dyes with large molar absorption coefficients and atmospheric pressure dying conditions.To increase the dyeability of PET fabrics, physical and chemical techniques have been tested to introduce various functional groups onto the fabric surface to increase their affinity with dyes. In terms of physical techniques, plasma, corona discharge, high energy irradiation, and laser irradiation1 have been studied but they generally require expensive equipment and high operating costs. Chemical methods, such as peroxide2 and redox3 initiators, have been tested for the grafting of olefinic monomers but they require long swelling times prior to thermal surface modification, which implies long processing times and is often accompanied by unfavorable damage to the fabrics.
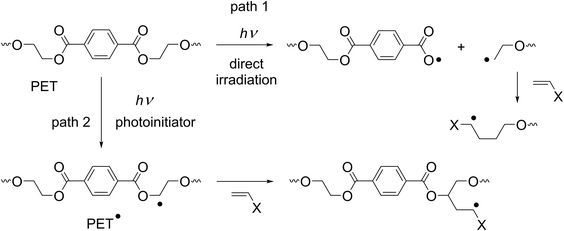
Photochemical methods have also been studied to increase the dyeability of PET fabrics. The direct irradiation of PET fabrics has been studied using lasers and excimer lamps. Chemical structural changes occur with intense laser light by a physical phenomenon, ablation,1 which results in some increase in dyeability4 but with considerable damage to the surface with significant morphological changes. To avoid such physical damage, lasers must be used with intensities below the ablation threshold. The direct irradiation of PET fabrics using a Xe2 (172 nm) excimer lamp has also been conducted in air but resulted only in a small increase in dyeability.5 Although the actual mechanism for direct irradiation is unclear, a photochemical cleavage of PET polymer chains by light absorption at the terephthalate moiety is proposed (Scheme 1, path 1).
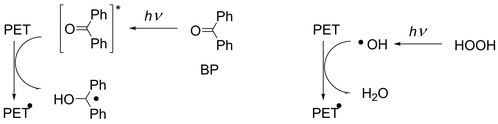
A more frequently studied photochemical method is the utilization of photoreactive compounds. Ozone was used as a photoreactive compound and caused nanoscale surface roughness on PET fabrics by UV irradiation but showed no improvement in the dyeability with disperse dyes.6 Photoinitiators are the most commonly used photoreactive compounds. Biacetyl has been used for the grafting of acrylic acid (AA) under nitrogen in the vapor phase, which resulted in an increase in the dyeability with basic and acidic dyes,7 but the most widely used photoinitiator is benzophenone (BP).8–11 Photochemically excited BP is known to abstract H atoms from aliphatic moieties of PET fabrics and generate carbon radicals on the fabric surface (Scheme 2, left). The resulting carbon radicals were reacted with various olefins, mostly those with electron-withdrawing groups, to induce grafting on the fabric surface. However, BP is insoluble in aqueous solutions, so vapor phase8 or organic solvents have been used for studies using BP. Organic solvents were used to prepare solutions for soaking fabrics,9–11 under inert gases8,9,11 or in air10 (Scheme 1, path 2). An increase in the dyeability of the grafted PET fabrics has been reported with direct,9 acid,11 and cationic10 dyes and crystal violet.8,9
However, the utilization of vapor phase or organic solutions is not practical for the textile industry because solvents used in the textile industry are generally limited to water. Therefore, the development of simple photochemical processes that can be used in aqueous environments, without requiring excessive energy and chemicals, is necessary for practical industrial use.
In this paper, we report on an efficient photochemical process for increasing the dyeability of PET fabrics with cationic dyes. This process involves simple room-temperature irradiation of PET fabrics soaked in aqueous solutions using a commercially available germicidal lamp (low-pressure mercury lamp without 185 nm emission) in air. Two techniques have been developed: (1) the grafting of AA using H2O2 instead of BP, and (2) the generation of carboxylic acid groups on the surface using only H2O2. In both techniques, instead of excited BP, carbon radicals are generated by H abstraction of photochemically generated OH radicals12 (Scheme 2, right) and the generated carbon radicals are used for grafting AA or are reacted with O2 to introduce carboxylic acid groups on the surface. The same dyeability with cationic dyes was obtained with techniques (1) and (2) but the latter has the advantage of requiring less chemicals.
An advantage of our method is that sufficient dyeing of PET fabrics can be accomplished with a reduced temperature without using high pressure, which indicates the use of less energy and safer processing conditions compared with those of conventional processes. Another advantage is that photochemical surface modification of the fabric for increasing their dyeability can be conducted using only H2O2 in contrast to conventional methods using organic solvents and initiators, and various olefins. This fact indicates that the process can be operated using only water as a solvent and require less and safer chemicals than usual processes.
2. Results and discussion
2.1. Photochemical treatment of PET fabrics
Photochemical treatment was conducted by 10 min of irradiation with a germicidal lamp (major emission: 254 nm13) in air of the fabric that had been soaked in various aqueous solutions. Three different solutions were tested, namely H2O (condition I), 0.5 M H2O2 (condition II), and 0.5 M AA + 0.5 M H2O2 (condition III). The irradiated fabrics were then dyed with a cationic dye (Cathilon Blue CD-FBLH). The dyeability was assessed by the K/S value,11,14 which is widely used in textile industries for the formulation of colors. It is defined as K/S = (1 − Rλ)2/2Rλ (Kubelka–Munk equation), where Rλ is the reflectance at the absorption maximum of the cationic dye.13 The K/S value is briefly proportional to the concentration of dye molecules, i.e. K/S = kC, where k is a constant and C is the concentration of the dye. The results are summarized in Table 1. The difference in fabric mass after photochemical treatment (ΔW), the tensile strength, and the whiteness of each fabric are also listed in Table 1.| Condition | ΔW (%) | Tensile strength (N) | Whiteness | K/S |
|---|---|---|---|---|
| a The difference in mass before and after photochemical treatment (before dying). b K/S values were calculated from the reflectance at λmax = 650 nm. c Irradiation conditions: germicidal lamp (1.07–1.08 mW cm−2), 10 min, in air. Reagents: H2O (condition I), 0.5 M H2O2 (condition II), 0.5 M AA + 0.5 M H2O2 (condition III). Dye: Cathilon Blue CD-FBLH. The average of 3–4 independent runs. The numbers in brackets are those measured on the reverse side. | ||||
| Original | — | 386 | 49 (49) | 0.86 (0.88) |
| I | −0.2 | 374 | 49 (49) | 0.96 (0.89) |
| II | 0 | 381 | 46 (48) | 2.12 (1.06) |
| III | −0.2 | 374 | 46 (47) | 2.02 (1.10) |
The appearance of the photochemically treated PET fabrics for all three conditions was the same as that before irradiation. The micrographs13 and whiteness measurements showed no change after photochemical treatment for all three conditions. In terms of fabric damage, tensile strength results indicate that no weakening of the fabrics occurred after three photochemical treatments.
Although no apparent change was observed in the fabrics after photochemical treatment, a considerable increase in the dyeability was observed with H2O2 (condition II) and AA + H2O2 (condition III), but only a small increase resulted with H2O (condition I). The increase in dyeability with H2O2 (condition II) was unexpected because the introduction of carboxylic acid groups by the grafting mechanism shown in Scheme 1 (path 2) does not proceed without AA. This increase was explained by the reaction of the carbon radicals with O2 (cf. section 2.4) and not by the direct irradiation of the PET fabrics (Scheme 1, path 1) because the increase of the dyeability with H2O (condition I) was small. The PET fabric treated with AA + H2O2 (condition III) showed no increase in mass (ΔW), which indicates that the degree of grafting of AA was small in air. This is in agreement with the result that the yield of the addition of the carbon radical to the olefins decreased with an increase in O2 concentration.12 The increase in dyeability was limited on the irradiated side and the increase on the reverse side was small. This is explained by the absorption of light by the PET fabric itself, which prevented light penetration to the reverse side and thus caused only a small change in the fabric surface.
2.2. Factors controlling the dyeability of PET fabrics using mixed aqueous solutions of H2O2 and acrylic acid (AA)
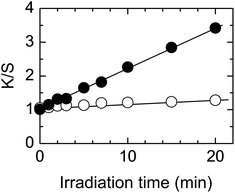
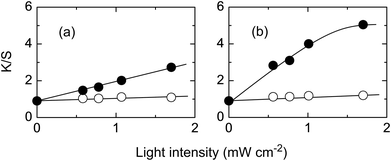
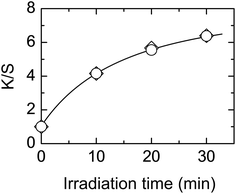
Fig. 1 shows the effect of irradiation time. The K/S values increase linearly with irradiation time indicating that the dyeability increases with irradiation time. As expected, only a small increase was observed on the reverse side.The effect of light intensity was also studied with results shown in Fig. 2. For a low H2O2 concentration, the K/S value increased linearly with light intensity (Fig. 2a). When the H2O2 concentration was increased eight-fold, the increase in K/S value was larger than that with low H2O2 concentration. The K/S value increased linearly at low light intensity but leveled off when the light intensity exceeded 1.5 mW cm−2 (Fig. 2b). These results indicate that the dyeability of PET fabric increases with light intensity.
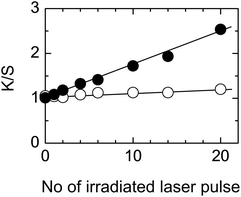
As the PET fabric dyeability increased with an increase in light intensity, a laser was used as a light source to facilitate the process. A KrF excimer (248 nm), which has a similar wavelength to the major emission of the germicidal lamp (254 nm), was used at an intensity below the ablation threshold to avoid physical changes (Fig. 3). The dyeability of the fabric increased with an increase in the number of laser pulses. The trend was the same as that obtained with the germicidal lamp shown in Fig. 1.
An irradiation of 20 laser shots corresponds to a total photon energy of 648 mJ cm−2 and gives a K/S value of 2.54 (Fig. 3), whereas irradiation with a germicidal lamp for 10 min corresponds to the same total photon energy of 648 mJ cm−2 and gives a similar K/S value of 2.26 (Fig. 1). These results indicate that the dyeability of PET fabrics depends on the total photon energy irradiated per unit area of the fabric, if the light source wavelength and reagent solution concentration are the same. This result is consistent with that obtained in Fig. 1 and 2. As the laser irradiation was conducted with a pulse repetition rate of 1 Hz, the processing time required for achieving the same dyeability was much faster with laser irradiation and the irradiation time can be shortened by increasing the repetition rate.
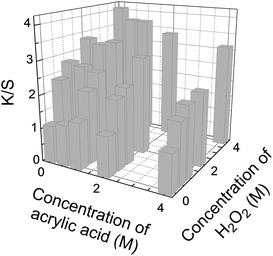
As the H2O2 concentration showed a considerable effect on the dyeability (Fig. 2), the effect of AA and H2O2 concentrations was investigated, with results shown in Fig. 4. A considerable increase in the dyeability was observed with an increase in H2O2 concentration, whereas a slight decrease was observed with an increase in AA concentration. The slight decrease with increasing AA concentration can be rationalized by a decrease in the concentration of OH radicals because of an increase in the filter effect of AA.
Results from Fig. 1 and 4 indicate an increase in dyeability with prolonged irradiation time and increased concentration of H2O2. Therefore, photochemical treatment of the PET fabric was conducted by 20 min irradiation with a germicidal lamp using (1) 4 M H2O2 and (2) 4 M AA + 4 M H2O2 (Fig. 5). The K/S values of the fabrics dyed with Cathilon Blue were 4.94 (1.16 on the reverse side) for condition (1) and 4.81 (1.15 on the reverse side) for condition (2). The fabrics were also dyed with Cathilon Red, with a K/S value of 4.98 (1.29 on the reverse side) for condition (1) and 4.99 (1.26 on the reverse side) for condition (2). The K/S values of the dyed original cloth were 0.86 for Cathilon Blue and 1.03 for Cathilon Red.
2.3. Factors controlling the dyeability of PET fabrics using H2O2 aqueous solutions
As the K/S value of the above condition (1) reached almost the same level as that of condition (2) (vide supra), photochemical treatment using only H2O2 was studied in detail. The effect of irradiation time showed that the K/S value increased with prolonged irradiation (Fig. 6, ◊). In contrast to the result in Fig. 1, the increase in Fig. 6 gradually slowed down with prolonged irradiation. This was attributed first to the decrease in H2O2 concentration with prolonged irradiation so that additional photochemical treatments were conducted by repeatedly soaking the fabric in fresh H2O2 followed by 10 min irradiation, thereby providing fresh H2O2 every 10 min (Fig. 6, ○). However, the result was the same as that for continuous irradiation with a single initial soaking (Fig. 6, ◊). This indicates that the decrease in H2O2 concentration was not the source of the gradual retardation in treatment with prolonged irradiation.The effect of H2O2 concentration was also investigated, with the result shown in Fig. 7. K/S increased rapidly at low H2O2 concentrations but slowed down at higher concentrations and started to decrease after reaching a maximum at ∼6.0 M.
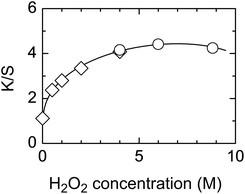 | ||
| Fig. 7 K/S values of dyed photochemically treated PET fabric as a function of H2O2 concentration. Symbols: data from Fig. 4 (◊), new data (○). K/S values were calculated from the reflectance at λmax = 650 nm. Irradiation conditions: germicidal lamp (1.07 mW cm−2), 10 min, in air. Reagent: aqueous H2O2. Dye: Cathilon Blue CD-FBLH. | ||
The results obtained in Fig. 6 and 7 indicate that the dyeability is dependent on the irradiation time and H2O2 concentration but they show slightly different features from those obtained using mixed aqueous solutions of H2O2 and AA.
2.4. Chemical structural change on the surface of photochemically treated fabrics
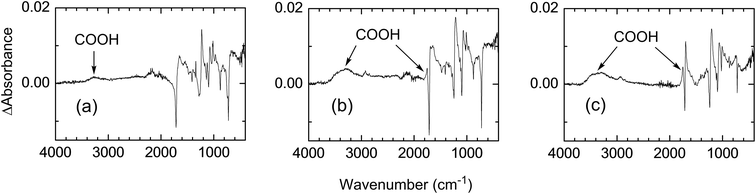
Fig. 8 shows the FTIR spectra of the PET fabrics treated with conditions I–III. The fabrics treated with H2O2 (condition II) and AA + H2O2 (condition III) showed a considerable increase in absorption at 3300 cm−1 and a small increase at 1800 cm−1, both of which were attributed to the absorption of carboxylic acid groups. The small absorption was attributed to the carboxylic acid moiety because the absorption of carboxylic acids generally appears at a slightly higher wavenumber than that of carboxylic esters. Although a considerable increase in dyeability was not observed with H2O (condition I), a slight increase in absorption was observed at 3300 cm−1, which probably corresponds to the absorption of the carboxylic acid group.A small difference in IR spectra between the fabric treated photochemically with AA + H2O2 (condition III) and the untreated fabric indicates that the degree of grafting, expected from Schemes 1 and 2, was smaller than in previous reports using other photoinitiators.7–11 This is also supported by the fact that the fabric mass did not increase by photochemical treatment. This is probably because of the lower concentration of AA than that used in previous reports and also because of the presence of O2. Previous studies were conducted mostly under an inert atmosphere to avoid reactions of O2 with carbon radicals generated on the PET fabric surface.
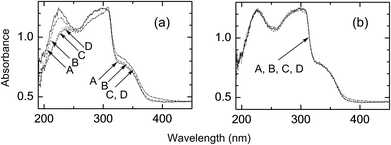
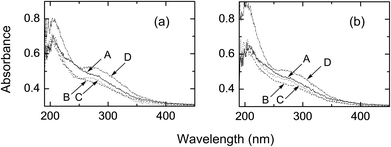
Fig. 9a shows the UV reflection spectra of PET fabrics on their irradiated side. The properties of the PET fabric treated with H2O (condition I) are almost the same as that of the original fabric. In contrast, fabrics treated with H2O2 (condition II) and AA + H2O2 (condition III) show an increase in absorption at 320–450 nm and a large decrease at <300 nm. The decrease in absorbance at ∼220 nm, which corresponds to an n→π* transition of carboxylic acids and esters, can be explained by the smaller molar absorption coefficient of acids than that of esters.15 This decrease supports the conversion of ester groups to acid groups, which is consistent with the result obtained in Fig. 8. The UV absorption spectra of photochemically treated PET fabrics with conditions I–III on their reverse side did not show any change from the original fabric (Fig. 9b).
To confirm the presence of carboxylic acid moieties on the surface of PET fabrics, methyl esterification of carboxylic acids was conducted on the modified fabrics using trimethylsilyldiazomethane (TMSCHN2) (eqn (1)),16 and the fabric was then dyed with a cationic dye (Table 2). A decrease in K/S values was observed from treatment with TMSCHN2 for H2O2 (condition II) and AA + H2O2 (condition III). This decrease is explained by the decrease in affinity between the cationic dyes and the carboxylic acid moieties by converting acids to their esters. This result supports the introduction of carboxylic acid groups by the photochemical treatment with H2O2 (condition II), even when carboxylic acid groups are not involved in modifying reagents.
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b
b
| Condition | Post treatment | |
|---|---|---|
| None | TMSCHN2 | |
| a K/S values were calculated from the reflectance at λmax = 650 nm. b Irradiation conditions: germicidal lamp (1.10 mW cm−2), 10 min, in air. Reagents: 0.5 M H2O2 (condition II), 0.5 M AA + 0.5 M H2O2 (condition III). Dye: Cathilon Blue CD-FBLH. The average of 2 independent runs. The numbers in brackets are those on the reverse side. | ||
| Original | 0.85 (0.85) | — |
| II | 2.27 (1.05) | 1.61 (0.95) |
| III | 2.03 (1.08) | 1.41 (0.93) |
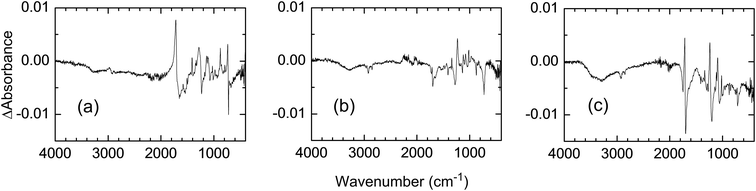
Fig. 10 shows the IR spectra of photochemically treated PET fabrics after TMSCHN2 treatment. The absorptions at 3300 and 1800 cm−1 decreased when the fabrics were treated with TMSCHN2. This result also supports the conversion of carboxylic acids to their methyl esters and is consistent with the results shown in Table 2.
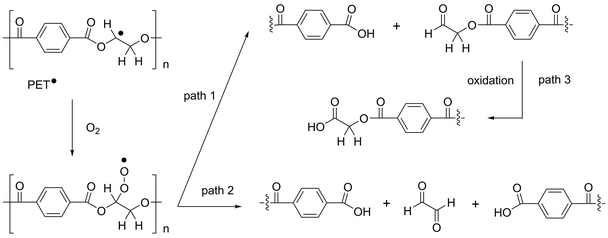
Although an increase in the dyeability was expected from AA + H2O2 (condition III), according to the reaction mechanism in Scheme 1, the increase with H2O2 (condition II) was unexpected. Most of the previous studies using photoinitiators have been conducted under an inert atmosphere (generally N2 or Ar) to avoid undesirable reactions with O2,7–9,11 and such reactions have not been considered in the study on the grafting of acrylates in air.10 Our results indicate that carboxylic acid moieties can be generated on the surface of PET fabrics by photochemical treatment using only H2O2. Carbon radicals react with O2 to form unstable peroxy radical species, which react further to yield carbonyl groups.17,18Scheme 3 shows plausible reaction paths for H2O2 (condition II) where PET radicals react with O2 to form stable species via paths 1 and/or 2 and by a similar reaction mechanism as indicated above.
To investigate the reaction mechanism in detail, the same photochemical treatments were conducted with cotton fabrics (Table 3). In contrast to PET fabrics, Table 3 shows that the dyeability of cotton fabrics did not increase with H2O2 (condition II). This result indicates that carboxylic acid groups were not generated from the aliphatic moieties of PET fabrics by further oxidation of the photochemically generated carbonyl groups (Scheme 3, path 3) but from terephthalate moieties in the fabrics. If carboxylic acid groups were formed via path 3 in Scheme 3, they should also be formed in cotton fabrics and result in an increase in the dyeability of the fabrics with H2O2 (condition II), which was not the case in Table 3. It should be noted that a considerable increase in cotton fabric mass (3.4%) was observed by photochemical treatment with AA + H2O2 (condition III). This indicates the efficient grafting of AA for introducing sufficient carboxylic acid groups on the fabrics and results in a significant increase in the dyeability of the cotton fabric, even in air. In contrast to the PET fabrics, the dyeability also increased on the reverse side because cotton fabric has no absorption at the wavelength of light used in the experiments. The photochemical reaction therefore proceeded efficiently with the light that penetrated through the fabric.
| Condition | ΔW (%) | Tensile strength (N) | Whiteness | K/S |
|---|---|---|---|---|
| a The change in mass before and after photochemical treatment (before dying). b K/S values were calculated from the reflectance at λmax = 650 nm. c Irradiation conditions: germicidal lamp (1.07–1.08 mW cm−2), 10 min, in air. Reagents: H2O (condition I), 0.5 M H2O2 (condition II), 0.5 M AA + 0.5 M H2O2 (condition III). Dye: Cathilon Blue CD-FBLH. The average of 3–4 independent runs. The numbers in brackets are those measured on the reverse side. | ||||
| Original | — | 176 | 55 (55) | 1.37 (1.27) |
| I | 0.5 | 195 | 57 (58) | 1.23 (1.19) |
| II | 0.4 | 173 | 58 (59) | 1.25 (1.18) |
| III | 3.4 | 171 | 52 (52) | 5.09 (12.0) |
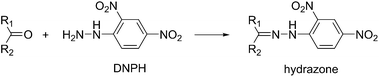
Scheme 3 indicates the formation of carbonyl groups via path 1, which occurs by a bond cleavage on one side of the ethylene glycol moiety. To confirm the presence of carbonyl groups on the fabric surface, photochemically treated fabrics were treated with 2,4-dinitrophenylhydrazine (DNPH), and their K/S values were measured (Table 4). DNPH is known to form yellow hydrazone with carbonyl groups (eqn (2)) so that an increase of the K/S value of hydrazone indicates the presence of carbonyl groups on the fabric surface. As shown in Table 4, the PET fabric showed only a slight increase in K/S values for conditions I–III. This result indicates that path 1 in Scheme 3 was not a major path for PET fabrics. Most probably, the cleavage of two bonds on both sides of the ethylene glycol moiety proceeded as shown in Scheme 3, path 2. Cotton fabrics, on the other hand, showed a large increase in the K/S value with H2O2 (condition II) and AA + H2O2 (condition III). This large increase indicates a considerable formation of carbonyl groups on the surface; however, the tensile strengths showed no weakening of the fabric for all three conditions (Table 3).
 | (2) |
| Condition | Polyester | Cotton |
|---|---|---|
| a K/S values were calculated from the reflectance at λmax = 370 nm. b Irradiation conditions: germicidal lamp (1.08 mW cm−2), 10 min, in air. Reagents: H2O (condition I), 0.5 M H2O2 (condition II), 0.5 M AA + 0.5 M H2O2 (condition III). Dye: DNPH. Average of 2 independent runs. The numbers in brackets are those on the reverse side. | ||
| Original | 0.64 (0.64) | 0.67 (0.66) |
| I | 0.71 (0.69) | 0.66 (0.61) |
| II | 0.93 (0.71) | 13.52 (3.91) |
| III | 0.90 (0.71) | 11.95 (4.66) |
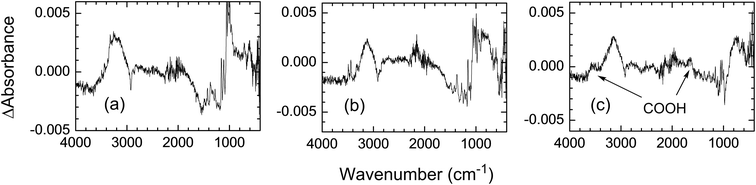
Fig. 11 shows the UV absorption spectra of photochemically treated cotton fabrics. Although original cotton fabric (A) and photochemically treated fabric with H2O (condition I) (B) showed no difference in the dyeability, a considerable decrease in the absorbance was observed at 190–450 nm for fabric B compared with that of A. The origin of this decrease is not clear at the moment but it is probably due to the decomposition of impurities remaining on the original cotton fabric.19 Cotton fabric treated with H2O2 (condition II) showed an increase in UV absorption at 270 nm and that with AA + H2O2 (condition III) showed a considerable increase in the wavelength range 190–400 nm with absorption maxima at 210 and 270 nm. The absorption maximum at 270 nm can be assigned to the n→π* transition of carbonyl groups and that at 210 nm to the n→π* transition of carboxylic acids.15 IR difference spectra showed a small absorption at 3400 and 1700 cm−1 for the fabric treated with AA + H2O2 (condition III) (Fig. 12). These absorptions correspond to carboxylic acid groups, in which the latter peak also includes absorption due to carbonyl groups. These results are in agreement with those in Tables 3 and 4.
3. Experimental
3.1. General aspects
Chemicals were used as purchased: 30% H2O2 aq., acrylic acid (stabilized with hydroquinone monomethyl ether, about 0.02%), MeOH, HCl (35–37%), and toluene were purchased from Wako Pure Chemical Industries, Ltd, DNPH from Kanto Chemical Co., Inc., TMSCHN2 in Et2O (2 M) from Sigma-Aldrich Co. LLC, and Cathilon Blue CD-FBLH and Cathilon Red CD-FBLH from Hodogaya Chemical Co., Ltd. Pure water was prepared using a Millipore Direct-Q ultrapure water system. Fabrics used in the experiments were polyester (71.8 g m−2, taffeta, purchased from Shikisensha Co., Ltd) and cotton (157 g m−2, G poplin (J6220), bleached and mercerized by Awazu Rensen Kogyo Co., Ltd).3.2. Photochemical treatment
A sheet of fabric (5 × 5 cm2) was padded in aqueous solutions of various reagents. The sheet was placed horizontally on a Petri dish and irradiated from the top with a light source at room temperature in air, washed thoroughly with running water for more than 1 h, and dried. The light sources used were a 20 W germicidal lamp (Panasonic Germicidal Lamp GL-20) or a Lambda Physik LPX210i [KrF (248 nm)] excimer laser. The light intensity of the germicidal lamp was measured using an Ushio UIT-150-A Ultraviolet Radiometer equipped with a UVD-S254 photodetector. The pulse energy of the laser was measured using a Gentec ED-500 joulemeter and a Gentec SOLO PE Laser Power & Energy Meter.3.3. Dyeing
To a 500 mL beaker were added 250 mL H2O, 6.5 mL acetic acid, 2.0 g cationic dye (Cathilon Blue CD-FBLH or Cathilon Red CD-FBLH), and 1.86 g sodium acetate. Compounds were dissolved by stirring at ∼60 °C. Fabric samples were added to the solution and the reaction temperature was raised from 60 °C to 80 °C in ∼10 min, from 80 °C to the boiling point in ∼10 min, maintained at the boiling point for ∼30 min, and reduced from the boiling point to ∼70 °C in ∼10 min. The fabric samples were washed with running water for ∼20 min, with a neutral detergent at ∼60 °C for ∼5 min, and then with running water for more than 1 h. The fabric samples were dried in a vacuum oven at room temperature. The dyed samples in each figure and table were dyed in the same batch, unless otherwise stated.3.4. Reaction of photochemically treated fabrics with TMSCHN2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 16
16
To a 250 mL beaker were added 10 mL MeOH, 35 mL toluene, and 2.0 mL of TMSCHN2 in Et2O (2 M). Fabric samples were added to the solution and reacted at room temperature for 40 min with occasional stirring. After removing the solution, the fabric samples were washed five times with excess MeOH and dried in a vacuum oven at room temperature.
3.5. Reaction of photochemically treated fabrics with DNPH
To a 500 mL beaker were added 150 mL MeOH and 1 g DNPH. After stirring for a few minutes at room temperature, 1 mL concentrated HCl and 150 mL MeOH were added to the solution. Fabric samples were added to the solution and reacted at room temperature for 1 h with occasional stirring. After removing the solution, the fabric samples were washed five times with excess MeOH and dried in a vacuum oven at room temperature.3.6. Measurements
Reflectance spectra were measured using a UV-vis spectrophotometer (Shimadzu UV-2400PC) equipped with an integration sphere (Shimadzu ISR-2200) using BaSO4 (Merck, for white standard DIN 5033) as a reference. K/S values were calculated from K/S = (1 − Rλ)2/2Rλ. The reflectance Rλ of the samples dyed with Cathilon Blue, Cathilon Red, and those treated with DNPH was measured at 650, 520, and 370 nm, respectively, which is the absorption maximum of each compound. The whiteness was determined as defined in JIS Z 8715 (CIE 1986c).ATR-FTIR measurements were conducted on a Shimadzu IRPrestige-21 spectrophotometer equipped with Dura SamplIR II ATR apparatus (Sens IR Technologies).
Fabric surfaces were studied using a KEYENCE VH-5000 digital microscope equipped with a VH-Z100 or VH-Z500 wide-range zoom lens, and the image focus merging software, Dynamic Eye REAL (Mitani Co.).
The fabric tensile strength was measured by the “breaking strength method A (raveled strip method)” defined in JIS L 1096 A (ISO 5081) using a Shimadzu Autograph AG-1000B.
4. Conclusion
PET fabrics are currently dyed using disperse dyes at high temperatures and pressures because of their low dyeability, which implies the use of a large amount of energy and pressurized vessels that are unsuitable for safe operations. A simple photochemical treatment of PET fabrics was conducted at room temperature under air using a commercially available germicidal lamp and H2O2 (and AA). A more than seven-fold increase in dyeability with cationic dyes was obtained under atmospheric pressure at reduced temperatures. The dyeability was dependent on the total photon energy irradiated per unit area, and concentrations of H2O2 and AA. The introduction of carboxylic acid groups on the fabric surfaces was responsible for the increase in the dyeability, which was confirmed by IR and UV measurements, and reactions using TMSCHN2.Acknowledgements
The authors thank Hodogaya Chemical Co., Ltd and Tokai Senko K. K. for providing the cationic dyes, Cathilon Blue CD-FBLH and Cathilon Red CD-FBLH, and Ms F. Ninomiya for the tensile strength measurements.Notes and references
- Reviews: (a) D. Knittel, W. Kesting and E. Schollmeyer, Polym. Int., 1997, 43, 231–239 CrossRef CAS; (b) D. Knittel, W. Kesting and E. Schollmeyer, Polym. Int., 1997, 43, 240–250 CrossRef CAS; (c) D. Knittel and E. Schollmeyer, Polym. Int., 1998, 45, 103–109 CrossRef CAS; (d) D. Knittel and E. Schollmeyer, Polym. Int., 1998, 45, 110–117 CrossRef CAS , and references cited therein.
- P. D. Kale, H. T. Lokhande, K. N. Rao and M. H. Rao, J. Appl. Polym. Sci., 1975, 19, 461–480 CrossRef CAS.
- Y. Avny, L. Rebenfeld and H.-D. Weigmann, J. Appl. Polym. Sci., 1978, 22, 125–147 CrossRef CAS.
- (a) M. S. Aronoff, Appl. Polym. Symp., 1977, 31, 397–405 CAS; (b) H. Watanabe and M. Yamamoto, J. Appl. Polym. Sci., 1999, 71, 2027–2031 CrossRef CAS.
- K. Gotoh, Sen'i Gakkaishi, 2008, 64, 199–204 CrossRef CAS.
- J. Jang and Y. Jeong, Dyes Pig., 2006, 69, 137–143 CrossRef CAS PubMed.
- R. P. Seiber and H. L. Needles, J. Appl. Polym. Sci., 1975, 19, 2187–2206 CrossRef CAS.
- Z. P. Yao and B. Rånby, J. Appl. Polym. Sci., 1990, 41, 1459–1467 CrossRef CAS.
- Z. Feng and B. Rånby, Angew. Makromol. Chem., 1992, 192, 113–125 CrossRef.
- W. Huang and J. Jang, Fibers Polym., 2009, 10, 27–33 CrossRef CAS PubMed.
- N. H. Mohamed, T. Bahners, A. Wego, J. S. Gutmann and M. Ulbricht, Appl. Surf. Sci., 2012, 259, 261–269 CrossRef CAS PubMed.
- A. Ouchi, C. Liu, M. Kaneda and T. Hyugano, Eur. J. Org. Chem., 2013, 3807–3816 CrossRef CAS.
- Original data are shown in ESI.†.
- P. Kubelka and F. Monk, Z. Tech. Phys., 1931, 12, 593–601 Search PubMed.
- H. H. Jaffé and M. Orchin, Theory and Application of Ultraviolet Spectroscopy, John Wiley & Sons, New York, 1962, ch. 9, section 9.5 Search PubMed.
- (a) N. Hashimoto, T. Aoyama and T. Shioiri, Chem. Pharm. Bull., 1981, 29, 1475–1478 CrossRef CAS; (b) A. Presser and A. Hüfner, Monatsh. Chem., 2004, 135, 1015–1022 CrossRef CAS PubMed.
- (a) T. Hyugano, S. Liu and A. Ouchi, J. Org. Chem., 2008, 73, 8861–8866 CrossRef CAS PubMed; (b) A. Ouchi, T. Hyugano and C. Liu, Org. Lett., 2009, 11, 4870–4873 CrossRef CAS PubMed.
- S. Z. Zard, Radical Reactions in Organic Synthesis, Oxford University Press, Oxford, 2003 Search PubMed.
- (a) A. Ouchi and H. Sakai, Green Chem., 2003, 5, 329–331 RSC; (b) A. Ouchi, H. Sakai, T. Oishi, T. Hayashi, W. Ando and J. Ito, Green Chem., 2003, 5, 516–523 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Emission spectra of a germicidal lamp; absorption spectra of Cathilon Blue, Cathilon Red, and hydrazone formed by DNPH treatment; the micrograph of photochemically treated PET and cotton fabrics. See DOI: 10.1039/c4gc01464b |
| This journal is © The Royal Society of Chemistry 2015 |