Environmental assessment of a bottom-up hydrolytic synthesis of TiO2 nanoparticles
Martina
Pini
*a,
Roberto
Rosa
b,
Paolo
Neri
a,
Federica
Bondioli
c and
Anna Maria
Ferrari
a
aDepartment of Sciences and Methods for Engineering, University of Modena and Reggio Emilia, Via Amendola, 2, 42100 Reggio Emilia, Italy. E-mail: martina.pini@unimore.it; Tel: +390522522089
bDepartment of Engineering “Enzo Ferrari”, University of Modena and Reggio Emilia, Via Vignolese, 905/A, 41125, Modena, Italy
cDepartment of Industrial Engineering, University of Parma, Parco Area delle Scienze, 181/A, Parma, Italy
First published on 18th September 2014
Abstract
A green metrics evaluation of the bottom-up hydrolytic sol–gel synthesis of titanium dioxide (TiO2) nanoparticles has been performed by following two different approaches, namely, EATOS software and LCA methodology. Indeed, the importance of engineered nanomaterials is increasing worldwide in many high-technological applications. Due to the as yet completely un-established environment and human health impact of nano-sized materials, the possibility of at least choosing a greener synthetic strategy through an accurate comparison of detailed environmental assessments will soon be of absolute importance in both the small and large scale production of these advanced inorganic materials. The present LCA study has been carried out following an ecodesign approach, in order to limit the environmental impacts and protect human health. The results of LCA analysis suggest that the highest environmental impact is mainly due to energy and the titanium isopropoxide precursor used in the synthesis process. Concurrently, software EATOS has been employed to calculate the environmental parameters that account for the environmental and social costs related to all the chemicals involved in the analyzed synthesis. As the EATOS approach is based purely on synthetic chemical mechanism considerations, thus neglecting any energy contributions, and its results cannot be directly compared to those arising from LCA analysis. However, similar and comparable outcomes are obtained by simply neglecting the energy contributions, broadening the application fields of the combined EATOS-LCA approach to the inorganic synthesis of engineered nanomaterials, highlighting the great potential of their synergy.
1. Introduction
Several evaluating parameters related to the environmental and human health impacts of a particular chemical process are gaining increasing interest and consideration alongside the traditionally employed ones, such as the yield, time and cost, as demanded by Green Chemistry, Green Engineering and Process Intensification developing philosophies.1–5At these latter regarding different metrics have been proposed over the last few decades,6,7 among which the E-factor8 and the mass index (MI),9,10 which are expressed respectively as eqn (1) and (2), have been the most studied and applied, leading to the design and development of the software, EATOS (Environmental Assessment Tool for Organic Syntheses) by Eissen and Metzger.11
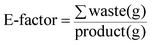 | (1) |
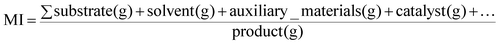 | (2) |
Indeed, this free of charge software12 allows the utilization of easily available data for calculations of the abovementioned mass metrics, and has been applied to several studies because it also allows a comparison of different synthetic strategies for the obtainment of a particular target compound.11,13–17
The most significant limitation of every mass metric, including EATOS, is the lack of any energy analysis together with its intrinsic character of being usually devoted to the gate-to-gate boundaries of a particular research laboratory or manufacturing plant. This means that several fundamental steps into the life cycle of employed chemicals, such as the extraction of raw materials, production, transportation, sales, distribution, use, and their final fate, are not considered by such mass metrics.7,18
All of these latter shortcomings can be overcome by applying the Life Cycle Assessment (LCA) methodology, which is based on a cradle-to-grave approach.19 Owing to the intrinsic highly comprehensive nature as well as to the difficulty in finding the necessary inventory data for the LCA, its use has been mainly limited to large scale production processes, instead of the early stages of research for innovative and greener synthetic routes, in which EATOS has already demonstrated its applicability.15 Nevertheless, numerous strategies have been proposed to simplify the LCA approach thus rendering it easily applicable to a laboratory scale. Among these artifices, for example, the possibility of substituting chemicals that are absent into the LCA software database with some analogues has been reported.20,21 In particular, in those works, different synthetic strategies have been evaluated and compared using both EATOS and LCA approaches. Noteworthy is the fact that similar assessments resulted, even though they are based two significantly different approaches.
Although the diffusion of the green chemistry developing philosophy usually involves less environmentally-friendly organic reactions, the significant environmental impact that nanotechnology related research activities are increasingly engendering should be considered, particularly considering that the environmental and human health effects of nano-sized materials are not yet fully established. In this last perspective, among the key issues related to the minimisation of the impact of nanotechnology on the environmental and on human health, which have been recently identified by Albrecht et al.22 a life cycle assessment and green chemistry metrics have been reported to be essential.
Therefore, the possibility of a greener approach for the synthesis of target engineered nanomaterials (being understood the desired particles size and shape) should be strongly recommended in order to pursue increasingly sustainable development.
LCA studies related to different nanomaterials have recently been reviewed23 including also a few studies necessarily related to titanium dioxide,24–27 which is the most studied and applied semiconductor and photocatalyst, owing to its unique physicochemical properties.28,29 In general, the preparation routes for this material produce anatase, rutile, or an amorphous solid, depending on the experimental synthetic conditions employed. Nanosized anatase is the most attractive crystalline form of titanium dioxide for advanced and high-technological applications mainly because of its higher stability and photocatalytic activity than rutile and brookite.
Consequently, the obtainment of anatase nanoparticles with high purity and a precisely controlled structure and particle size is the main purpose of optimizing synthetic methods, and many approaches have been proposed, including inert gas condensation,30 sol–gel method31,32 and hydrothermal synthesis.33 Among these, the sol–gel technique is most frequently applied for the synthesis of anatase nanoparticles with sizes ranging from 5 nm to several micrometers and a large variety of crystal shapes.
Aim of the present work was to apply two different green metrics evaluation tools, namely EATOS software and a detailed LCA study, to the hydrolytic sol–gel synthesis of TiO2 nanoparticles, produced according to the method patented by one of the most important Italian companies, a supplier of chemicals for building and further industrial sectors,34,37 and following an ecodesign approach. The obtained results were then compared to evaluate the applicability of these combined approaches to the inorganic synthesis of engineered nanomaterials.
Indeed, similar to what has recently begun to be accepted by a part of the organic chemistry community, an environmental impact evaluation should always accompany any new synthetic strategy proposed for the synthesis of inorganic engineered nanomaterials, and the same should also be valid for already established and recognized preparation procedures with the present manuscript representing a pioneering work in that precise direction. Being the recently developed synthetic approach for the obtainment of engineered nanomaterials, based on a meticulous reaction mechanism (thus no longer based on prolonged solid state diffusion-controlled high temperature treatments), it should not be surprising that an environmental assessment of their chemical synthesis can be, as in the case of the present work, evaluated using EATOS software, i.e., a tool originally developed for organic syntheses.
2. Experimental
The environmental performance of the hydrolytic synthesis of anatase TiO2 nanoparticles was evaluated using the LCA methodology (according to the ISO 14040/44)35,36 and EATOS tool, according to the reaction mechanism considerations reported in the following paragraph.2.1. Hydrolytic sol–gel synthesis of TiO2 nanoparticles
The hydrolytic sol–gel synthesis of anatase TiO2 nanoparticles was performed according to the procedure recently patented and actually employed by Colorobbia S.p.A., one of the most important Italian suppliers of chemicals for the building and industrial sectors for the preparation of aqueous TiO2 suspensions.34,37 It is well known37,38 that in the hydrolytic sol–gel syntheses of oxide nanomaterials, water acts both as a solvent and as a true reactant, with the reaction mechanism involving subsequent hydrolysis and condensation reactions. All of the possible hydrolytic reactions to which the organic metal precursors can undergo are reported in eqn (3)–(6), in which titanium isopropoxide is considered a titanium precursor. Eqn (7) can alternatively be used to summarise and group all the previous reactions.| Ti(OiPr)4 + H2O → Ti(OiPr)3OH + iPrOH | (3) |
| Ti(OiPr)3OH + H2O → Ti(OiPr)2(OH)2 + iPrOH | (4) |
| Ti(OiPr)2(OH)2 + H2O → TiOiPr(OH)3 + iPrOH | (5) |
| TiOiPr(OH)3 + H2O → Ti(OH)4 + iPrOH | (6) |
| Ti(OiPr)4 + nH2O → Ti(OiPr)4−n(OH)n + niPrOH | (7) |
The next condensation reactions can obviously occur between both hydroxylated metal species, leading to a Ti–O–Ti bond with the release of a water molecule (eqn (8)), and between a hydroxide and an alkoxide leading to the formation of a Ti–O–Ti bond with the release of an isopropyl alcohol molecule (eqn (9)).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OH + HO–Ti Ti–OH + HO–Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) → → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–O–Ti Ti–O–Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + H2O + H2O | (8) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–OiPr + HO–Ti Ti–OiPr + HO–Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) → → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ti–O–Ti Ti–O–Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) + iPrOH + iPrOH | (9) |
The chemical reactivity of the metal alkoxide towards hydrolysis and condensation mainly depends on the electrophilic nature of the metal atom, its ability to increase the coordination number, the steric hindrance of the alkoxy group, as well as the polarity, the dipole moment and the acidity of the solvent.39 Therefore, the major problem of aqueous sol–gel methods based on the hydrolysis and condensation of a molecular precursor is control over the reactions rates. For most transition metal oxide precursors, these reactions are too fast, resulting in the loss of morphological and structural control over the final oxide material. One of the possibilities of decreasing and adjusting the reactivity of the precursor is the use of organic additives, which can act as chelating ligands that would modify the reactivity of the precursors.
In this specific case, for each Ti–O–Ti bridge formed, a water or an alcohol molecule is generated, and considering the product TiO2 as the repetitive unit of the inorganic network and taking into account the octahedral coordination of Ti atoms and the trigonal-planar one of each oxygen atom, the stoichiometric reaction describing the overall hydrolytic sol–gel synthesis of TiO2 nanoparticles can be expressed as eqn (10).
| Ti(OiPr)4 + 2H2O → TiO2 + 4iPrOH | (10) |
The experimental synthetic procedure followed37 exploited the use of concentrated nitric acid and Triton X-100 to control the alkoxide reactivity as well as the hydrolysis and condensation reactions rates; the latter also acting as dispersant agent for the as synthesised nanocrystals. The previous reaction (eqn 10), however, assumes that the complete hydrolysis of the metal precursor occurs first, leading to the formation of four molecules of isopropyl alcohol, with subsequent condensation reactions occurring only between the tetra hydroxylated species, leading to the liberation of two water molecules for each TiO2 repetitive unit formed.
To be as accurate as possible when comparing the two green metrics evaluation procedures (i.e. EATOS and LCA), eqn (11) was considered. This is slightly different from eqn (10) because eqn (11) considers the water in the reaction products.
| Ti(OiPr)4 + 4H2O → TiO2 + 2H2O + 4iPrOH | (11) |
2.2. Description of the life cycle of the bottom-up hydrolytic synthesis of TiO2 nanoparticles
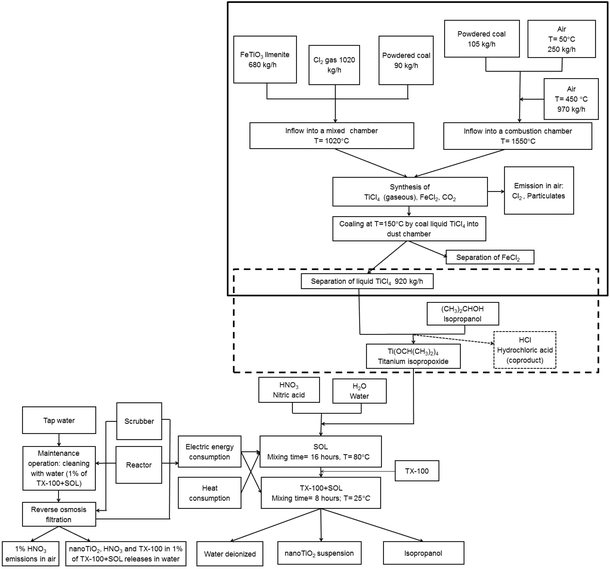
Fig. 1 shows the production process of the considered bottom-up hydrolytic synthesis of a TiO2 nanoparticles suspension. In particular, the process consists of two main steps: the sol preparation and the addition of auxiliary materials to prevent the flocculation of titanium dioxide. Because titanium isopropoxide, which is required for the sol preparation, has been not present in LCA databases, the representation of its synthesis route from titanium tetrachloride has been included in the present LCA study.43 The same also applies to the synthesis of the TiCl4 precursor, which a further step backward to its preparation from ilmenite.43| Chemicals | wt% |
|---|---|
| Titanium isopropoxide | 23.22 |
| Water | 73.40 |
| Nitric acid 63% | 2.38 |
| Triton X-100 | 1 |
| Total | 100 |
| Recycled isopropanol (co-product) | 12 |
| Remaining 88% | % |
| TiO2 nanoparticles suspension (main product) | 85.71 |
| H2O (co-product) | 14.29 |
| Total | 100 |
The following assumptions were considered:
• During the production and purification steps, the release of HNO3 into the air occurs. In particular, 1% of the total amount of material has been assumed to be emitted.
• During the maintenance operations, particularly in the purification process, the % of the TiO2 nanoparticles suspension produced and composed of TiO2, HNO3, TX-100 and H2O was assumed to be released into the cleaning water.
Owing to the above-mentioned emissions, a reverse osmosis filter equipped with a scrubber system and a scrubber system solely were considered in the maintenance operations and production process, respectively. The scope was the reduction of the emissions to water and air. In particular, the scrubber system can capture the HNO3 emissions, while the titanium dioxide nanoparticles present in the obtained aqueous suspension are entrapped by the reverse osmosis filter. The efficiency of the filter in the production process is 99.97%. The installation of a reverse osmosis filter with high efficiency reduces the environmental impact according to the ecodesign approach.
Regarding the end of life treatments, the part of filter that captured the emissions, together with the entrapped chemical substances, were disposed of in a residual material landfill.
The physical–chemical properties of the TiO2 nanoparticles suspension are reported in Table 2. In particular, the amount of TiO2 nanoparticles in the aqueous suspension results was 6 wt%, which was used to calculate the chemical reaction effective yield of 69.34%, which will be considered in the following EATOS calculations (see section 4).
| Physical and chemical properties | Amount |
|---|---|
| TiO2 concentration (%w/w) | 6 |
| Density (g ml−1) | 1.15 |
| Viscosity 20 °C (mPa s−1) | 2 |
| Nanoparticle size (nm) | 30 |
| Polydispersity index (pdI) | 0.25 |
| pH | 5.5 |
3. Life cycle assessment (LCA)
3.1. Aim definition
The aim of this LCA study was to assess the environmental impacts of the hydrolytic sol–gel synthesis of a titanium dioxide nanoparticles suspension produced according to the procedure recently patented and actually employed by Colorobbia S.p.A. for the preparation of aqueous TiO2 suspensions,34,37 to identify its environmental loads.3.2. System, functional unit and function of the system
The represented system is a multi-output process, which is based on the mass allocation criteria. The functional unit is represented by both the main product, i.e., 0.75425 kg of the TiO2 nanoparticles suspension and two co-products, namely 0.12 kg of recovered isopropanol and 0.12575 kg of recovered water.3.3. System boundaries
Fig. 1 highlights the system boundaries for the analysis, which includes the upstream phases, from raw material extraction and chemicals production, to the finished product packaging, thus obtaining “a cradle to the gate” overview. The production, maintenance and disposal of facilities, as well as the environmental burdens related to the production and disposal of packaging materials and other auxiliary materials have been also included in the present study. Emissions into the air and water, as well as disposal of the part of filter that captures the nanoparticles or the volatile chemical compounds, such as nitric acid, were taken into account. The transportation of chemical materials, facilities and packaging materials to the company that used them and the transportation of waste materials to a treatment facility have been also considered. In addition, the energy consumption required in all life cycle steps of the TiO2 nanoparticles suspension production were also evaluated.3.4. Data quality and impact assessment methodology
The data for the study was collected both directly from the authors of the patented procedure (primary data)37 and from scientific literature (secondary data). In particular, the primary data, referring to the optimized method for the preparation of aqueous dispersion of TiO2 nanoparticles,34,37 consisted of the composition and physical/chemical properties of the TiO2 nanoparticles suspension and energy consumption. Where the data was somehow missing, the study was completed based on the secondary data obtained from the Ecoinvent database that exploited them to model the background processes (land use, materials production, fuel and electricity production, and transport). The analysis is performed using the SimaPro 7.3.3 software and IMPACT 2002+ evaluation method to assess the environmental impacts.3.5. Life cycle inventory
In the LCA study, the quality and credibility of the results largely depend on the quality of the data included in the Life Cycle Inventory (LCI) stage. In accordance with the ILCD Handbook,41 the inventory must state, in a specific and reliable way, all the inputs in the form of material and energy resources and all the outputs in the form of air emissions, emissions into water and soil, as well as the solid waste that is generated, for each of the stages of the life cycle of the system being studied (according to ISO/TS 14048).42 The inventory data was modelled in SimaPro 7.3.3, taking the Ecoinvent database v2.2 as a reference to configure the inventory of some chemicals (i.e. nitric acid and TX-100), natural gas, electricity, heat, transport, infrastructure, machinery, and waste treatments. In those cases, where the chemicals were missing, such as the case of titanium isopropoxide (reagent) and titanium tetrachloride (titanium isopropoxide precursor), they were created using literature data.43 The same approach was followed for the vacuum system and the purification processes.3.6. Impact assessment
The life cycle impact assessment (LCIA) results were modeled using the IMPACT 2002+ method with Simapro 7.3.3 to determine the environmental impacts related to the emissions released and the resources consumed in the system under study.44 This impact assessment method covers more impact categories than other methods and includes more substances, but the following additions and modifications were implemented to describe the system considered in a more representative manner:• Land use was estimated by considering the basic indicators of both land occupation and transformation. In the present study Transformation, to forest intensive, normal, Transformation, to forest intensive and Transformation, to arable were introduced.
• Mineral extraction was characterised in consideration of some additional resources, such as silver, gravel, sand, lithium, bromine and water in ground, derived from the category Minerals of Eco-indicator 99 with the same characterisation factors.45
• A radioactive waste category was added. In particular, both this kind of waste and its occupied volume were evaluated considering the same characterization and normalization factors of EPID 2003 method.46 This category allows the possible damage of the electric energy mix, which also includes the electricity generated by nuclear plants, to be taken into account. This latter kind of energy produces radioactive waste, which needs to be safely managed and disposed.
Concerning the toxicity of nanoparticles, it is known that there are currently several major uncertainties and knowledge gaps with regard to the behavior, chemical and biological interactions and toxicological properties of engineered nanomaterials.47 Despite this, a preliminary attempt to assess the toxicity of TiO2 nanoparticles released in water on freshwater ecosystem and human health has been done. In particular, the IMPACT 2002+ method has been further exploited for this purpose as follows:
- Particulates, <100 nm, in freshwater (a representative substance of the damage on freshwater ecosystem) has been introduced in a new impact category named nanoTiO2 ecotoxicity in freshwater, which refers to a new damage category with the same name and with a characterization factor calculated by Salieri et al.48
- NanoTiO2Human toxicity, in freshwater (a representative substance of a local damage on human health, considering the area of Emilia Romagna region in the north-east of Italy) has been introduced in a new impact category called NanoTiO2carcinogens in freshwater, which refers to a new damage category with the same name and with a new calculated damage assessment factor.49 This factor has been determined considering the Anandanl and Kumar study, which evaluated the target organ toxicity dose (TTD) value for human liver cell toxicity in stream water of 8.33 μg L−1 for the TiO2 nanoparticles.50 The new damage factor was determined by the Eco-indicator 99 calculation method for carcinogenic substances. In particular, it consists of three main steps: fate analysis, effect analysis and damage analysis. It considers an emission released into freshwater during the purification of water contaminated by nanoparticles of 1 kg per year, a water body volume of Reggio Emilia province of 9 × 106 m3 and a calculated nanoparticles concentration of 1.111 × 10−7 kg m−3. The damage assessment factor obtained was 7.14 × 10−6 DALY kg−1 of TiO2 nanoparticles released into water.
4. EATOS calculations
The list of starting substances considered in the assessment made using EATOS and the categories to which they have been considered to belong are reported in Table 4. Table 4 summarizes the yield of the synthetic procedure as well as the useful amounts of obtained coupled products; the latter accounting for their possibility to be recovered and eventually recycled. All of the amounts reported in the two above-mentioned tables are in accordance with the functional unit considered in LCA analysis (i.e. the amount of TiO2 obtained from 1 kg of starting materials).The amount of solvent has been considered to be completely recyclable (see Table 3), while the useful quantities of isopropyl alcohol and the remaining water (i.e. the second coupled product) have been settled in accordance to what is indicated by the patented procedure37 and off course to the amounts used in the LCA assessment, as reported in Table 1. In detail, 120 g constituted the effectively recovered amount of isopropyl alcohol, while the maximum useful amount of the coupled product water has been established according to the yield of the reaction, assuming thus a maximum value of 69.34% of that theoretically obtainable. This yield value corresponds to a water amount which is significantly lower with respect to what is indicated (Table 1) and considered in the LCA framework. The reason needs to be found in the fact that what is considered by LCA as the water co-product, indeed refers to the amount of water recovered after the synthetic procedure, the latter comprising both the effective water co-product and part of the water originally intended as the solvent.
| Substance | Category | Molecular weight (g mol−1) | Quantity (g) |
|---|---|---|---|
| Ti(OiPr)4 | Key substrate | 284.2308 | 232.2 |
| H2O | Substrate | 18.0152 | 58.87 |
| H2O | Solvent | 18.0152 | 675.13 (recyclable quantity = 100%) |
| HNO3, 63% | Catalyst | 63.0128 | 23.8 |
| Triton X-100 | Auxiliary material | 646.8572 | 10 |
| Substance | Category | Molecular weight (g mol−1) | Useful quantity (% or g) | Yield (%), referred to the key substrate |
|---|---|---|---|---|
| TiO2 | Product | 79.8788 | — | 69.34 |
| H2O | Coupled product | 18.0152 | 69.34% | — |
| C3H8O | Coupled product | 60.0956 | 120 g | — |
The software EATOS allows calculating four important environmental parameters: the mass index MI (eqn (2)), the environmental factor E (eqn (1)) together with EI_in and EI_out corresponding to the former ones in which each substance quantity is multiplied by its specific total weighting factor, Qtot, which represents the mean value calculated among the different weighting factors Qi values. In detail, the weighting factors allow evaluating and examining the specific chemical reaction with particular regard to the potential environmental and human health relevance of each substance employed.51 The index i in the Qi notation, accounts for the ith weighting category among the claiming of resources, risk, human toxicity, chronic toxicity, ecotoxicology, ozone creation, air pollution, accumulation, degradability, greenhouse effect, ozone depletion, nitrification, and acidification. Each Qi can assume values ranging from 1 to 10 according to specific classifications internally made by the software algorithms. The EATOS software also allows selecting the opportune significance to associate with each weighting category.
The relevant information that determines the Qi value for that particular substance can be easily found in the Material Safety Data Sheet (MSDS) from the supplier. In particular, in this work, the MSDS considered those available on the Sigma Aldrich website,52 and regarding the price, the largest amount available was selected.
5. Results and discussion
The Life Cycle Impact Assessment (LCIA) was performed by both a midpoint and endpoint assessment. The midpoint indicators are considered to be linked to the cause-effect chain (environmental mechanism) of an impact category. Common examples of midpoint characterization factors include the ozone depletion potentials, global warming potentials, and photochemical ozone (smog) creation potentials. The endpoint indicators are instead considered to be linked to the cause-effect chain for all categories of impacts (e.g., human health impacts, in terms of disability adjusted life years for carcinogenicity, climate change, ozone depletion, photochemical ozone creation, or impacts in terms of changes in biodiversity, etc.).535.1. LCA results
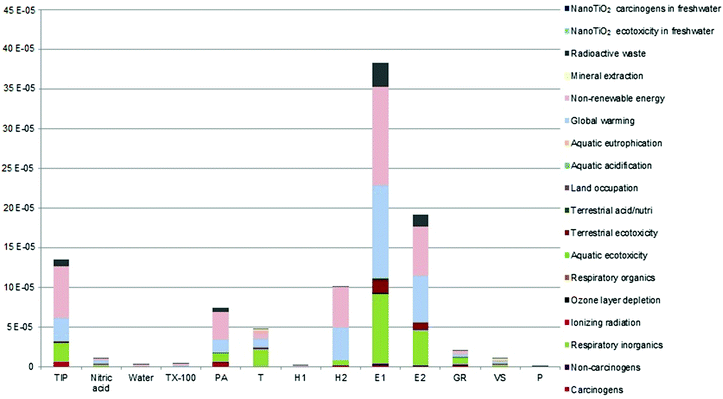
The single score damage is 988.87 μPt for 1 kg of TiO2 nanoparticles suspension produced. As Fig. 2 shows, the electric energy consumption to mix the colloidal solution (sol) for 16 hours (indicated as E1) and the one necessary to mix the sol and TX-100 for 8 hours (indicated as E2) are the two contributions that are mainly responsible for the total damage (38.76% and 19.38% respectively), followed by titanium isopropoxide (TIP) production (13.65%) and by the heat consumption (indicated as H2) to maintain the solution at 80 °C (10.33%). A midpoint category interpretation was conducted because it is more appropriate to evaluate the environmental impacts of the various substances counted in the life cycle inventory. The impact categories considered are global warming, non-renewable energy, mineral extraction, carcinogens, non-carcinogens, respiratory inorganics, respiratory organics, aquatic ecotoxicity, terrestrial ecotoxicity, ionising radiation, ozone layer depletion, terrestrial acidification, aquatic acidification, aquatic eutrophication, radioactive waste, land occupation, nanoTiO2 ecotoxicity in freshwater, and nanoTiO2 human toxicity.Table 5 summarises the outcomes of the midpoint and endpoint analysis that are explained in detail hereafter. In particular, for midpoint analysis, the contributions of the main impact categories on the total damage are reported.
| Midpoint results | Endpoint results | ||||
|---|---|---|---|---|---|
| Impact category | Unit | Total | Damage category | Unit | Total |
| Carcinogens | kg C2H3Cl eq. | 4.43 × 10−2 | Human health | DALY | 1.68 × 10−6 |
| Non-carcinogens | kg C2H3Cl eq. | 1.63 × 10−2 | |||
| Respiratory inorganics | kg PM2.5 eq. | 2.13 × 10−3 | |||
| Ionizing radiation | Bq C-14 eq. | 48 | |||
| Ozone layer depletion | kg CFC-11 eq. | 3.95 × 10−7 | |||
| Respiratory organics | kg C2H4 eq. | 1.06 × 10−3 | |||
| Aquatic ecotoxicity | kg TEG water | 1.70 × 102 | Ecosystem quality | PDF m2 year | 0.503 |
| Terrestrial ecotoxicity | kg TEG soil | 52.8 | |||
| Terrestrial acid/nutri | kg SO2 eq. | 4.39 × 10−2 | |||
| Land occupation | m2 org.arable | 2.91 × 10−2 | |||
| Aquatic acidification | kg SO2 eq. | 1.27 × 10−2 | |||
| Aquatic eutrophication | kg PO4 P-lim | 8.17 × 10−4 | |||
| Global warming | kg CO2 eq. | 2.86 | Climate change | kg CO2 | 2.863 |
| Non-renewable energy | MJ primary | 55 | Resources | MJ primary | 55.15 |
| Mineral extraction | MJ surplus | 1.53 × 10−1 | |||
| Radioactive waste | kg | 6.37 × 10−5 | Radioactive waste | kg | 6.37 × 10−5 |
| NanoTiO2 ecotoxicity in freshwater | kg | 6.63 × 10−9 | NanoTiO2 ecotoxicity in freshwater | PAF m3 day | 1.86 × 10−9 |
| NanoTiO2 carcinogens in freshwater | kg | 6.63 × 10−9 | NanoTiO2 carcinogens in freshwater | DALY | 4.73 × 10−14 |
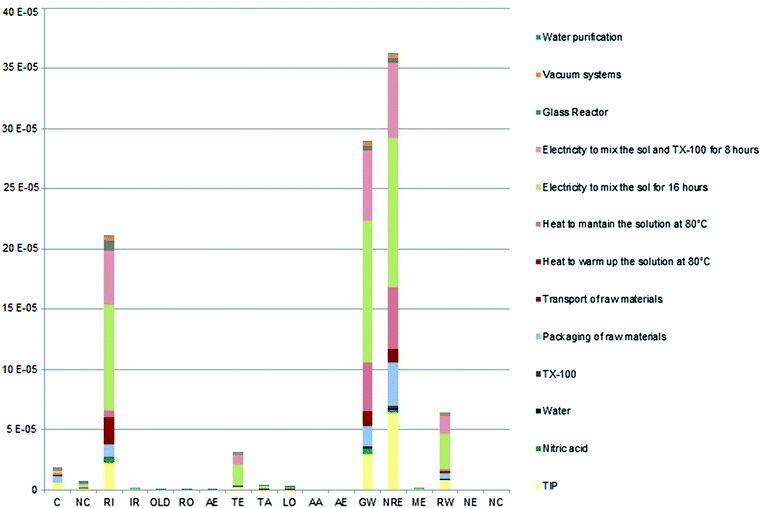
Fig. 3 shows that the most significant contribution to the total damage is due to the non-renewable energy impact category, which is primarily affected by natural gas (52.31%), crude oil (25.58%) and hard coal (11.23%) emissions. For all types of emissions, the electric energy consumption to mix the sol for 16 hours is the process that produces the major environmental load (33.09%, 25.5%, and 49.18% respectively). In particular, in this process, the emissions are mainly caused by the natural gas and crude oil production and the hard coal mining respectively.
The second major contribute to the total damage is generated by the global warming impact category, mainly due to GHG (greenhouse gas) emissions (96.17%), which are for the 41.24% belonging to the electric energy consumption to mix the sol for 16 hours and in particular that generated by the natural gas used to produce the electricity.
In respiratory inorganics, the major contributions are due to the following emissions to the air: 37.3% of NOx, 28.91% of SO2 and 18.61% of particulates <2.5 μm. All of these are mainly due to the electric energy consumption to mix the sol for 16 hours (38.77%, 49.65% and 45.54% respectively), and in particular, that generated for the first two (NOx and SO2) during heavy fuel oil combustion while the latter is during hard coal combustion.
In the radioactive waste impact category, the volume occupied by low-active radioactive waste contributes 65.31% of the total damage due to the electric energy production by nuclear power plants.
Regarding the nanoTiO2 ecotoxicity in freshwater and nanoTiO2 carcinogens in freshwater impact categories, the damage is totally due to the release of 6.63 μg of particulates, <100 nm (anatase TiO2 nanoparticles) and 6.63 μg nanoTiO2human toxicity in freshwater during the purification of contaminated water, which are obtained by the maintenance operation (reactor cleaning, see paragraph 2.2.3).
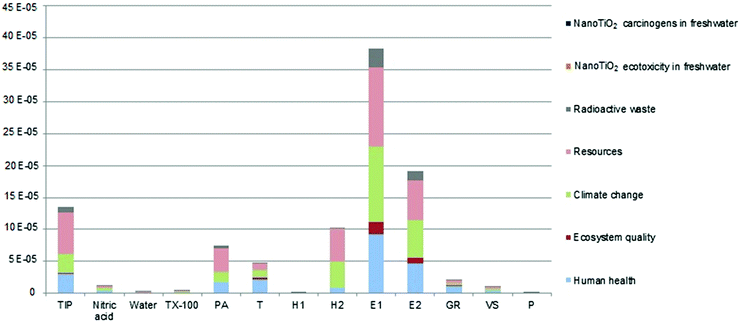
The endpoint analysis highlights (Fig. 4) that the total damage is affected for 23.9% the human health (2.36 × 10−4 Pt), for 36.7% the resources (3.63 × 10−4 Pt), for 29.24% the climate change (2.89 × 10−4 Pt), for 3.71% the ecosystem quality (3.67 × 10−5 Pt), for 6.44% the radioactive waste (6.37 × 10−5 Pt), for 3.36 × 10−6% the nanoTiO2 ecotoxicity in freshwater (3.32 × 10−11 Pt) and for 6.75 × 10−7% the nanoTiO2 carcinogens in freshwater (6.67 × 10−12 Pt). In the latter two cases, the damage is restrained because, as mentioned before, the LCA study has been set in an ecodesign approach, thus installing a specific filter for nanoparticles with high efficiency (99.97%) to limit the nanoparticle releases.
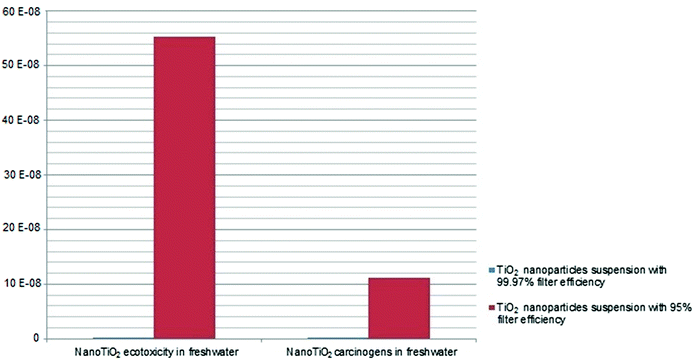
Fig. 5 shows that the variation of the sole filter efficiency from 99.97% to 95% leads to an increase in the damage in nanoTiO2 ecotoxicity in freshwater and nanoTiO2 carcinogens in freshwater impact categories equal to 165.67%.
Furthermore, life cycle cost analysis was carried out. The internal and external costs were assessed. The first takes into account the costs of all input materials and the latter considers the environmental costs caused by the hydrolytic synthesis of TiO2 nanoparticles.
The results for 1 kg of the TiO2 nanoparticles suspension show that internal and environmental costs values are 29.125 euro and 1.289 euro respectively, which are mainly caused by electric energy consumption.
5.2. EATOS results
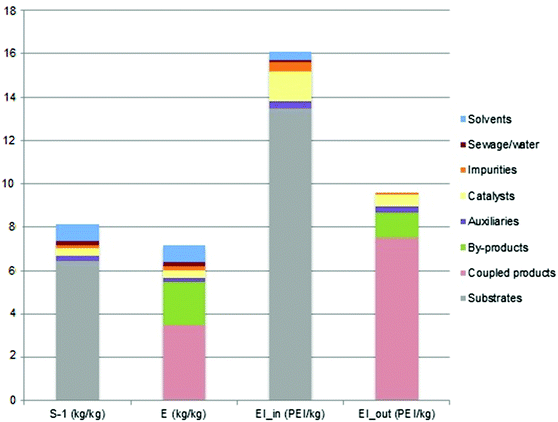
The results from the EATOS software in terms of the evaluation of histograms for the metrics MI, E-factor, EI_in and EI_out are reported in Fig. 6. The green metric EI_out parameter, expressed in Potential Environmental Impact (PEI; the larger its value, the worse the process will impact on the environment) per kg of product, accounts for the environmental damage potentially induced by the reaction waste. In particular, as detailed in Table 6, its total value of 9.6195 is mainly attributed to coupled products, because only a limited amount of isopropyl alcohol is recovered. The category by-products accounts for the fact that as the yield of the synthetic process is equal to 69.34, some unspecified by-products need to be considered.| Category | Substance | S−1 (kg of starting material/kg of product) | E (kg of waste/kg of product) | EI_in (PEI kg−1) | EI_out (PEI kg−1) |
|---|---|---|---|---|---|
| Substrates | Titanium isopropoxide and water | 6.4728 | 0.0402 | 13.4997 | — |
| Coupled products | Isopropanol and water | — | 3.4604 | — | 7.5233 |
| By-products | Unspecified | — | 1.9723 | — | 1.18 |
| Auxiliaries | Triton X-100 | 0.2278 | 0.2278 | 0.3418 | 0.2848 |
| Catalysts | Nitric acid | 0.3416 | 0.3416 | 1.3665 | 0.5124 |
| Impurities | Unspecified | 0.1587 | 0.1587 | 0.3968 | 0.119 |
| Sewage/water | Water + unspecified | 0.2006 | 0.2006 | 0.1003 | — |
| Solvents | Water | 0.7691 | 0.7691 | 0.3845 | — |
| Total | 8.1706 | 7.1707 | 16.0896 | 9.6195 |
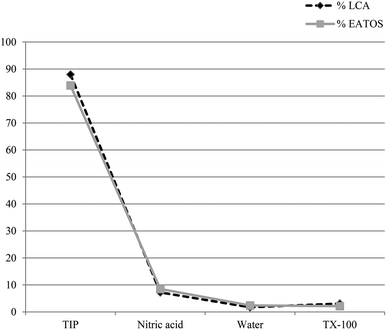
More interestingly, in the particular framework of a direct comparison with the LCA results, the potential environmental and human health relevance of each substance employed in the total inward materials flux related to the hydrolytic synthesis of anatase TiO2 nanoparticles suspension should be considered. In this regard, the green metrics parameter EI_in reaches a total value of 16.0896 PEI kg−1, which is 83.9% due to the titanium isopropoxide precursor, 8.5% to the catalyst, 2.1% to auxiliary materials, and 2.4% to the solvent (i.e. water). Noteworthy is that this latter finding is in agreement with the previously discussed LCA results. Indeed, by simply neglecting the energy contributions, the LCA outcomes show that the total damage is mainly caused by titanium isopropoxide for 87.98% and by nitric acid for 7.22%.
According to this latter approximation, Fig. 7 reports the comparison between the LCA and EATOS outcomes in terms of the main factors affecting the single score damage and EI_in respectively, revealing a tremendously similar trend.
It needs to be specified that for the present EATOS analysis, the weighting categories considered have been those in which the corresponding values for at least one substance have been found and consequently inserted, i.e. human toxicity, chronic toxicity, ecotoxicology, and accumulation (to which an influence of 25% has been assigned). The possibility of Q = 0 assignment has also been considered.
A further possibility offered by the EATOS software, is to calculate and present the different entry items, such as substrates, solvents, etc., by the so-called economic index, i.e. COST INDEX (CI). The different contributions to the CI (expressed in eur kg−1) deriving from the different input materials used for synthesis under consideration are detailed in Table 7.
| Substance category | CI (EUR kg−1) |
|---|---|
| Substrates | 454.948 |
| Auxiliaries | 8.9115 |
| Catalysts | 17.6381 |
| Impurities | 13.6392 |
| Solvent | 7.9986 |
| Sewage/water | 2.0866 |
| Total | 505.222 |
6. Conclusions
The environmental assessment of the bottom-up hydrolytic sol–gel synthesis of anatase TiO2 nanoparticles was concurrently performed by the software EATOS and by LCA methodology and, and despite the different philosophies on which they are based, similar conclusions can under some conditions be drawn.EATOS software was originally developed for organic chemists and mainly applies to fine chemical processes, relying on the data that is easily available from the material safety data sheets. On the contrary, life cycle assessment is a much more detailed and versatile method, suitable to assess industrial scale procedures, but it is barely adaptable to small scale ones,54 owing to its intrinsic time-consuming nature and to the difficulty to source necessary inventory data. In particular, for chemical processes, the databases to which the LCA methodology refers to, are still significantly inadequate. Therefore, when the required chemical is not included in a database, it is necessary either to consider chemicals with similar properties, among those comprised in the databases, or to recreate the synthesis of that particular substance ex novo.
Despite its immediacy, EATOS suffers from the major strong approximation of neglecting any energetic contribution. The typical limitations and drawbacks of both EATOS and LCA highlight their complementary character, which has been extensively exploited in environmental and human health assessments of several organic reactions.
The present work represents, to the best of authors’ knowledge, the first example, in which the synergy between LCA methodology and EATOS software has been applied to a green metrics evaluation of the inorganic synthesis of engineered nanomaterials.
In detail, the studied hydrolytic sol–gel synthesis route generates a suspension of TiO2 nanoparticles with high purity and crystallinity according to the patented procedure.37 The evaluated ecodesign approach (based on the installation of 99.97% efficiency filter and the use of closed reactor) together with the aqueous solvent employed and the low processing temperature, contribute to the good fit of this synthetic protocol in a green chemistry perspective.
The LCA results showed that most environmental loads are generated by the electric energy consumption (58.14%), followed by titanium isopropoxide (13.65%) and heat consumption to maintain the solution at 80 °C (10.33%). A better environmental performance can be achieved by the following:
• using renewable energy sources (e.g. solar power, geothermal, biomass, etc.) to reduce the non-renewable energy consumption.
• using microwave dielectric heating of reaction mixture, which has recently been significantly employed not only in organic chemistry but also in inorganic synthesis of a wide variety of engineered nanomaterials,55 because of its intrinsic advantages over conventional heating techniques based on heat transfer mechanisms (rather than on energy transfer ones).
• substituting titanium tetraisopropoxide with a different metal oxide precursor that is more environmentally friendly and less costly.
In this regard, the use of EATOS software alone, can furnish the first reliable approximation of an environmental assessment of synthetic strategies employing different metal oxide precursors. Indeed, in the present study, neglecting any energy contributions, the comparison between the results obtained using LCA methodology and EATOS software resulted in similar conclusions. In particular, they had very similar environmental impacts with the more affecting factor being titanium isopropoxide, followed by nitric acid with the aqueous solvent and auxiliary triton X-100 showing similar close contributions.
In conclusion EATOS provides fast and functional results that are comparable to the LCA results when energy consumption is neglected. Furthermore, EATOS is a free of charge and user-friendly software, so it is particularly suitable when a choice among different synthetic strategies needs to be considered for the preparation of the desired engineered nanomaterial.
This study represents the first example of the application of EATOS software (originally developed for the synthesis of fine chemicals) to the inorganic synthesis of transition metal oxide nanostructures, and the natural progression of the research activities in this direction, actually in progress, will involve a comparison among the most widely employed synthetic strategies for obtaining the desired inorganic nanocrystals. Nevertheless, a deeper investigation by a complete LCA study should be carried out because the LCA methodology provides more detailed and accurate results. Indeed midpoint and endpoint analysis give complete knowledge on (i) the impact and damage categories that mainly affect the total environmental and human health impacts of the studied process, (ii) the substances that cause these impacts and (iii) the compartments (air, soil, water, raw) that result in more damage.
The main conclusion of the present work, which be a recommendation to inorganic chemists and materials scientists worldwide, is to always combine an environmental assessment with any new proposed strategy for the synthesis of engineered nanomaterials, so that the strict requirement of using the most environmentally friendly procedure could very soon accompany traditional requests of a desired size and shape.
The assessment by means of easily available and accessible EATOS tool, although able to furnish trustworthy indications, should be integrated by a complete life cycle assessment study, thereby allowing a consideration of the impacts of the energy consumption, which usually represents the most impacting parameter of the entire process.
Acknowledgements
Authors thank Colorobbia S.p.A. for their support in the Life Cycle Inventory Analysis. This study has been supported by “ARACNE” project, Bando Regione Emilia Romagna, Italy.References
- P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Design through the 12 principles of green engineering, Environ. Sci. Technol., 2003, 37, 94A–101A CrossRef.
- T. Van Gerven and A. Stankiewicz, Structure, energy, synergy, time-the fundamentals of process intensification, Ind. Eng. Chem. Res., 2009, 48, 2465–2474 CrossRef CAS.
- A. Stankiewicz and J. A. Moulijn, Process intensification: transforming chemical engineering, Chem. Eng. Prog., 2000, 96, 22–34 CAS.
- J. F. Jenck, F. Angterberg and M. J. Droescher, Products and processes for a sustainable chemical industry: a review of achievements and prospects, Green Chem., 2004, 6, 544–556 RSC.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Metrics to “green” chemistry-which are the best?, Green Chem, 2002, 4, 521–527 RSC.
- C. Jiménez-Gonzalez, D. J. C. Constable and C. S. Ponder, Evaluating the “Greenness” of chemical processes and products in the pharmaceutical industry-a green metrics primer, Chem. Soc. Rev., 2012, 41, 1485–1498 RSC.
- R. A. Sheldon, The E factor: fifteen years on, Green Chem., 2007, 9, 1273–1283 RSC.
- T. Hudlicky, D. A. Frey, L. Koroniak, C. D. Claeboe and L. E. Brammer Jr., Toward a “reagent-free” synthesis-tandem enzymatic and electrochemical methods for increased effective mass yield (EMY), Green Chem, 1999, 1, 57–59 RSC.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Using the right green yardstick: why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- M. Eissen and J. O. Metzger, Environmental performance metrics for daily use in synthetic chemistry, Chem. – Eur. J., 2002, 8, 3581–3585 CrossRef.
- M. Eissen and J. O. Metzger, EATOS, Environmental Assessment Tool for Organic Syntheses; the software can be obtained free of charge via http://www.chemie.uni-oldenburg.de/oc/metzger/eatos.
- M. Eissen, K. Hungerbuehler, S. Dirks and J. Metzger, Green Chem., 2003, 5, G25–G27 RSC.
- A. Corradi, C. Leonelli, A. Rizzuti, R. Rosa, P. Veronesi, R. Grandi, S. Baldassari and C. Villa, New “green” approaches to the synthesis of pyrazole derivatives, Molecules, 2007, 12, 1482–1495 CrossRef CAS.
- S. Protti, D. Dondi, M. Fagnoni and A. Albini, Assessing photochemistry as a green synthetic method. Carbon-carbon bond forming reactions, Green Chem., 2009, 11, 239–249 RSC.
- C. Villa, R. Rosa, A. Corradi and C. Leonelli, Microwaves-mediated preparation of organoclays as organic-/bio-inorganic hybrid materials, Curr. Org. Chem., 2011, 15, 284–295 CrossRef CAS.
- W. Piang-Siong, P. de Caro, C. Lacaze-Dufaure, A. S. C. Sing and W. Hoareau, Effect of catalytic conditions on the synthesis of new aconitate esters, Ind. Crops Prod., 2012, 35, 203–210 CrossRef CAS PubMed.
- M. G. T. C. Ribeiro and A. A. S. C. Machado, Grenness of chemical reactions-limitations of mass metrics, Green Chem., Lett. Rev., 2013, 6, 1–18 CrossRef CAS.
- L. Tufvesson, P. Tufvesson, J. Woodley and P. Borjesson, Life cycle assessment in green chemistry: overview of key parameters and methodological concerns, Int. J. Life Cycle Assess., 2013, 18, 431–444 CrossRef CAS PubMed.
- D. Ravelli, S. Protti, P. Neri, M. Fagnoni and A. Albini, Photochemical technologies assessed: the case of rose oxide, Green Chem., 2011, 13, 1876–1884 RSC.
- D. Ravelli, D. Dondi, M. Fagnoni and A. Albini, Titanium dioxide photocatalysis: an assessment of the environmental compatibility for the case of the functionalization of heterocyclics, Appl. Catal., B, 2010, 99, 442–447 CrossRef CAS PubMed.
- M. A. Albrecht, C. W. Evans and C. L. Raston, Green chemistry and the health implications of nanoparticles, Green Chem., 2006, 8, 417–432 RSC.
- R. Hischier and T. Walser, Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps, Sci. Total Environ., 2012, 425, 271–282 CrossRef CAS PubMed.
- N. Osterwalder, C. Capello, K. Hungerbuhler and W. J. Stark, Energy consumption during nanoparticle production : how economic is dry synthesis, J. Nanopart. Res., 2006, 8, 1–9 CrossRef CAS.
- G. F. Grubb and B. R. Bakshi, Energetic and environmental evaluation of titanium dioxide nanoparticles, IEEE International Symposium on Electronics and the Environment, IEEE, San Francisco, USA, 2008.
- G. F. Grubb and B. R. Bakshi, Life cycle of titanium dioxide nanoparticle production, J. Ind. Ecol., 2010, 15, 81–95 CrossRef PubMed.
- G. F. Grubb and B. R. Bakshi, Appreciating the role of thermodynamics in LCA improvement analysis via an application to titanium dioxide nanoparticles, Environ. Sci. Technol., 2011, 45, 3054–3061 CrossRef CAS PubMed.
- D. P. Macwan, P. N. Dave and S. Chaturvedi, A review on nano-TiO2 sol-gel type syntheses and its applications, J. Mater. Sci., 2011, 46, 3669–3686 CrossRef CAS.
- Y. F. Chen, C. Y. Lee, M. Y. Yeng and H. T. Chiu, The effect of calcination temperature on the crystallinity of TiO2 nanopowders, J. Cryst. Growth, 2003, 274, 363–370 CrossRef.
- J. Rubio, J. L. Oteo, M. Villegas and P. Duran, Characterization and sintering behavior of submicrometre titanium dioxide spherical particles obtained by gas-phase hydrolysis of titanium tetrabutoxide, J. Mater. Sci., 1997, 32, 643–652 CrossRef CAS.
- S. M. Gupta and M. Tripathi, A review on the synthesis of TiO2 nanoparticles by solution route, Cent. Eur. J. Chem., 2012, 10(2), 279–294 CrossRef CAS.
- A. B. Corradi, F. Bondioli, B. Focher, A. M. Ferrari, C. Grippo, E. Mariani and C. Villa, Conventional and microwave-hydrothermal synthesis of TiO2 nanopowders, J. Am. Ceram. Soc., 2005, 88, 2639–2641 CrossRef CAS PubMed.
- P. Alphonse, A. Varghese and C. Tendero, Stable hydrosols for TiO2 coatings, J. Sol-Gel Sci. Technol., 2011, 56(3), 250–263 CrossRef.
- http://www.colorobbia.it/ .
- ISO 14040, Environmental management – Life cycle assessment – Principles and framework, International Standards Organization, ISO, 2006 Search PubMed.
- ISO 14044, Environmental management – Life cycle assessment – Requirements and guidelines, International Standards Organization, ISO, 2006 Search PubMed.
- G. Baldi, M. Bitossi and A. Barzanti, Method for the preparation of aqueous dispersions of TiO2 in the form of nanoparticles, and dispersions obtainable with this method, US Pat., 0317959 A1, 2008 Search PubMed.
- M. Niederberger and G. Garnweitner, Organic reaction pathways in the nonaqueous synthesis metal oxide nanoparticles, Chem. – Eur. J., 2006, 12, 7282–7302 CrossRef CAS PubMed.
- M. Niederberger and N. Pinna, Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application, Springer-Verlag, London, 2009, ch. 2, pp. 7–18 Search PubMed.
- Life Cycle Inventories, Ecoinvent Database, Version 2.0, 2010. http://www.ecoinvent.ch/ Search PubMed.
- EUROPEAN COMMISSION JOINT RESEARCH CENTRE, Institute for Environment and Sustainability, Analysis of existing Environmental Impact Assessment Methodologies for use in Life Cycle Assessment, ILCD Handbook, European Union, 1st edn, 2010 Search PubMed.
- ISO/TS 14048, Environmental management – Life cycle assessment – Data documentation format, International Standards Organization, ISO, 2002 Search PubMed.
- L. Piccolo, B. Calcagno and M. Ghirga, Method for preparing titanium tetrachloride, US Pat., 3787556, 1974 Search PubMed.
- O. Jolliet, M. Margni, R. Charles, S. Humbert, J. Payet, G. Rebitzer and R. Rosenbaum, IMPACT 2002+: A new life cycle impact assessment methodology, Int. J. Life Cycle Assess., 2003, 8(6), 324–330 CrossRef.
- M. Goedkoop and R. Spriensma, The Eco-indicator 99 – A damage oriented method for Life cycle Assessment, Methodology Report, Pré Consultants B.V., Amersfoort, 2001 Search PubMed.
- J. Potting and M. Hauschild, The EDIP 2003 methodology – Background for spatial differentiation in life cycle impact assessment, Methodology Report, Danish Environmental Protection Agency, Copenhagen, 2004 Search PubMed.
- C. Som, M. Bergesb, Q. Chaudhryc, M. Dusinskad, T. F. Fernandese, S. I. Olsenf and B. Nowack, The importance of life cycle concepts for the development of safe nanoproducts, Toxicology, 2010, 269, 160–169 CrossRef CAS PubMed.
- B. Salieri, Ph.D. Thesis, University of Bologna, 2013 Search PubMed.
- M. Pini, P. Neri and A. M. Ferrari, Determination of the potential damage and benefits of TiO2 nanoparticles by Life Cycle Assessment methodology, Internal Technology Report RT-07, University of Modena and Reggio Emilia, 2013 Search PubMed.
- A. Anandanl and A. Kumar, Exposures to TiO2 and Ag Nanoparticles: What are Human Health Risks?, Sci. Soc., 2011, 9(2), 155–162 Search PubMed.
- Environmental Assessment Tool for Organic Syntheses, EATOS, user manual version 1.1, by M. Eissen and J.O. Metzger, available online free of charge at http://www.metzger.chemie.uni-oldenburg.de/eatos/eatosmanual.pdf.
- The Materials Safety Data Sheet (MSDS) of all the substances considered in this study can be found at the website: http://www.sigma-aldrich.com.
- J. C. Bare, P. Hofstetter, D. W. Pennington and A. H. Udo de Haes, Life Cycle Impact Assessment Workshop Summary Midpoints versus Endpoints: The Sacrifices and Benefits, Int. J. Life Cycle Assess., 2000, 5(6), 319–326 CrossRef.
- D. Ravelli, S. Protti, P. Neri, M. Fagnonia and A. Albini, Photochemical technologies assessed: the case of rose oxide, Green Chem., 2011, 13, 1876 RSC.
- M. Baghbanzadeh, L. Carbone, P. D. Cozzoli and C. O. Kappe, Microwave-assisted synthesis of colloidal inorganic nanocrystals, Angew. Chem., Int. Ed., 2011, 50, 11312–11359 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |