Open Access Article
Open Access ArticleFolate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays
Sabine
Mönch†
a,
Michael
Netzel‡
b,
Gabriele
Netzel§
b,
Undine
Ott
c,
Thomas
Frank
d and
Michael
Rychlik
*ef
aLehrstuhl für Lebensmittelchemie, Technische Universität München, Lise-Meitner-Str. 34, D-85350 Freising, Germany
bDepartment of Human Nutrition, Institute of Nutrition, Friedrich Schiller Universität Jena, Dornburgerstr. 29, D-07743 Jena, Germany
cKuratorium für Dialyse und Nierentransplantation e.V., Zur Lämmerlaide 1, D-07751 Jena, Germany
dPrivate Consultant, D-65812 Bad Soden, Germany
eChair of Analytical Food Chemistry, Technische Universität München, Alte Akademie 10, D-85350 Freising, Germany. E-mail: michael.rychlik@tum.de; Fax: +49-8161-71 4216; Tel: +49-8161-71 3153
fBIOANALYTIK Weihenstephan, Research Center for Nutrition and Food Sciences, Technische Universität München, Alte Akademie 10, D-85350 Freising, Germany
First published on 3rd November 2014
Abstract
Different sources of folate may have different bioavailability and hence may impact the standard definition of folate equivalents. In order to examine this, a short term human study was undertaken to evaluate the relative native folate bioavailabilities from spinach, Camembert cheese and wheat germs compared to pteroylmonoglutamic acid as the reference dose. The study had a single-centre, randomised, four-treatment, four-period, four-sequence, cross-over design, i.e. the four (food) items to be tested (referred to as treatments) were administered in sequences according to the Latin square, so that each experimental treatment occurred only once within each sequence and once within each study period. Each of the 24 subjects received the four experimental items separated by a 14-day equilibrium phase and received a pteroylmonoglutamic acid supplement for 14 days before the first testing and between the testings for saturation of body pools. Folates in test foods, plasma and urine samples were determined by stable isotope dilution assays, and in urine and plasma, the concentrations of 5-methyltetrahydrofolate were evaluated. Standard non-compartmental methods were applied to determine the biokinetic parameters Cmax, tmax and AUC from baseline corrected 5-methyltetrahydrofolate concentrations within the interval from 0 to 12 hours. The variability of AUC and Cmax was moderate for spinach and oral solution of pteroylmonoglutamic acid but high for Camembert cheese and very high for wheat germs. The median tmax was lowest for spinach, though tmax showed a high variability among all treatments. When comparing the ratio estimates of AUC and Cmax for the different test foods, highest bioavailability was found for spinach followed by that for wheat germs and Camembert cheese. The results underline the dependence of folate bioavailability on the type of food ingested. Therefore, the general assumption of 50% bioavailability as the rationale behind the definition of folate equivalents has to be questioned and requires further investigation.
Introduction
The vitamins of the folate group play a crucial role as coenzymes in the metabolism of one-carbon groups, and are decisively involved in DNA synthesis, amino acid metabolism and methylations.1 However, intake of folate from natural sources is considered to be below the human dietary recommendations. Low dietary intake of folate is associated with the risk of neural tube defects2 and is suspected to be associated with the development of certain forms of cancer,3 Alzheimer's disease4 and cardiovascular disease.5 Over 50 countries have introduced mandatory folate fortification with pteroylmonoglutamic acid administration implemented in 1998 in the USA and Canada and in Australia in September 2009. The benefits of this fortification program with regard to neural tube defects have been obvious, as their incidence in Canada decreased by up to 3.8 cases per 1000 births from 1998 to 2002.6 However, discussions about the safety of this measure are still ongoing since reports on increased incidence of colon cancer in some countries with mandatory folate fortification7 alternate with such on no significant effect on any kind of cancer.8 The molecular cause is suggested to be a high plasma level of pteroylmonoglutamic acid that may lead to neoplastic transformations and formation of adenomas due to its effect on DNA synthesis9 and DNA methylation.10 Moreover, pteroylmonoglutamic acid supplementation in rats has stimulated the progression of aberrant crypt foci (ACF), the earliest precursor of colorectal cancer.11 In a human study, pteroylmonoglutamic acid supplementation decreased the cytotoxicity of circulating natural killer cells potentially affecting the destruction of neoplastic cells.12 Therefore, many countries in the EU have refused mandatory fortification and favour the consumption of foods endogenously high in folates or increasing endogenous folate content in foods. However, apart from folate content alone, bioavailability appears to be the challenge if folate supply from foods is intended to be increased.The current dietary recommendations are based on the studies of Sauberlich et al.,13 who determined in a long-term study a 50% bioavailability of food folates relative to pteroylmonoglutamic acid. However, this generalization has been questioned because of recent human studies such as the short-term study performed by Prinz-Langenohl et al.,14 who determined a folate bioavailability of spinach ranging between 89–113% relative to pteroylmonoglutamic acid. Moreover, in a long-term study Brouwer et al.15 found a 98% folate bioavailability for citrus fruits and vegetables relative to pteroylmonoglutamic acid. This finding may also be due to enhanced stability of 5-methyltetrahydrofolic acid (5-CH3-H4folate) in concurrent presence of ascorbic acid.16 Even when 5-CH3-H4folate was used as the reference dose, bioavailabilities ranging between 99–120% for broccoli and strawberries were found by Witthöft et al.17
In preparation of this investigation, we performed a pilot study on folate bioavailability by using stable isotope dilution assays for analysis of plasma folates and an area under the curve (AUC) approach.18 However, the plasma monitoring time of 6 hours after the intake of a pteroylmonoglutamic acid supplement as the reference dose was found to be too short, as the plasma level did not return to the baseline. Additionally, the analytical tools for this model have recently been improved and were extended to the analysis of folates in urine and erythrocytes.19 5-Methyltetrahydrofolate (5-CH3-H4folate) is the folate derivative normally found in the circulation, and in addition, is the predominant type of folate present in food. However, in case of fortification or when supplements of pteroylmonoglutamic acid are used, non-metabolised pteroylmonoglutamic acid may be found in blood circulation.20
Based on these considerations and preliminary studies, the aim of the present study was to assess in a short-term human study the relative bioavailability of folates in several foods rich in folates by recording 5-CH3-H4folate levels in plasma post-dose for 12 hours. As the test foods, spinach, wheat germs, and a low-fat Camembert cheese were chosen. Moreover, the suitability of analysing folate levels in urine for assessing bioavailability was evaluated.
Materials and methods
Chemicals
The following chemicals were obtained commercially from the sources given in parentheses: rat serum (Biozol, Eching, Germany), chicken pancreas (Difco, Sparks, MD, USA), acetic acid, acetonitrile, sodium phosphate dibasic dihydrate, formic acid, hexane, methanol, potassium phosphate monobasic, sodium hydroxide, sodium chloride, (Merck, Darmstadt, Germany), alpha-amylase, ammonium formate, ascorbic acid, pteroylmonoglutamic acid, 4-morpholineethanesulfonic acid (MES), 2-mercapto ethanol, protease type XIV, sodium acetate, (Sigma, Deisenhofen, Germany), (6S)-tetrahydrofolic acid, calcium (6S)-5-methyltetrahydrofolate, 10-formylfolic acid, (6S)-5-formyltetrahydrofolic acid (Schircks, Jona, Switzerland). The solvents were at least of analytical-reagent grade.[2H4]-5-Methyltetrahydrofolic acid, [2H4]-5-formyltetrahydrofolic acid, [2H4]-tetrahydrofolic acid, [2H4]-10-formylfolic acid and [2H4]-pteroylmonoglutamic acid were synthesised as reported recently.21
Ammonium formate buffer consisted of ammonium formate (10 g L−1) and ascorbic acid (1 g L−1) adjusted to pH 3.2.
Eluting solution for SPE was a mixture of aqueous sodium chloride (5%) and aqueous sodium acetate (100 mmol L−1) containing ascorbic acid (1%).
Foods
Frozen spinach and low-fat Camembert cheese were purchased at local supermarkets in the City of Jena, Germany. The spinach was cooked as described on the label prior consumption. Wheat germs were obtained from a local retail store in the City of Erding, Germany. The pteroylmonoglutamic acid solution was prepared by suspending pteroylmonoglutamic acid (3.96 mg) in tap water, which was then alkalised with diluted sodium hydroxide until all solids were dissolved and then adjusted to pH 7 with diluted hydrochloric acid followed by adjustment to volume (1 L) with tap water. In the context of this study these four test items are referred to as treatments.Food analysis
Foods were analysed according to the validated stable isotope dilution assay described by Mönch and Rychlik.22 Quality control was performed by assessing recovery, precision, linearity, LOD, LOQ and the analysis of dried, mixed vegetables as certified reference material.23Plasma
Plasma samples were analysed using phenyl SPE cleanup similar to that described by Pfeiffer and coworkers.20 Aliquots of plasma (400 μL) were spiked with [2H4]-5-methyltetrahydrofolic acid (5 ng) and then overlaid with ammonium formate buffer (600 μL) and equilibrated for 30 min at room temperature and subjected to cleanup on phenyl SPE cartridges (Discovery DSC-ph, 100 mg, 1 mL, Varian, Darmstadt, Germany). Folates were eluted from SPE columns with 0.5 mL of elution solution.Urine
Urine was pooled from 24 h and aliquots were analysed for 5-CH3-H4folate according to Mönch et al.19Human study
The study protocol was approved by the Ethics Committee of the Friedrich Schiller University Jena, Faculty of Medicine (code 1415-09/04). Each subject gave his written informed consent prior to participation. Twenty four healthy, non-smoking Caucasian volunteers participated in the study (12 men and 12 women, mean (±standard deviation [SD]) age 24.1 (±2.30) years, and mean (±SD) body mass index 22.6 (±2.98) kg m−2).Before inclusion, the subjects underwent a screening evaluation regarding their medical history. Participants adhered to their usual diet, but they received a vitamin supplement with 800 μg of pteroylmonoglutamic acid for 14 days before the first testing and between the testings, which was discontinued two days prior to the start of the study. This “saturation” was done to improve uniformity among subjects and subsequently the precision of bioavailability estimates.24,25
The study had a single-centre, randomised, four-treatment, four-period, cross-over design. There were four treatment sequences in accordance with the Latin square, so that each experimental treatment occurred only once within each sequence and once within each period. Each subject had the following four experimental treatments separated by a 14-days equilibrium phase: 1294 nmol sum of folates via Camembert cheese (200 g), 534 nmol sum of folates via wheat germs (50 g), 1185 nmol sum of folates via heated spinach (500 g), and 852 nmol pteroylmonoglutamic acid via orally administered pteroylmonoglutamic acid solution (95 mL) serving as reference treatment. The order in which the treatments were given was randomised.
Between 8:00 and 9:00 a.m., after an overnight fast, volunteers took the test meals or drank the test solution, respectively, together with one slice of toast bread. During the experimental treatment periods (24 hours), the consumption of water was allowed ad libitum, and two further standardised and virtually folate-free meals consisting of wheat bread (9 slices per 500 g), butter (100 g), honey (250 g), apple sauce (355 g), and apricot jam (225 g) were offered for lunch and dinner. All food items were common brands and purchased at local supermarkets in the City of Jena, Germany.
Between the test periods, i.e., during the equilibrium phase, the participants were instructed to take the folate supplementation as mentioned above while keeping their normal dietary habits unchanged. For the determination of the biokinetic profile of 5-CH3-H4folate in plasma, venous blood samples were drawn predose, as well as 1, 2, 3, 4, 5, 6, 8, 10, 12 and 24 hours after the administration of the dose. Each blood sample (9 mL) was collected in an EDTA-coated tube (Sarstedt, Nuernbrecht, Germany). Plasma and red blood cells were obtained by centrifuging the blood for 10 min at 2000g and 4 °C. In addition, the volunteers collected the complete postdose urine for 24 h into 2 L opaque brown urine containers which were stored refrigerated during the collection periods. Plasma, red blood cells and urine samples were stored frozen at −24 °C until further preparation and analysis.
Statistics and biokinetic calculations
Concentrations of 5-CH3-H4folate in urine and plasma were evaluated. The individual pre-dose 5-CH3-H4folate plasma concentration of each treatment day was used as a baseline for the calculation of the AUC. To avoid negative AUC values, which can result in some cases if plasma 5-CH3-H4folate concentration falls below baseline, the positive AUC was used, i.e., all values dropping below the individual pre-dose level were discarded.Standard noncompartmental methods were applied to determine the biokinetic parameters:26Cmax (observed maximum concentration), tmax (time of Cmax), AUC from baseline corrected 5-CH3-H4folate concentrations (limited within the interval from 0 to 12 h). The range of biokinetic evaluation was limited to 12 hours postdose, because it became obvious during data review that 5-CH3-H4folate concentrations increased from 12 to 24 h post-dose (see also Fig. 1). The AUC was calculated according to the linear trapezoidal rule. The amount of 5-CH3-H4folate excreted into urine from time zero up to 24 h (Ae0–24) was determined by multiplying the 5-CH3-H4folate concentration with the volume of the 24 h urine sample. The fraction of orally administered folate excreted into urine (‘%Excretion’) was calculated by dividing Ae0–24 through the respective dose administered. Concentrations below the limit of quantification (LOQ) were set to zero.
The primary biokinetic parameters for inferential statistics were Cmax and AUC after logarithmic data transformation. Prior to logarithmic transformation, the Cmax and AUC values were normalised to dose (i.e., assuming dose-proportionality) since no equimolar doses were administered. The data were analyzed with a linear mixed effects model with fixed terms for treatment, period, sequence and sex, and random term for subject within sequence-by-sex:
| Log(Parameter) = Sequence + Subject(Sequence × Sex) + Period + Treatment + Sex + Error, |
For Cmax and AUC, estimate and 90% confidence interval (CI) for the ratio of treatment means (test/reference) were obtained by computing estimate and 90% CI for the contrast giving the difference between treatment means within the linear mixed effects model framework, and then converting to ratio of geometric means by the antilog transformation. Equivalence was concluded if the 90% CI for the ratio was entirely within the 0.80 to 1.25 equivalence reference interval.
The secondary PK parameter was ‘%Excretion’. It was subjected to the same linear mixed effects model analysis as the primary PK parameters.
The level of statistical significance was fixed at p < 0.05. No adjustment of the alpha-level was made for multiple analyses.
Results
Due to their high folate content, spinach, low-fat Camembert cheese and wheat germs were chosen as the test foods.27 Total folate content and vitamer distribution was determined by stable isotope dilution assays the results of which confirmed the high folate content of the test foods and the principal vitamer distribution of spinach as the only food with literature data available (Table 1).21,28| Food | Tetrahydrofolate (μg per 100 g) | 5-Methyl-tetrahydrofolate (μg per 100 g) | 5-Formyl-tetrahydrofolate (μg per 100 g) | 10-Formylfolate (μg per 100 g) | Pteroylmonoglutamic acid (μg per 100 g) | Sum of folates (nmol per 100 g) |
|---|---|---|---|---|---|---|
| a n.d. not detectable. | ||||||
| Spinach | 10.3 | 72.2 | 6.6 | 15.6 | n.d. | 237 |
| Camembert cheese | 144.7 | 46.2 | 54.5 | 40.2 | n.d. | 647 |
| Wheat germs | 36.0 | 30.6 | 313.6 | 65.2 | 25.8 | 1068 |
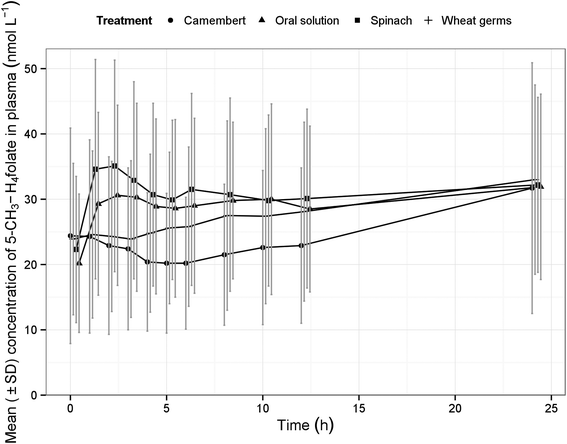
After administration of the test foods, the mean plasma concentrations of 5-CH3-H4folate were determined as displayed in Fig. 1. It is worth noting that the widely varying, mean plasma concentrations partially rose again after 4–6 h post-dose. However, this increase is not attributable to the intermediate consumption of the low-folate lunch, which was provided after the 4 hour blood sample was drawn, as it contained less than 5% of the folate dosage of the treatments. The low-folate dinner was provided after the 10 hour blood sample has been drawn. Apart from 5-CH3-H4folate, further folate vitamers in plasma were not considered as they occurred only intermittent in traces and always below their LOQ.
Table 2 summarises the biokinetic parameters of baseline corrected 5-CH3-H4folate. The variability of AUC and Cmax was moderate for spinach and oral solution of pteroylmonoglutamic acid (CV% 30–60%), but high for Camembert cheese (CV% 60–90%) and very high for wheat germ (CV% >90%). The time to attain the maximum concentrations (tmax) was highly variable among treatments. However, the median of tmax was lower by trend for spinach than for the pteroylmonoglutamic acid solution. This finding is in line with recent findings from a double-label ileostomy study which showed a lower tmax for labelled 5-CH3-H4folate than for labelled pteroylmonoglutamic acid.29
| Parameterb | Camembert | Wheat germs | Spinach | Oral solution |
|---|---|---|---|---|
| a Tabulated values are arithmetic mean ± SD (CV%) [geometric mean] of n = 24 subjects except for tmax where values are median (min, max). b AUC, Cmax and tmax were determined/calculated within the 0 to 12 hour interval. c AUC is the positive AUC, i.e., concentrations falling below the individual predose values were discarded. | ||||
| C max (nmol L−1) | 4.47 ± 3.93 (88) [3.52] | 7.94 ± 8.04 (101) [4.45] | 17.7 ± 9.78 (55) [14.9] | 15.1 ± 6.82 (45) [13.5] |
| t max (h) | 5.00 (1.00, 12.03) | 10.00 (1.00, 12.02) | 3.00 (1.00, 12.00) | 4.52 (0.98, 12.15) |
| AUC (nmol h L−1)c | 20.6 ± 16.3 (79) [12.5] | 43.8 ± 51.5 (118) [19.3] | 123 ± 66.3 (54) [96.3] | 113 ± 55.2 (49) [93.8] |
The results of the linear mixed effects model analysis on AUC and Cmax of baseline corrected 5-CH3-H4folate (ratio estimates [geometric mean] with 90% confidence limits) are summarised in Table 3. Camembert and wheat germs were not bioequivalent to pteroylmonoglutamic acid solution in terms of dose-normalised Cmax and AUC of 5-CH3-H4folate, since the treatment ratio estimate and 90% CI were outside the predefined 80–125% acceptance range (Table 3). Spinach was also not bioequivalent since the treatment ratio estimate and 90% CI of dose-normalised Cmax and AUC of 5-CH3-H4folate did not fall completely within the predefined 80–125% acceptance range (Table 3). The statistical tests on model effects revealed that the model treatment effect was proven as statistically significant for AUC (p < 0.001) and Cmax (p < 0.001), whereas sex, sequence and period effects were not. Regardless of the statistic evaluation it is worth comparing the ratio estimates given in Table 3 for the different test foods. From both AUC and Cmax the highest bioavailability is indicated for spinach, which appears higher than that from wheat germs and lowest bioavailability from Camembert cheese can be assumed.
| Dependent | Reference | Test | Lower 90% CI | Ratio [%Ref] | Upper 90% CI |
|---|---|---|---|---|---|
| ln(AUC) | Oral solution | Camembert | 5.1 | 8.8 | 15.4 |
| Oral solution | Wheat germs | 19.5 | 33.0 | 56.0 | |
| Oral solution | Spinach | 43.4 | 73.0 | 122.9 | |
| ln(Cmax) | Oral solution | Camembert | 11.9 | 17.4 | 25.5 |
| Oral solution | Wheat germs | 36.3 | 52.1 | 74.8 | |
| Oral solution | Spinach | 55.7 | 79.6 | 113.7 |
The percentage of 5-CH3-H4folate excreted into urine relative to the respective dosage (‘%Excretion’) was 1.7%, 2.7%, 6.8% and 21.8% after administration of Camembert, wheat germs, spinach and pteroylmonoglutamic acid solution, respectively, with variabilities (%CV) between 50 and 108%. The ‘%Excretion’ was subjected to the linear mixed effects model analysis as secondary analysis. As the point estimate (geometric mean treatment ratio [% of Reference] of ‘%Excretion’) and the 90% confidence intervals fell outside the prespecified 80–125% acceptance interval, average bioinequivalence of folate originating from Camembert, wheat germs and spinach vs. oral pteroylmonoglutamic acid solution was proven for the urine data (results no shown). The model treatment effect was proven as statistically significant. There was also a significant period effect of unknown cause, but no sequence and sex effect. Thus, the analysis of the urine data – except for spinach – point in the same direction as the corresponding plasma data.
In the context of this study the individual predose concentration of 5-CH3-H4folate was defined as baseline. To prove absence of a diurnal rhythm in plasma folate levels, which would have led possibly to a more complicated correction of the baseline, the folate levels of an additional subject were analyzed over 24 hours under a virtually folate-free diet. The provided food (for the virtually folate-free diet) as well as all other standardised conditions such as facility, medical personnel, blood and urine collection procedure were identical to that of the actual study days. Since there was little variation in the subject's folate levels, the concentrations varied between 13.8 and 17.3 nmol L−1 during 24 hours, the baseline correction procedure used in this study seemed to be justified.
Discussion
An increase in plasma folate concentration at late sampling times after folate dosage normally is not observed in pharmacokinetic investigations.30,31 However, it is known that the individual folate levels also depend on the time of the last food intake. Earlier studies performed by Pietrzik et al. found that plasma concentrations increase to a multiple of the initial value under fasting conditions.30 The latter authors attributed this increase to the suppression of bile production and excretion as they also analysed serum bilirubin, which is an indicator of hepatic excretion and which showed exact the same behaviour. It remains unclear whether the volunteers were in part fasting and, therefore, showed the unexpected pharmacokinetics. In order to exclude these obvious effects at the last sampling time of 24 h, the kinetic evaluation was limited to the range from 0 to 12 h.In contrast to the original plans for the study protocol no equimolar doses of folate/pteroylmonoglutamic acid were administered to the subjects. This was compensated by dividing the plasma values by the dose. From a similar study on the bioavailability of spinach folates14 it can be deduced that in the chosen dose range no deviation from dose-proportionality should occur, so that this approach is certainly justified. Comparison of the dose-normalised AUC between test (food folate) and ‘reference’ pteroylmonoglutamic acid has been accepted as a valuable indicator of absorption, provided the post-dosing plasma measurement test period is long enough to capture ≥80% of the whole AUC (extrapolated to infinity). In the majority of cases, the determination of the whole AUC was not possible due to increasing 5-CH3-H4folate concentrations toward the end of the study (Fig. 1). Therefore, the terminal elimination phase could not be determined reliably in this study, which, however, would have been needed for a correct biokinetic evaluation. The range for AUC determination was limited to 12 hours post-dose to cover at least the initial absorption and metabolism of pteroylmonoglutamic acid. In consideration of this, it remains open whether the use of the urine data had some advantage over plasma data, since the absorption phase was satisfactorily covered. It was also shown that estimates of ‘%Excretion’ were subject to a high degree of variability, and cannot be taken as more reliable than those obtained from plasma concentration-time profiles.32 Thus, urinary excretion is not recommended as a substitute for blood concentration data; rather, these studies should be used in conjunction with blood level data for confirmatory purposes.33
Bioavailabilities of folates from the foods used in this study could not be calculated when using the model applied as the kinetics of plasma 5-methyltetrahydrofolic acid response to food folates is different to that from pteroylmonoglutamic acid as shown by Wright et al.34 and recently by a dual-label ileostomy model.29 However, relative bioavailabilities can be estimated from the model presented if the following four conditions are fulfilled: (1) that physiological doses of folates and pteroylmonoglutamic acid are initially reduced and then methylated in the intestine and the liver and that essentially only 5-methyltetrahydrofolic acid appears thereafter in circulation, as it is the case for absorbed physiological doses of all naturally-occurring reduced folates; (2) that plasma 5-methyltetrahydrofolic acid response derives entirely from (biotransformed) newly absorbed folate;35 (3) generally, dose-proportionality of absorption existed in the dose-range investigated; and (4) saturation of the subjects with pteroylmonoglutamic acid does not significantly alter folate absorption. Under these assumptions it can be stated that the relative bioavailability of folate from spinach was higher than that from wheat germs and Camembert cheese. It is further assumed that through the chosen study design (randomised cross-over) any systematic errors are reduced as far as possible. Nevertheless, due to the limitations outlined above the results of this study should be interpreted with due caution.
Conclusions
The results presented underline the dependence of folate bioavailability on the type of food ingested. Therefore, the general assumption of 50% bioavailability as the rationale behind the definition of folate equivalents has to be questioned and requires further investigation.36 Moreover, the high individual variation in response to folate intake has to be underlined and is one cause for complexity and expense of human studies. Although we have no further evidence, more detailed screening for genetic mutations of e.g. methylene tetrahydrofolate reductase (MTHFR), dihydrofolate reductase, reduced folate carrier (RFC) as well as for status of vitamin B12 and stratification of the subjects in subsequent studies could alleviate this problem. As remarked above, conclusions of the study presented here are restricted as folate saturation was applied and calculation was hampered by the 5-CH3-H4folate baseline levels of our subjects. The reasons for the differences of folate bioavailability are not clear yet. Several hypotheses such as (a) different kinetics and bioavailabilities of the folate vitamers and particularly of the polyglutamate forms, (b) presence of deconjugase inhibitors and (c) entrapping of folates in the food matrix have been proposed. The use of new models such as a dual ileostomy model may circumvent the model restrictions mentioned above.29 However, the latter study was limited to folates added to foods as monoglutamates and it is known that absorption of the abundant polyglutamic forms in foods is also dependent on deconjugase activity in the jejunal mucosa. Therefore, bioavailability studies require further improvement in the future to adjust more detailed and substantiated recommendations for dietary folate intake.Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (RY, 19/7-1; NE 1188/1-1).Notes and references
- J. Selhub Folate, J. Nutr., Health Aging, 2002, 6, 39–42 Search PubMed.
- A. F. Czeizal and J. Dudas, N. Engl. J. Med., 1992, 327, 1832–1835 CrossRef PubMed.
- M. Caudill, J. Food Sci., 2003, 69, SNQ55–SNQ59 Search PubMed.
- D. A. Snowdon, C. L. Tully, C. D. Smith, K. P. Riley and W. R. Markesbery, Am. J. Clin. Nutr., 2000, 71, 993–998 CAS.
- K. Robinson, Heart, 2000, 83, 127–130 CrossRef CAS.
- Ph. De Wals, F. Tairou, M. I. Van Allen, S.-O. Uh, R. B. Lowry, B. Sibbald, J. A. Evans, M. C. Van den Hof, P. Zimmer, M. Crowley, B. Fernandez, N. S. Lee and T. Niyonsenga, N. Engl. J. Med., 2007, 357, 135–142 CrossRef CAS PubMed.
- J. B. Mason, A. Dickstein, P. F. Jacques, P. Haggarty, J. Selhub, G. Dallal and I. H. Rosenberg, Cancer Epidemiol. Biomarkers Prev., 2007, 16(7), 1325–1329 CAS.
- S. E. Vollset, R. Clarke, S. Lewington, M. Ebbing, J. Halsey and E. Lonn, et al. , Lancet, 2013, 381(9871), 1029–1036 CrossRef CAS.
- B. F. Cole, J. A. Baron and R. S. Sandler, et al., Folic Acid for the Prevention of Colorectal Adenomas: A Randomized Clinical Trial, J. Am. Med. Assoc., 2007, 297(21), 2351–2359 CrossRef CAS PubMed.
- F. Coppede, Cancer Lett., 2014, 342, 238–247 CrossRef CAS PubMed.
- G. M. Lindzon, A. Medline, K.-J. Sohn, F. Depeint, R. Croxford and Y.-I. Kim, Carcinogenesis, 2009, 30(9), 1536–1543 CrossRef CAS PubMed.
- A. M. Troen, B. Mitchell and B. Sorensen, et al. , J. Nutr., 2006, 136, 189–194 CAS.
- H. E. Sauberlich, M. J. Kretsch, J. H. Skala, H. L. Johnson and P. C. Taylor, Am. J. Clin. Nutr., 1987, 46, 1016–1028 CAS.
- R. Prinz-Langenohl, A. Bronstrup, B. Thorand, M. Hages and K. Pietrzik, J. Nutr., 1999, 129, 13–916 Search PubMed.
- I. A. Brouwer, M. Van Dusseldorp, C. E. West, S. Meyboom, C. M. G. Thomas, M. Duran, K. H. Van Het Hof, T. K. A. B. Eskes, J. G. A. J. Hautvast and R. P. M. Steegers-Theunissen, J. Nutr., 1999, 129, 1135–1139 CAS.
- M. Lucock, Z. Yates, L. Boyd, C. Naylor, J.-H. Choi, X. Ng, V. Skinner, R. Wai, R. Kho, S. Tang, P. Roach and M. Veysey, Eur. J. Nutr., 2013, 52, 569–582 CrossRef CAS PubMed.
- C. M. Witthöft, L. Strålsjö, G. Berglund and E. Lundin, Scand. J. Nutr., 2003, 47, 6–18 CrossRef.
- M. Rychlik, M. Netzel, I. Pfannebecker, T. Frank and I. Bitsch, J. Chromatogr., B: Biomed. Appl., 2003, 792, 167–176 CrossRef CAS.
- S. Mönch, M. Netzel, G. Netzel and M. Rychlik, Anal. Biochem., 2010, 398, 150–160 CrossRef PubMed.
- C. M. Pfeiffer, Z. Fazili, L. McCoy, M. Zhang and E. W. Gunter, Clin. Chem., 2004, 50, 423–432 CAS.
- A. Freisleben, P. Schieberle and M. Rychlik, J. Agric. Food Chem., 2002, 50, 4760–4768 CrossRef CAS PubMed.
- S. Mönch and M. Rychlik, J. Agric. Food Chem., 2012, 60, 1363–1372 CrossRef PubMed.
- C. Ringling and M. Rychlik, J. Agric. Food Chem., 2013, 236, 17–28 CAS.
- J. F. Gregory, J. Nutr., 2001, 131, 1376S–1382S CAS.
- T. Tamura and E. L. Stokstad, Br. J. Haematol., 1973, 25, 513–532 CrossRef CAS PubMed.
- W. Cawello, Parameters for compartment-free pharmacokinetics, Shaker, Aachen, 1999, pp. 20–25 Search PubMed.
- M. Rychlik, J. Food Compos. Anal., 2004, 17, 475–483 CrossRef CAS PubMed.
- G.-F. Zhang, S. Storozhenko, D. Van Der Straeten and W. E. Lambert, J. Chromatogr., A, 2005, 1078, 59–66 CrossRef CAS PubMed.
- V. Oehrvik, B. Buettner, M. Rychlik, E. Lundin and C. M. Witthoeft, Am. J. Clin. Nutr., 2010, 92, 532–538 CrossRef CAS PubMed.
- K. Pietrzik, M. Hages and T. Remer, J. Micronutr. Anal., 1990, 7, 207–222 CAS.
- R. Prinz-Langenohl, S. Brämswig, O. Tobolski, Y. M. Smulders, D. E. C. Smith, P. M. Finglas and K. Pietrzik, Br. J. Pharmacol., 2009, 158, 2014–2021 CrossRef CAS PubMed.
- R. E. Notari, Biopharmaceutics and Clinical Pharmacokinetics, Dekker M Inc, New York, 1987, p. 189 Search PubMed.
- R. Chereson, in Basic Pharmacokinetics, ed. M. C. Makoid, P. Vuchetich and U. V. Banakar, The Virtual University Press, Omaha, 1996, pp. 1–117 Search PubMed.
- A. Wright, P. M. Finglas, J. R. Dainty, C. Wolfe, D. J. Hart, D. M. Wright and J. F. Gregory, J. Nutr., 2005, 135, 619–623 CAS.
- A. J. A. Wright, J. R. Dainty and P. M. Finglas, Br. J. Nutr., 2007, 98, 667–675 CrossRef CAS PubMed.
- L. B. Bailey, Nutr. Rev., 1998, 56, 294–299 CrossRef CAS PubMed.
Footnotes |
| † Current address: BIOANALYTIK Weihenstephan, Research Center for Nutrition and Food Sciences, Technische Universität München, Alte Akademie 10, D-85350 Freising, Germany. |
| ‡ Current address: Centre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation (QAAFI), The University of Queensland, 39 Kessels Road, Coopers Plains, QLD 4108, Australia. |
| § Current address: ARC Centre of Excellence in Plant Cell Walls, Centre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation (QAAFI), The University of Queensland, St. Lucia, QLD 4072, Australia. |
| This journal is © The Royal Society of Chemistry 2015 |