Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: influence of water quality parameters†
Amisha D.
Shah‡
a,
Zheng-Qian
Liu
ab,
Elisabeth
Salhi
a,
Thomas
Höfer
c,
Barbara
Werschkun
d and
Urs
von Gunten
*ae
aEawag, Swiss Federal Institute of Aquatic Science and Technology, Überlandstrasse 133, CH-8600 Dübendorf, Switzerland. E-mail: vongunten@eawag.ch; Fax: +41 58 765 5210; Tel: +41 58 765 5270
bSchool of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
cDepartment Exposition, Federal Institute for Risk Assessment, Max-Dohrn-Strasse 8-10, D-10589 Berlin, Germany
dWissenschaftsbüro, Naunynstrasse 30, D-10997 Berlin, Germany
eSchool of Architecture, Civil and Environmental Engineering (ENAC), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
First published on 30th April 2015
Abstract
As ocean-going ships begin implementing chemical disinfection to treat ballast water, the potential formation of disinfection by-products (DBPs) is an important issue of concern. This is especially critical since ballast waters are often saline, and the information regarding DBP formation under these conditions is limited. This study exposed representative ballast waters (synthetically-made and natural freshwaters, brackish waters, and seawaters) to ozone, free chlorine, and peracetic acid (PAA) treatment where various water quality parameters and treatment conditions were varied to assess DBP formation. DBPs including bromate, trihalomethanes (THMs), and haloacetic acids (HAAs) were affected by changes in salinity (especially bromide concentration), dissolved organic matter (DOM) concentration/type, and oxidant type/dose. Temperature effects (22 ± 2 °C or 4 ± 2 °C) were limited for THMs and HAAs formation but were greater for bromate formation (only ozone) in waters containing high bromide levels. Interestingly, bromate formation during ozonation was rapid (complete formation in <15 min) but not linearly proportional to the bromide concentration when its molar concentration exceeded the molar ozone dose. Its formation was well predicted using a simplified kinetic model, previously applied to freshwaters, incorporating known reactions of ozone with bromide to form bromate. Kinetic studies indicated that increased bromide concentrations in brackish waters/seawaters led to higher Br-DBPs formation (brominated THMs and HAAs) for all oxidants tested. These included bromoform (CHBr3), dibromoacetic acid (DBAA), and tribromoacetic acid (TBAA), which formed for all three oxidants but also dibromochloromethane (CHBr2Cl) and bromoacetic acid (MBAA) which formed during chlorination and PAA treatment or for only PAA treatment, respectively. Approximately 50% formation of the DBPs occurred within 24 h of the typical 5 day ballast water treatment holding time. The oxidant dosage experiments, when normalized to the DOM concentration, indicated that for the brackish waters/seawaters, formation typically overlapped each other and that outliers were driven by the specific UV absorbance (SUVA) values. In addition, formation of CHBr3 and TBAA was found to correlate well with each other for all three oxidants, indicating that they are derived from similar DOM precursors while CHBr3 and TBAA correlated to DBAA formation to a lesser degree, similar to observations found in chlorinated freshwaters.
Water impactAs ocean-going ships begin to apply chemical disinfectants to treat ballast waters before their discharge into the surrounding waters, this study aimed to evaluate the extent of disinfection by-product (DBP) formation. Key water quality parameters and treatment conditions driving DBP formation in such waters were ascertained given that previous land-based testing rendered varied results. This study identified several important parameters (e.g. salinity, DOM type/concentration, and oxidant type/dose) that affected DBP formation. Brominated DBPs dominated in high bromide-containing waters and the effects of temperature were limited. Overall, these results help target the most important factors involved, which can then be used for predictive purposes, so that ocean-going ships can minimize DBP formation during ballast water treatment. |
Introduction
Over the last 40 years, the formation of disinfection by-products (DBPs) in drinking water and wastewater has been investigated, beginning with the discovery of trihalomethane formation in the 1970s.1 Since this time, more than 600 individual DBPs have been identified,2 many of which are halogenated due to the prolific use of disinfectants such as free chlorine, which is well known to halogenate organic water matrix moieties. Research has also shown that the type of halogenated products can be significantly influenced by the presence of other halide ions such as bromide and iodide in the water matrix. These halides can react with free chlorine to form secondary oxidants such as HOBr and HOI, which can then in turn react with various organic moieties to form brominated and iodinated DBPs.3,4 For non-impaired freshwater resources, the bromide and iodide concentrations are typically low ([Br−]mean = 100 μg L−1 (ref. 5); [I−] = 0.5–10 μg L−1 (ref. 6)) leading to relatively low concentrations of brominated and iodinated DBPs. However, these values must be weighted against the fact that brominated and iodinated DBPs have been shown to be significantly more cytotoxic and genotoxic than their chlorinated analogues.2This information is especially important given that chemical disinfectants are also used to treat saline waters such as seawater used for swimming pools7,8 and for desalination,9,10 cooling waters used in coastal power plants11,12 and salt-impacted freshwaters.13,14 Numerous studies have found that chlorination of these waters can lead to the formation of various DBPs such as trihalomethanes (THMs), haloacetic acids (HAAs), halophenols, and nitrogen-based halogenated DBPs.7–17
In recent years, the disinfection of ballast waters on board ocean-going ships has emerged as an important application where saline waters are disinfected. Disinfection of such waters is being considered since it has been well known for decades that ballast waters can actively transport invasive species which can cause human and ecological harm.18 To resolve this problem, the “International Convention for the Control and Management of Ships' Ballast Water and Sediments (BWM Convention)” was adopted in 2004 to initiate the formation of regulatory and technical guidelines in which chemical disinfectants could be applied to treat these waters.18 As of May 2013, the International Maritime Organization's Marine Environment Protection Committee (MEPC) has approved 31 ballast water management systems (BWMS) that use active substances (most of which are chemical disinfectants) within their treatment protocol.18 These systems include the application of chlorine, ozone, peracetic acid (PAA), and chlorine dioxide, and it is this order (from the former to the later) in which they are also predominantly used.18 Such BWMSs have also reported the effects of disinfectant application in marine waters through land-based testing to assess DBP formation. These tests indicate that for several BWMSs using chlorination and ozonation, brominated DBPs and bromate are formed at levels that exceed by far those typically observed in freshwater treatment scenarios.19
To better evaluate DBP formation in saline waters, it is important to understand that several key differences exist between seawaters and freshwater with regards to their chemical makeup. First, salinities differ significantly such that in freshwaters, the mean chloride and bromide concentrations are 10.6 mg L−1 (300 μM)20 and 100 μg L−1 (1.3 μM; see above), respectively, and for iodide, the concentrations range from 0.5–10 μg L−1 (0.004–0.08 μM)6 while seawaters contain mean values of 19.2 g L−1 (0.540 M) chloride, 67 mg L−1 (840 μM) bromide, and 60 μg L−1 (0.5 μM) iodide.21 Thus, the halide concentrations in seawater are factors of 1800, 672, and 6–120× higher for chloride, bromide, and iodide in seawaters than in freshwaters, respectively. Additionally, the concentration and chemical composition of the dissolved organic matter (DOM), measured as dissolved organic carbon (DOC), varies considerably between these two water types. For seawaters, the DOC concentration is typically low ([DOC] = 0.4–1 mg L−1 C in surface and deep ocean waters22), whereas fresh surface waters typically contain 1–20 mg L−1 of DOC.23 Seawater DOM has also been characterized to contain a significant fraction of aliphatic moieties such as neutral sugars, amino sugars, and amino acids although greater than 50% of it still remains uncharacterized.22 Alternatively, freshwater DOM typically contains higher fractions of chemical constituents that contain phenolic-type moieties (e.g. humic acids), which are well known precursors for THM formation.24,25 Ballast waters are typically a mixture of these water types since they are frequently taken up in estuaries, coastal areas or industrial ports that experience significant terrestrial and anthropogenic impacts on the marine water body.
With this in mind, the objectives of this study were to better understand how such chemical differences in seawaters and brackish waters as compared to freshwaters could affect DBP formation under typical ballast water treatment conditions. Synthetic waters along with nine natural waters were selected (freshwaters, brackish waters, and seawaters) and treated with chlorine, ozone, and PAA. Within this experimental matrix, a series of kinetic and dosage experiments was performed to investigate bromate, THMs, and HAAs formation under varying water quality/treatment conditions such as salinity, DOC concentration/type, and temperature (22 °C and 4 °C). Iodinated DBPs (I-DBPs) (e.g. iodate and iodinated THMs and HAAs) were not monitored in these experiments due to analytical constraints especially in the high salinity brackish waters and seawaters. Additional parameters such as HOBr were monitored since HOBr is known to be the dominant secondary oxidant formed during ozonation and chlorination of bromide-containing waters.26 Overall, these tests were designed to determine which water quality parameters or treatment conditions drive DBP formation in saline waters.
2. Experimental methods
2.1 Standards and reagents
The standards and reagents used included 2,6-dichlorophenol (2,6-DCP), dichloroacetic acid-2-13C (13CHCl2COOH; DCAA-13C), bromoacetic acid-1-13C (CH2Br13COOH; MBAA-13C), 2-bromobutyric acid (2-BrBu), sodium sulfite, 2-Cl phenol, and trans-cinnamic acid, which were purchased from Sigma-Aldrich and 4-Br-2,6-dichlorophenol (4-Br-2,6-DCP), which was purchased from Fluorochem (Derbyshire, UK). Ascorbic acid was purchased from Merck. Phenol and 4-Cl phenol were purchased from Fluka. Sodium hypochlorite (NaOCl; free chlorine; 6–14%; reagent grade) and PAA (32 wt.%; <6% H2O2; 40–45% acetic acid; [PAA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [H2O2]0 (M/M) = 1
[H2O2]0 (M/M) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3–0.3827) were purchased from Sigma-Aldrich. The THM analytical standard mix was purchased from Supelco and contained chloroform (CHCl3), bromodichloromethane (CHBrCl2), dibromochloromethane (CHBr2Cl), and bromoform (CHBr3). The EPA 552.2 HAA analytical standard mix was also purchased from Supelco and contained bromoacetic acid (MBAA), bromochloroacetic acid (BCAA), bromodichloroacetic acid (BDCAA), chloroacetic acid (MCAA), dibromochloroacetic acid (DBCAA), dibromoacetic acid (DBAA), dichloroacetic acid (DCAA), tribromoacetic acid (TBAA), and trichloroacetic acid (TCAA). These and other chemicals such as NaH2PO4, Na2HPO4, NaOH, H2SO4, MeOH, NaHCO3, and H3BO3 were of reagent grade or higher. Reagent water (18.2 MΩ cm) was generated from a Barnstead™ Nanopure™ (Thermo Scientific) water purification system.
0.3–0.3827) were purchased from Sigma-Aldrich. The THM analytical standard mix was purchased from Supelco and contained chloroform (CHCl3), bromodichloromethane (CHBrCl2), dibromochloromethane (CHBr2Cl), and bromoform (CHBr3). The EPA 552.2 HAA analytical standard mix was also purchased from Supelco and contained bromoacetic acid (MBAA), bromochloroacetic acid (BCAA), bromodichloroacetic acid (BDCAA), chloroacetic acid (MCAA), dibromochloroacetic acid (DBCAA), dibromoacetic acid (DBAA), dichloroacetic acid (DCAA), tribromoacetic acid (TBAA), and trichloroacetic acid (TCAA). These and other chemicals such as NaH2PO4, Na2HPO4, NaOH, H2SO4, MeOH, NaHCO3, and H3BO3 were of reagent grade or higher. Reagent water (18.2 MΩ cm) was generated from a Barnstead™ Nanopure™ (Thermo Scientific) water purification system.
2.2 Water samples
Samples were collected from nine water sources that range in salinity from freshwater (F) to brackish water (B) and to seawater (S). These waters were either collected from a river source, its corresponding estuary, or from the open ocean off the coast of The Netherlands (NE), Norway (NOR), and Germany (DE) (for locations see Fig. S1, (ESI†) and GPS coordinates in Table S1 (ESI†)). Three waters of varying salinity were taken from each country and labeled as follows according to the parameters above: (i) NE-F, (ii) NE-B, (iii) NE-S, (iv) NOR-F, (v) NOR-B, (vi) NOR-S, (vii) DE-B1 (first German brackish water), (viii) DE-B2 (second German brackish water), and (ix) DE-S (Table S1, ESI†). The raw waters were collected and shipped to Dübendorf, Switzerland, where they were filtered through pre-combusted 0.7 μM nominal pore size glass-fiber filters and held at 4 °C until use. The water quality parameters of these nine waters including DOC concentration, SUVA, pH, alkalinity, ammonia concentration and salinity (chloride and bromide) are presented in Table S1 (ESI†).2.3 Experimental setup
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [H2O2]0 (M/M) = 1
[H2O2]0 (M/M) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3–0.38, as stated above). The PAA stock concentration was quantified by quenching the diluted stock with excess iodide to form triiodide, which was then measured spectrophotometrically at 352 nm (ε = 2
0.3–0.38, as stated above). The PAA stock concentration was quantified by quenching the diluted stock with excess iodide to form triiodide, which was then measured spectrophotometrically at 352 nm (ε = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5433 M−1 cm−1) and done so immediately (<1 min) to avoid interferences from H2O2.31
5433 M−1 cm−1) and done so immediately (<1 min) to avoid interferences from H2O2.31
Additional parameters were also monitored over these time periods including the primary oxidant residual concentration and secondary oxidant formation (i.e. HOBr). For further description of the corresponding methods see section 2.4 analytical methods. For the ozonation experiments, the residual ozone concentration was only measured for the short-term experiments (i.e. bromate measurement over 15 min) whereas HOBr was monitored for all experiments. For the free chlorine experiments, samples were similarly quenched to measure HOBr but also included measurement of the free chlorine residual concentration. For the PAA experiments, neither the PAA residual concentration nor HOBr were measured due to experimental complications with the HOBr quenching agent, 2,6-DCP, which precipitated over these extended reaction times. Table S2 (ESI†) lists a summary of the specific DBPs and parameters monitored for each type of kinetic experiment. No kinetic experiments were conducted for the DE waters. Kinetic model calculations were performed to predict/understand the formation of bromate and HOBr during ozonation over 12 min for the synthetic water experiments containing bromide (1.25–840 μM) at pH 8 using the Kintecus® software.32
2.4 Analytical methods
THMs were measured by gas chromatography with electron capture detection (GC-ECD) with headspace injection (GC-8000 Fisons). Samples (5 mL) were filled in 10 mL headspace vials and heated/shaken for 30 min at 80 °C. Gas phase samples (250–1000 μL) were then injected with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 to 1
50 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 split ratio onto a Rtx-624 (30 m × 0.32 mm × 1.8 μm) column. The injection port temperature was held at 180 °C. The column oven was held at 40 °C for 5 min, ramped to 240 °C at 5 °C min−1 and then held for 5 min. The method detection limits (MDLs) for CHCl3, CHBrCl2, CHBr2Cl, and CHBr3 were 3.9 ± 1.2 μg L−1, 6.1 ± 1.9 μg L−1, 3.4 ± 1.1 μg L−1, and 5.8 ± 1.8 μg L−1, respectively.
100 split ratio onto a Rtx-624 (30 m × 0.32 mm × 1.8 μm) column. The injection port temperature was held at 180 °C. The column oven was held at 40 °C for 5 min, ramped to 240 °C at 5 °C min−1 and then held for 5 min. The method detection limits (MDLs) for CHCl3, CHBrCl2, CHBr2Cl, and CHBr3 were 3.9 ± 1.2 μg L−1, 6.1 ± 1.9 μg L−1, 3.4 ± 1.1 μg L−1, and 5.8 ± 1.8 μg L−1, respectively.
HAAs were measured by capillary ion chromatography (Thermo Dionex ICS-4000) with tandem mass spectrometry (Thermo TSQ-Vantage) (IC-MS/MS) following a modified approach from the EPA 557 method.36 The capillary IC was run using an AS 24A column at a 12 μL min−1 flow rate using eluent run in gradient mode from 7 to 65 mM KOH. The tandem MS analysis was conducted using electrospray ionization in the negative mode with a HESI vaporizer temperature of 150 °C, sheath gas pressure of 25 (unitless), transfer capillary temperature of 250 °C, and spray voltage of 2250 V. The parent and product masses, collision energies, and S-lens RF amplitude values for the HAAs and internal standards are listed in Table S4 (ESI†). The method detection limit (MDL) for standards in nanopure water were 0.1–0.2 μg L−1 (MCAA; MBAA; DCAA; BCAA; TCAA, and TBAA) and 0.02–0.05 μg L−1 (DBAA; DCBAA; DBCAA). However, the MDL values increased due to the dilution of sample waters. Freshwaters, brackish waters, and seawaters were diluted by 10, 100, and 200 times leading to MDLs of 1–2, 10–20 μg L−1, and 20–40 μg L−1 for DCAA, BCAA, TCAA, and TBAA and 0.2–0.5, 2–5, and 4–10 μg L−1 for MCAA, MBAA, DBAA, DCBAA and DBCAA, respectively.
3. Results and discussion
3.1 Effect of salinity
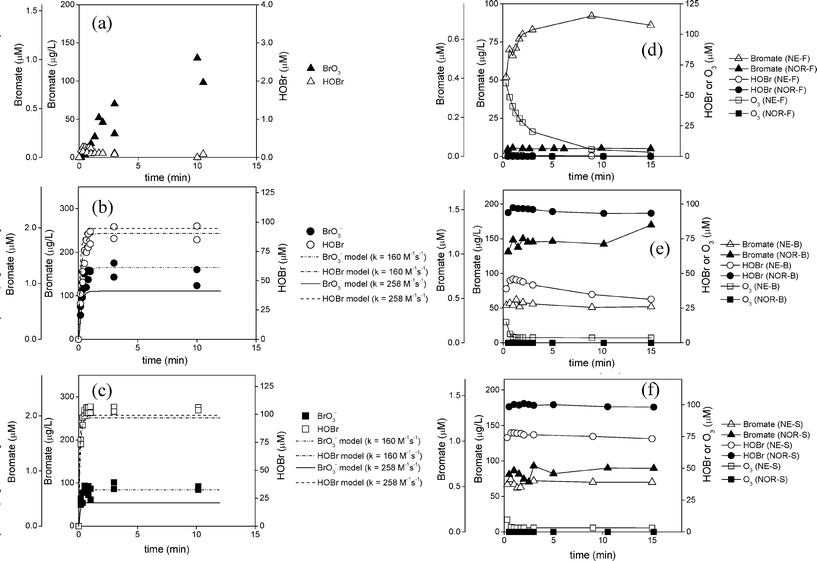
The bromate concentrations formed for the synthetic brackish water and seawater are not surprising given the known and simplified reaction mechanism for bromate formation in DOM-free waters (reactions (1)–(3), neglecting hydroxyl radical reactions due to the fast consumption of ozone by bromide in saline waters). Ozone first reacts with bromide to form hypobromite (OBr−) (reaction (1)). About one fourth of the OBr− is then further oxidized by ozone to form bromite (BrO2−) (reaction (2), for complete reaction see ESI,† Table S5) which then further reacts with ozone to form bromate (BrO3−) (reaction (3), simplified, see Table S5† for explanation).
O3 + Br− ⇄![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BrOOO− → OBr− + O2 k = 160–258 M−1s−1 (ref. 44, 45) BrOOO− → OBr− + O2 k = 160–258 M−1s−1 (ref. 44, 45) | (1) |
| O3 + OBr− → BrO2− + O2 k = 100 M−1s−1 (ref. 29) | (2) |
| BrO2− + O3 → BrO3− + O2 k > 1 × 105 M-1s-1 (ref. 29) | (3) |
At high bromide concentrations as in the synthetic waters B and S, a rapid and quantitative conversion of ozone to hypobromite (OBr−) can be expected during ozonation given that ozone is the limiting reagent under these conditions ([O3]0 = 104 μM; [Br−]0,B = 420 μM; [Br−]0,S = 840 μM). This is supported by results in Fig. 1b and c where 97 and 106 μM HOBr/OBr− is formed rapidly (<1 min), which is within the error of the ozone dose (104 μM). For waters containing high bromide levels, ozone is almost completely consumed in reaction (1), leaving only a limited residual ozone concentration for reactions (2) and (3), which is reflected in the limited bromate formation of <1.5 μM for B and S waters. For low-bromide containing waters such as F (Fig. 1a), this is not the case, and a larger ozone residual remains to further oxidize OBr− to BrO3−. Furthermore, under these conditions, hydroxyl radicals can also become more important in the bromate formation mechanism.37
To validate these conclusions, model simulations of HOBr and bromate formation were performed with Kintecus®32 using reactions (1)–(3) under conditions equivalent to the synthetic water experiments for B and S waters (conditions and list of reaction equations are provided in Table S5, ESI†). Since two rate constants have been reported for reaction (1) (160 (ref 44) and 258 (ref. 45) M−1 s−1), separate simulations were performed using either value (Fig. 1b and c). They were not performed for the fresh water because radical reactions were not incorporated into this model but are necessary to accurately predict bromate formation given that they are known to increase its formation in such low-bromide containing waters.40
For B and S waters, the modeling results for HOBr formation indicated that its formation was well characterized by these reactions over the assessed bromide concentration range and only a slight difference was observed when using either 160 or 258 M−1 s−1 as values for the rate constants for reaction (1) (Fig. 1b and c, dashed lines). This is in contrast to the model results for bromate formation whereby decreasing the rate constant for reaction (1) from 258 to 160 M−1 s−1 significantly increased the predicted bromate formation to generate a final bromate concentrations of 1.3 μM (165 μg L−1) and 0.66 μM (84 μg L−1) for the B or S waters, respectively (Fig. 1b and c, solid lines for 258 M−1 s−1 and dashed-dotted lines for 160 M−1 s−1). Such model results are expected given that a lower value for the second order rate constant for reaction (1), would lead to less ozone consumption through reaction (1), which in turn leaves a greater residual ozone to form bromate through reactions (2) and (3). Overall the model predicts bromate formation for both the brackish water (Fig. 1b) and seawater (Fig. 1c) reasonably well.
Additional experiments were also conducted with the NE and NOR waters which ranged in salinity to include freshwater (NE-F and NOR-F), brackish water (NE-B and NOR-B), and seawater (NE-S and NOR-S) to evaluate bromate formation during ozonation. These waters were ozonated ([O3]0 = 5.0 mg L−1; 104 μM) at 22 ± 2 °C over 15 min at the natural pH values and HOBr, bromate and the ozone residual were measured (Fig. 1d–f). In general, bromate concentrations ranged between 5 to 170 μg L−1 after 15 min (Fig. 1d–f). The rate of bromate formation for these waters was similar to the synthetic water experiments shown in Fig. 1a–c. Bromate rapidly reached maximum concentrations in ≤30 s in all brackish waters and seawaters (Fig. 1e and f) while it was more gradual for the NE freshwater (NE-F) where bromate reached 92 μg L−1 after 9 min (Fig. 1d).
Water salinity also had a strong impact on the extent of bromate formation. For the freshwaters, NE-F and NOR-F, the bromate concentrations formed were 86 and 5 μg L−1, respectively (Fig. 1d). This significant disparity in bromate formation between these two waters is clearly demonstrated by the initial bromide concentrations, the ozone consumption rate, and the slight pH difference between the two waters, a factor which is known to affect bromate formation40 (Fig. 1d). For the NE-F water with a bromide concentration of 0.3 mg L−1 (3.75 μM, Table S1, ESI†) the ozone consumption was relatively slow, which led to a formation of 86 μg L−1 bromate (Fig. 1d), which was similar to the results obtained for the synthetic fresh water. Alternatively, the bromate formation for the NOR-F was particularly low at 5 μg L−1 due to its low initial bromide concentration (0.044 mg L−1 (0.6 μM); Table S1, ESI†) and its high DOC (8.8 mg L−1 C; Table S1, ESI†), which led to a fast ozone consumption and a low ozone exposure (Fig. 1d).
For the brackish waters, NE-B and NOR-B, 62 and 170 μg L−1 of bromate were formed, respectively (Fig. 1e). The NOR-B brackish water, which contained 54.5 mg L−1 (681 μM) bromide (Table S1, ESI†) exhibited a factor 1.9 higher bromate concentrations than its seawater counterpart (NOR-S, bromide 68.5 mg L−1 (856 μM)) (Fig. 1e–f), a trend that was similarly observed for the synthetic waters. However, this trend was not matched for the NE-B water, which formed similar levels of bromate as its seawater counterpart (NE-S) (Fig. 1e–f). This is likely a result of not only the initial bromide concentration but also the presence of DOM, which can react with ozone and HOBr to incur an additional demand on these oxidants. The NE-B water contained 38.4 mg L−1 (480 μM) bromide and 3.6 mg L−1 C DOC (Table S1, ESI†) and only about 62 μg L−1 bromate was formed at pH 7.9 (Fig. 1e), which is low compared to the synthetic brackish water ([Br−]0 = 33.5 mg L−1 (420 μM) which formed 155 μg L−1 bromate at pH 8.0 (Fig. 1b). This lower bromate level for the NE-B water is likely driven by the presence of DOM, which appears to significantly consume the intermediate HOBr (Fig. 1e). This is clearly demonstrated in the case of the HOBr demand when comparing the maximum 46 μM HOBr formed experimentally (Fig. 1e) to the 94–97 μM HOBr that is predicted to form with the same bromide concentration but without DOM through model simulations (Fig. S2, ESI†; model calculations performed using Kintecus®,32 complete procedure and conditions in Table S5, ESI†).
The seawaters NE-S and NOR-S, which contain 62.6 mg L−1 (782 μM) and 68.5 mg L−1 (856 μM) bromide (Table S1, ESI†), respectively, formed relatively low bromate at 70 and 90 μg L−1, respectively. This matches the synthetic seawater results in that low bromate formation is caused by the rapid ozone consumption (complete consumption in <2 min) by bromide (reaction (1); Fig. 1f) and limited ozone remains to further oxidize OBr− to bromate.
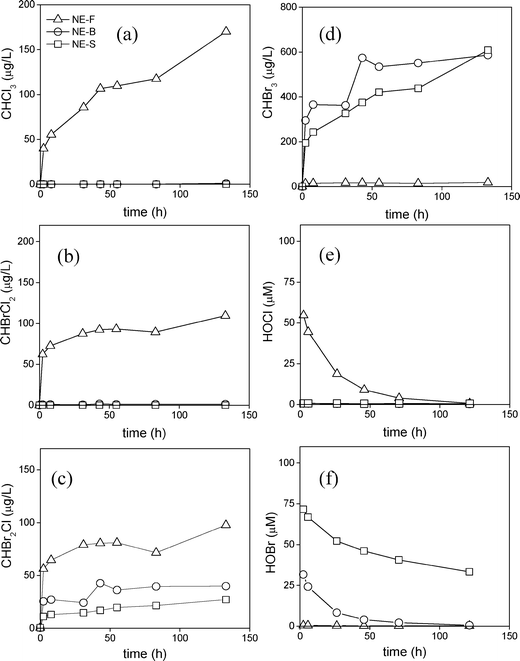
For ozonation, no THM formation was observed for the NE-F water and no CHCl3 and CHBrCl2 formation was observed for any of the three NE waters tested. CHBr3 was the predominant THM formed for both the NE-B and NE-S waters with concentrations of 460 and 550 μg L−1 after 5 days (Fig. S3a†), respectively, whereas minor concentrations of CHBr2Cl (7 and 16 μg L−1 for NE-B and NE-S waters, respectively) were detected (Fig. S3b†). The predominant formation of CHBr3 is expected given that ozone reacts with bromide to form HOBr (reaction (1)), which is a known brominating agent that can react with various DOM moieties to form brominated DBPs including CHBr3.44,46 This is supported by the initial HOBr concentrations formed for the NE-B and NE-S waters which are either completely consumed or consumed by approximately 50% after 120 h, respectively (Fig. S3c†). The formation of CHBr2Cl may be caused by the formation of radical halide species (RHS, e.g. BrCl˙−) which could partially brominate and chlorinated DOM precursors. Such RHS species have been similarly observed to generate low yields (<1%) of halophenols during UV/H2O2 treatment of phenol in simulated seawater.47 CHBr3 formation in the NE-B and NE-S waters rapidly increased up to 210 and 150 μg L−1 after 1 h, but then steadily increased up to 460 and 550 μg L−1, respectively, after the 120 h (5 days) holding time (Fig. S3a†). This two phase kinetics has been similarly observed in other waters for chlorination reactions.48 It is a result of highly reactive DOM moieties forming THMs quickly during an initial phase followed by less reactive DOM moieties forming THMs slowly in a second phase.48
PAA treatment showed similar behavior in regard to THMs formation. No or ≤detection limit (d.l.) concentrations of CHCl3 and CHBrCl2 were formed, respectively. CHBr3 formation was observed at up to 920 and 870 μg L−1 (Fig. S4a†) and CHBr2Cl formation was observed at up to 110 and 70 μg L−1 (Fig. S4b†) for the NE-B and NE-S waters after 120 h, respectively. These results are a direct consequence of the PAA reactivity with bromide and chloride to form secondary oxidants such as HOBr and HOCl, respectively. A previous study indicated that PAA can react with bromide to form HOBr with a second order rate constant for the reaction of PAA with bromide of kPAA/Br− = 0.24 ± 0.02 M−1 s−1.27 Therefore, the HOBr formation is relatively slow compared to the analogous reactions of ozone and free chlorine.27 This low rate constant is partially compensated by the high applied PAA dose ([PAA]0 = 150 mg L−1 (2.0 mM)), subsequently leading to higher CHBr3 formation for the NE-B and NE-S waters compared to ozonation (Fig. S3a†) or as discussed later, for chlorination (Fig. 2), in which a 19 times lower dose of 104 μM was applied. Alternatively, PAA can also react with chloride to form the secondary oxidant HOCl.27 The rate constant for this reaction is very low (k = (1.47 ± 0.58) × 10−5 M−1 s−1) and even though the chloride concentration in seawater is about 640 times higher than bromide, the fraction of PAA reacting with chloride is only about 4%. Such limited formation of chlorine is therefore responsible for ≤d.l. concentrations of higher chlorinated THMs (CHCl3 and CHBrCl2) compared to the higher brominated THMs (Fig. S4, ESI†). Further observations from these PAA experiments also indicate that CHBr2Cl and CHBr3 formation for the NE-B is slightly higher than the NE-S water (Fig. S4, ESI†), likely a result of the higher DOM concentration found in the NE-B water. One additional factor related to THMs formation during PAA treatment is that they can be significantly minimized if the PAA solution contains a greater H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA molar ratio (e.g. 2
PAA molar ratio (e.g. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of H2O2
1 molar ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA) than the one used for these experiments (0.3–0.38
PAA) than the one used for these experiments (0.3–0.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, Sigma-Aldrich PAA), as observed previously.27 Hydrogen peroxide reduces both HOBr and HOCl and can therefore minimize formation of THMs.27 This indicates that the initial H2O2 concentrations in such reactions must be evaluated so that its influence on THMs formation can be delineated.
1 molar ratio, Sigma-Aldrich PAA), as observed previously.27 Hydrogen peroxide reduces both HOBr and HOCl and can therefore minimize formation of THMs.27 This indicates that the initial H2O2 concentrations in such reactions must be evaluated so that its influence on THMs formation can be delineated.
A more complete array of chlorinated and brominated THMs is formed during chlorination ([free chlorine]0 = 7.4 mg L−1). Specifically, the chlorination of NE-F, NE-B, and NE-S waters led to 170 μg L−1, <d.l., and <d.l. of CHCl3 (Fig. 2a), 110 μg L−1, <d.l. and <d.l. of CHCl2Br (Fig. 2b), 100, 40 and 27 μg L−1 of CHBr2Cl (Fig. 2c) and, 19, 590, and 608 μg L−1 of CHBr3 (Fig. 2d) after 120 h, respectively. These values indicate a clear transition from chlorinated to brominated THM formation when the bromide concentration increases from fresh to brackish waters or seawaters with significantly higher bromide levels. This is expected given that free chlorine is converted to HOBr, following its reaction with bromide (reaction (4)), and HOBr is a known brominating agent.26
HOCl + Br− ⇄![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HOBr + Cl− k = 1550 M-1s-1 (ref. 49) HOBr + Cl− k = 1550 M-1s-1 (ref. 49) | (4) |
This conversion from free chlorine to HOBr in waters containing high bromide levels is also displayed indirectly in Fig. 2e and f where residual free chlorine is only present in the NE-F water (Fig. 2e), while HOBr is present in the NE-B and NE-S waters over 120 h (Fig. 2f). Similar observations were made in bromide-enhanced freshwaters, in which the transition from chlorinated to brominated THMs was also observed during chlorination by increasing the bromide levels46 and also during chlorination of seawaters used in swimming pools where brominated THMs rather than chlorinated THMs predominantly formed.7,8 A comparison of the kinetic results for chlorine and ozone (Fig. 2 and S3†) indicate that the trends and extent of formation for CHBr3 for the NE-B and the NE-S water are very similar. This is caused by the rapid transformation of the primary oxidants to the secondary oxidant HOBr, which then mainly leads to CHBr3 formation.
During ozonation up to 54 and 57 μg L−1 DBAA and 50 and 20 μg L−1 TBAA after 120 h for the NOR-B and NOR-S waters were formed, respectively (Fig. S5, ESI†). Brominated HAA formation is expected given the high bromide concentration in these two waters (NOR-B: [Br−]0 = 54.5 mg L−1; NOR-S: [Br−]0 = 68.5 mg L−1; Table S1, ESI†), which reacts with ozone to the secondary oxidant HOBr (reaction (1)), as discussed above. HOBr can then further react with various DOM moieties to generate brominated HAAs. The corresponding HOBr consumption is approximately 50% of its initial concentration over 120 h (Fig. S5c, ESI†). DBAA and TBAA formation was roughly two to three times higher for the NOR-B than the NOR-S water. This is likely due to the greater DOC concentration (2.3 mg L−1 C; Table S1, ESI†) and higher SUVA value (2.0 L mg−1 m−1); Table S1, ESI†) of the NOR-B water as compared to the NOR-S water (1.2 mg L−1 C; 1.0 L mg−1 m−1); Table S1, ESI†) (see further discussions below). Notably, no MBAA was formed during ozonation of the NOR-B or NOR-S waters, and no DBAA and TBAA was formed during ozonation of the NOR-F water due to its low bromide concentration ([Br−]0 = 0.044 mg L−1; Table S1, ESI†).
During PAA treatment (([PAA]0 = 150 mg L−1 (2 mM)) all three brominated HAAs, MBAA, DBAA, and TBAA formed. As similarly noted above, the HAAs formation for these experiments is considerable given the low molar ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA of the Sigma-Aldrich PAA mixture used. If a PAA mixture with a higher molar ratio were applied, HAAs formation would be significantly inhibited, as observed previously.27 Thus given this difference, MBAA formed here in all three waters at 19, 60, and 48 μg L−1 for the NOR-F, NOR-B, and NOR-S waters, respectively after 120 h (Fig. S6a, ESI†), but DBAA and TBAA only formed in the NOR-B and NOR-S waters. DBAA concentrations fluctuated slightly up to 360 and 300 μg L−1 over 66 h but then significantly increased up to 620 and 410 μg L−1 (Fig. S6b†) after 120 h in the NOR-B and NOR-S waters, respectively. Similarly, TBAA concentrations of 140 and 60 μg L−1 were measured after 120 h in the NOR-B or NOR-S waters, respectively (Fig. S6c†). Three main conclusions can be drawn from these results: (i) PAA treatment of the NOR-B water leads to greater formation of MBAA, DBAA, and TBAA compared to NOR-S water (Fig. S6a–c†), which is likely due to the DOC concentration and qualitative DOM differences (i.e. SUVA) of these two waters (as described above), (ii) DBAA is the dominant brominated HAA formed for the high-bromide waters (NOR-B and NOR-S) while MBAA is the main brominated HAA formed for the NOR-F water (Fig. S6a–c†). This finding seems reasonable given that the NOR-F water has very low bromide and subsequent HOBr concentrations to react with HAA precursors, whereas NOR-B and NOR-S water contain much higher bromide and subsequent HOBr concentrations to react with HAA precursors to form higher brominated HAAs. (iii) Compared to ozonation, PAA treatment generates significantly higher concentrations of MBAA, DBAA, and TBAA for the high-bromide waters (NOR-B and NOR-S; Fig. S5 and S6, ESI†). These results correspond to the analogous comparison for CHBr3 formation (see discussion above). This suggests that while the HOBr formation rate is considerably lower for PAA than for ozone, it is compensated by the higher PAA dose.
PAA of the Sigma-Aldrich PAA mixture used. If a PAA mixture with a higher molar ratio were applied, HAAs formation would be significantly inhibited, as observed previously.27 Thus given this difference, MBAA formed here in all three waters at 19, 60, and 48 μg L−1 for the NOR-F, NOR-B, and NOR-S waters, respectively after 120 h (Fig. S6a, ESI†), but DBAA and TBAA only formed in the NOR-B and NOR-S waters. DBAA concentrations fluctuated slightly up to 360 and 300 μg L−1 over 66 h but then significantly increased up to 620 and 410 μg L−1 (Fig. S6b†) after 120 h in the NOR-B and NOR-S waters, respectively. Similarly, TBAA concentrations of 140 and 60 μg L−1 were measured after 120 h in the NOR-B or NOR-S waters, respectively (Fig. S6c†). Three main conclusions can be drawn from these results: (i) PAA treatment of the NOR-B water leads to greater formation of MBAA, DBAA, and TBAA compared to NOR-S water (Fig. S6a–c†), which is likely due to the DOC concentration and qualitative DOM differences (i.e. SUVA) of these two waters (as described above), (ii) DBAA is the dominant brominated HAA formed for the high-bromide waters (NOR-B and NOR-S) while MBAA is the main brominated HAA formed for the NOR-F water (Fig. S6a–c†). This finding seems reasonable given that the NOR-F water has very low bromide and subsequent HOBr concentrations to react with HAA precursors, whereas NOR-B and NOR-S water contain much higher bromide and subsequent HOBr concentrations to react with HAA precursors to form higher brominated HAAs. (iii) Compared to ozonation, PAA treatment generates significantly higher concentrations of MBAA, DBAA, and TBAA for the high-bromide waters (NOR-B and NOR-S; Fig. S5 and S6, ESI†). These results correspond to the analogous comparison for CHBr3 formation (see discussion above). This suggests that while the HOBr formation rate is considerably lower for PAA than for ozone, it is compensated by the higher PAA dose.
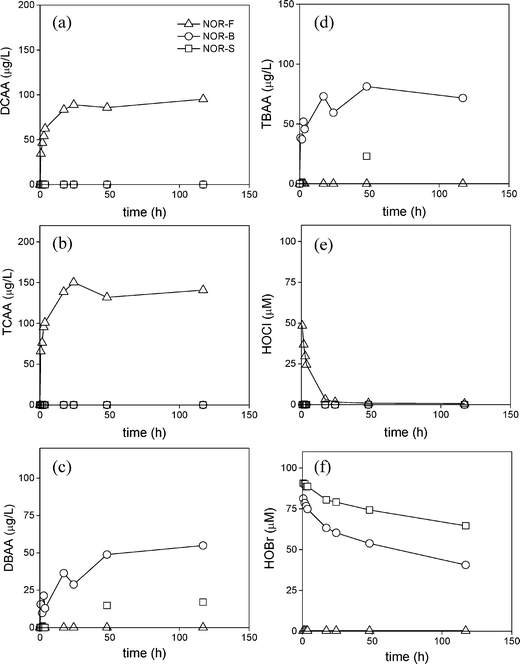
Chlorination ([free chlorine]0 = 7.4 mg L−1 (104 μM)) of the NOR waters led to a formation of DCAA, TCAA, DBAA, and TBAA over 120 h (Fig. 3). Again, due to the low bromide level for the NOR-F water, no brominated HAA formation was observed but rather only chlorinated HAAs (90 μg L−1 DCAA and 140 μg L−1 TCAA) (Fig. 3a, b). These observations are consistent with THM experiments, in which (i) only chlorinated THMs and no brominated THMs were formed for similar chlorination conditions, (ii) the residual free chlorine concentration was relatively high for the initial 24 h for this water in comparison to the NOR-B and NOR-S waters (Fig. 3e) and (iii) no HOBr formation was observed over 120 h (Fig. 3f). Alternatively, DBAA and TBAA formation was dominant in the high-bromide waters (NOR-B and NOR-S; Fig. 3c and d) in which HOBr was present rather than free chlorine (Fig. 3e and f). For these waters, DBAA formation was up to 55 and 15 μg L−1 and TBAA formation was up to 72 and 13 μg L−1 in the NOR-B and NOR-S waters after 120 h, respectively (Fig. 3c and d). In agreement with ozonation experiments, NOR-B formed greater concentrations of DBAA and TBAA as compared to NOR-S (Fig. 3c and d). These observations are comparable to previous studies, in which DBAA and TBAA were the predominant HAAs formed when coastal seawaters or seawaters used in swimming pools were chlorinated.7,11,50
3.2 Effect of DOM content
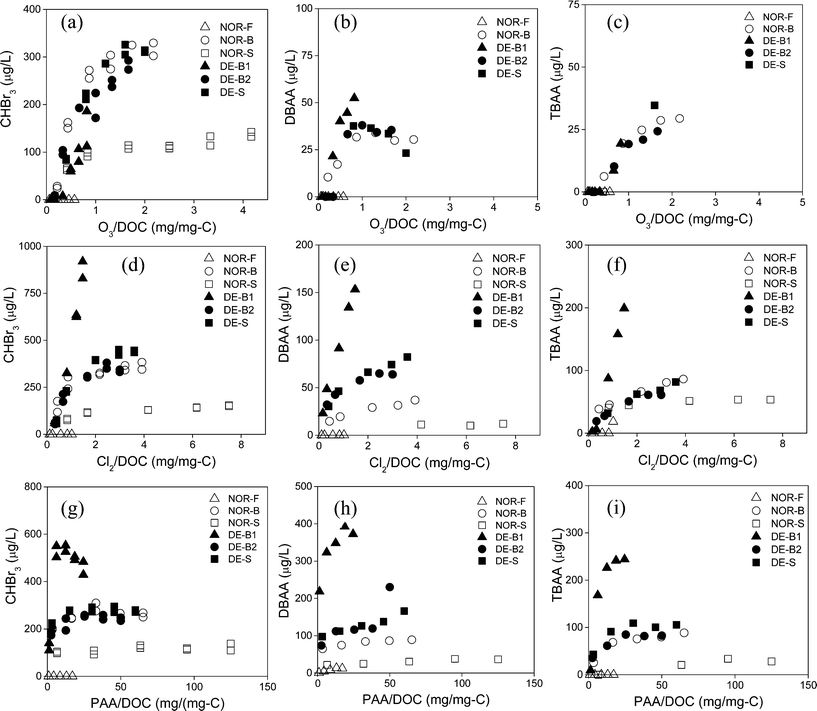
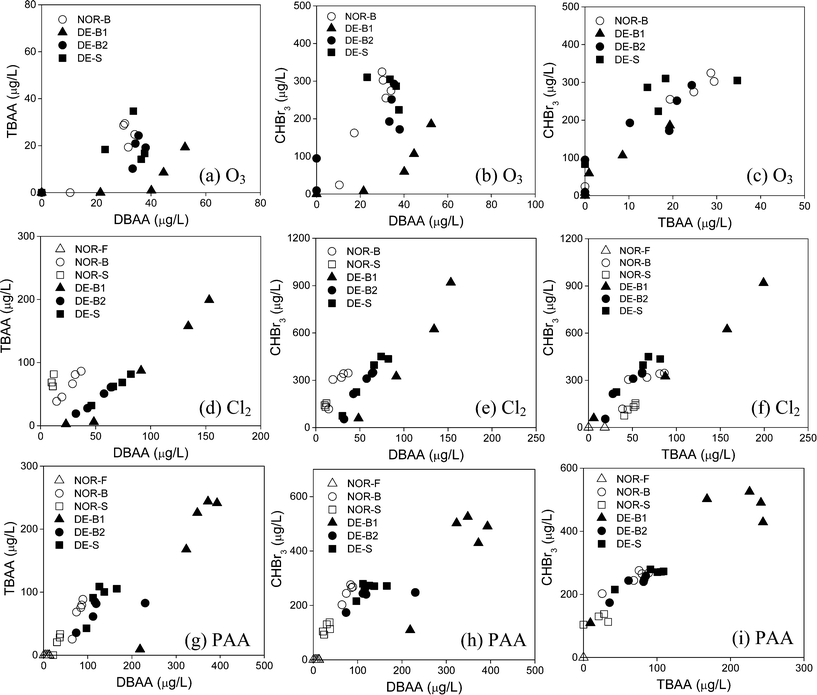
The DOM content (measured as DOC) of natural waters is an important parameter governing THM and HAA formation. Of the nine natural waters tested in this study, it was possible to evaluate how DOM affected THMs and HAAs formation using six of these waters (NOR- and DE- F, B, S) by performing a series of dosage-based experiments. These six natural waters had DOC concentrations in the range of 1.2 to 8.8 mg L−1 C (Table S1, ESI†), with NOR-F having a particularly high value (8.8 mg L−1 C). This is not surprising given that many Norwegian freshwaters have high DOC levels due to various environmental factors.51,52 Differences in typical seawater and freshwater chemical composition are also well represented in these six waters by their SUVA values, which are an indication for the aromatic content of the DOM of the selected waters. For the NOR waters, the SUVA values show a decrease in the order fresh water > brackish water > seawater, indicating a decreasing aromaticity (Table S1, ESI†). This trend is not as clearly defined for the DE waters though (Table S1, ESI†).For the dosage experiments, the waters were exposed to ozone (0.5–5.0 mg L−1 (10–104 μM)), free chlorine (1–9 mg L−1 Cl2 (14–126 μM)), and PAA (7.5–150 mg L−1 (0.1–2 mM)) for 22 ± 4 h (18–26 h) at 22 ± 2 °C. The measured THM and HAA concentrations were plotted against the specific oxidant dose, which is the oxidant dose divided by the DOC concentration of each water (mg per mg C). Fig. 4 shows plots for the formation of CHBr3, DBAA, and TBAA for experiments with ozone, chlorine and PAA with NOR and DE waters. No data were plotted for DBAA and TBAA formation during ozonation of the NOR-S water since the concentrations were <d.l. (Fig. 4b and c). Additional plots were similarly generated for the CHBr2Cl results obtained during chlorination and PAA treatment and MBAA results obtained during PAA treatment of NOR and DE waters (Fig. S7, ESI†). Several conclusions can be drawn from the results presented in Fig. 4: (i) very low concentrations of all three brominated DBPs are formed in the NOR-F water as a result of its low bromide concentration (0.044 mg L−1). The bromide concentration governs the Br-DBP formation in this water rather than the DOM type. (ii) The NOR-S water forms significantly less CHBr3, DBAA, and TBAA as a function of the specific oxidant dose for all three oxidants (Fig. 4). This is likely a result of low SUVA (1.0 L mg−1 m−1) of this seawater reflecting a low reactivity towards bromine. This pattern is also observed for the CHBr2Cl and MBAA results presented in Fig. S7, ESI.† (iii) The oxidant/DOC normalized formation of CHBr3, DBAA, and TBAA is very similar for NOR-B, DE-B1, DE-B2 and DE-S during ozonation (Fig. 4a–c). However, the selected DBPs are significantly higher for DE-B1 water for chlorination and PAA treatment (Fig. 4d–i). For chlorination and PAA treatment, these elevated Br-DBP patterns for the DE-B1 water are likely driven by its higher SUVA value ((3.1 L mg−1 m−1), Table S1, ESI†) as compared with the other waters (1.4–2.2 L mg−1 m−1) for NOR-B, DE-B2, and DE-S (Table S1, ESI†). This effect is likely blurred during ozonation because of the low bromide level in the DE-B1 water, which leads to a slower conversion of bromide to HOBr and thus a longer lifetime of ozone (t1/2 (O3) ≈ 30 s for 12 mg L−1 Br−, t1/2 (O3) ≈ 8 s for 40 mg L−1 Br−). The residual ozone can then partially oxidize bromide-reactive DOM moieties (represented by SUVA53) and subsequently reduce Br-DBP formation. (iv) NOR-B, DE-B2, and DE-S have similar SUVA and high bromide levels, which leads to a similar extent of CHBr3, DBAA, TBAA (Fig. 4) and CHBr2Cl and MBAA (Fig. S7, ESI†) formation for all oxidants if normalized to the specific oxidant dose. Such findings indicate that these waters likely contain similar DOM moieties serving as precursors for DBP formation.
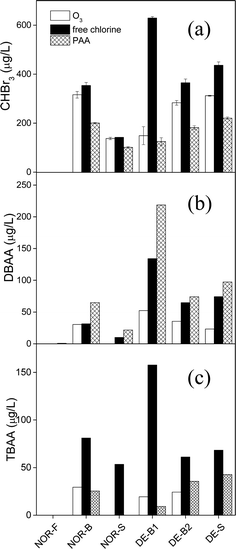
3.3 Comparison of oxidants
The type of chemical oxidant that is applied during disinfection can have a direct impact on the types and concentrations of DBPs generated. To evaluate this effect, experiments were performed in which the same oxidant dose of 104 μM was applied to the six NOR and DE waters for ozonation, chlorination, and PAA treatment over 22 ± 4 h (18–26 h) at 22 ± 2 °C. These molar oxidant doses translate to 5.0 mg L−1 ozone, 7.4 mg L−1 free chlorine, and 7.8 mg L−1 PAA. Fig. 5 shows the results from these experiments for CHBr3, DBAA, and TBAA formation. These DBPs were chosen because they had mainly been formed in the six selected waters. However, for the NOR-S water, no bars are presented for DBAA and TBAA for certain oxidants since their concentrations were present at <d.l. Bromate formation was excluded from Fig. 5 because its formation during ozonation is well described in Fig. 1, and neither chlorination nor PAA treatment was found to generate bromate under these conditions.Fig. 5 indicates that chlorination yielded slightly to significantly higher CHBr3, DBAA, and TBAA formation than ozonation for the NOR-B, NOR-S, and all DE waters (NOR-F led to no CHBr3 for any of the three oxidants because of the low bromide level). This is expected since ozone can react partially with the Br-DBP precursors, which reduces the formation of the corresponding DBPs. This is especially pronounced for the DE-B1 water as discussed above. Ozonation is also a known treatment option for the reduction of THM formation in water treatment during post-chlorination.29 In highly saline waters, this effect is much smaller due to the fast consumption of ozone by bromide. By comparison, PAA treatment resulted in the lowest formation of CHBr3 (Fig. 5a) as well as low TBAA formation when compared to chlorination (Fig. 5c). Overall, the CHBr3 formation follows the trend chlorine > ozone > PAA (Fig. 5a) for the NOR-B, NOR-S, and all DE waters. TBAA formation is similarly high during chlorination but a less pronounced and mixed effect for ozone and PAA for these same waters was observed, generally leading to chlorine ≫ ozone ≈ PAA (Fig. 5c). In contrast, PAA treatment results in the highest formation of DBAA for the NOR-B, NOR-S, and all DE waters, leading the overall formation of DBAA to follow the trend PAA > chlorine > ozone (Fig. 5b). This might be a consequence of the low oxidizing capacity of PAA towards DBAA-precursors as compared to free chlorine and ozone, which then leaves these precursors to react with HOBr to form DBAA. This finding also implies that the precursors for DBAA formation differ from the precursors for CHBr3 and TBAA formation, which well matches similar conclusions from previous studies where freshwaters or bromide-containing synthetic waters were chlorinated.54,55
Additional insights on oxidant behavior towards DBP formation can be gained by assessing their cross-correlations (Fig. 6). In this case, the results were taken from the dosage experiments of the six NOR and DE waters which were exposed to varying concentrations of ozone (0.5–5.0 mg L−1 (10–104 μM)), free chlorine (1–9 mg L−1 Cl2 (14–126 μM)), and PAA (7.5–150 mg L−1 (0.1–2 mM)) for 22 ± 4 h (18–26 h) at 22 ± 2 °C. The formation of CHBr3, DBAA, and TBAA at different oxidant doses were plotted against each other to establish correlations between (i) TBAA and DBAA formation (Fig. 6a, d, g) (ii) CHBr3 and DBAA formation (Fig. 6b, e, h) and (iii) CHBr3 and TBAA formation (Fig. 6c, f, i) for all three oxidants. Similarly as above, some cross-correlations for the NOR-F and NOR-S waters in particular could not be made since either no Br-DBP formation or <d.l. levels were observed. Most of the results indicate fairly good linear correlations between all three relationships tested. This is especially true for CHBr3 and TBAA formation for all three oxidants (Fig. 6c, f, i) and further suggests that both their precursors respond proportionally to increases in ozone, free chlorine, and PAA doses and the formation of these DBPs is related to each other. This finding is well supported by previous research which has proposed that CHCl3 and TCAA formation can occur through hydrolysis and oxidative hydrolysis, respectively, following chlorination of the same NOM-derived precursors (see ESI,† Scheme S1 for mechanism).55 However, these pathways differ for DCAA formation which is derived from different DOM precursors, as similarly suggested above and shown in Scheme S1 (ESI†).55 This scheme also indicates that tri-halogenated HAAs are not formed by directly halogenating di-halogenated HAAs, which was also supported through additional experimental evidence which found that the molar fraction of di-halogenated or tri-halogenated HAAs formation did not change with increasing HOBr doses (generated by bromide reaction with free chlorine) when testing synthetic waters.54 Our data further compliment this observation so that in general, CHBr3 and TBAA correlated more closely with each other than DBAA with either CHBr3 or TBAA (Fig. 6). This is especially true during ozonation where fairly poor correlations are observed between DBAA and TBAA (Fig. 6a) and DBAA and CHBr3 (Fig. 6b).
3.4 Temperature dependence
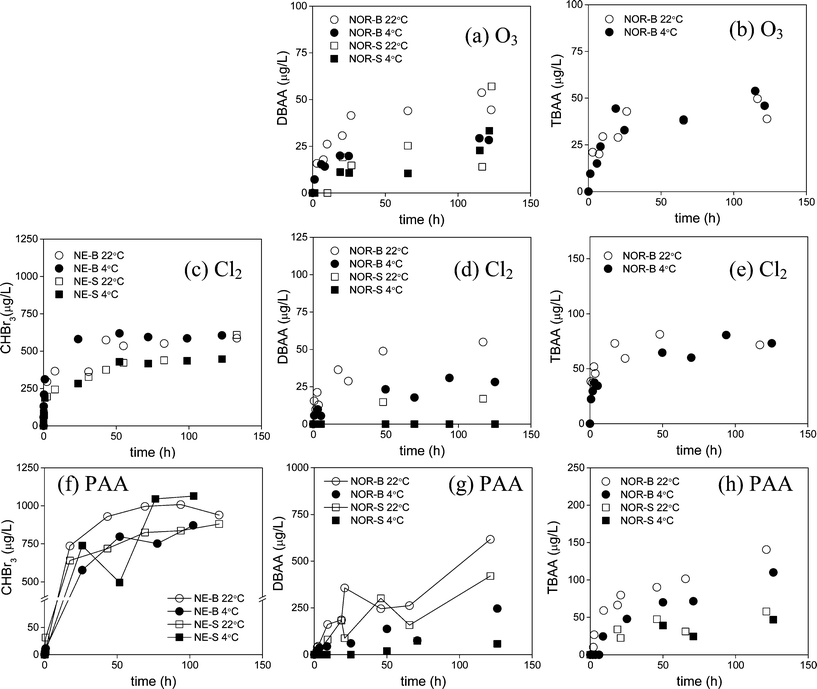
A series of kinetic experiments were performed to evaluate the effect of temperature on bromate, THM, and HAA formation for ozonation, chlorination, and PAA treatment of the NE and NOR waters. Two temperatures, 22 ± 2 °C or 4 ± 2 °C, were selected to represent the typical range of temperatures found in ballast waters. The effect of temperature on bromate formation as a function of time over 15 min is shown in Fig. S8 (ESI†). The effect of temperature on THM and HAA formation was either (i) plotted as a function of time over 5 days (Fig. 7) or (ii) as a function of the HOBr exposure determined over 5 days (Fig. S9, ESI†). CHBr3, DBAA, and TBAA were the selected DBPs to be plotted in these latter figures (Fig. 7 and S9, ESI†) since they were the dominantly formed for the three oxidants evaluated.The effect of temperature on bromate formation was observed specifically for the NE-F, NOR-B, and NOR-S waters and was found to have a varying influence depending on water type. Bromate formation for the NOR-B and NOR-S waters decreased by up to a factor of 3 (Fig. S8b and c, ESI†) but was relatively equivalent for the NE-F water (Fig. S8a, ESI†) when the temperature decreased from 22 ± 2 to 4 ± 2 °C after 15 min. Such patterns are likely a result of the impact that temperature has on (i) HOBr formation and consumption (Fig. S8d–f†) and (ii) the ozone residual concentration (Fig. S8g–i, ESI†) which are the two important reactants involved in forming bromate (eqn (1)–(3)). In general, the temperature decrease was found to slightly increase HOBr formation over 15 min (Fig. S8d–f, ESI†) but also slightly stabilize the HOBr concentration and slows down its further consumption, which is especially observed for the NE-F water (Fig. S8d, ESI†). In addition, the ozone residual concentration is affected by temperature but the extent of this effect varies depending on the bromide concentration of the waters. Waters containing high bromide levels (NOR-B, and NOR-S) exhibited little to no effect of temperature since the ozone is quickly consumed by bromide (Fig. S8h–i, ESI†) whereas in the NE-F water with a low bromide level, the ozone residual increases substantially when the temperature is lowered (Fig. S8g, ESI†). Overall, these combined effects directly influence the impact that the temperature has on bromate formation. For the NOR-B and NOR-S waters, there is little difference in the HOBr concentrations (Fig. S8e–f, ESI†), but the reactions to form bromate (reactions (2) and (3)) become slower (Fig. S8b–c, ESI†). The ozone residual concentration does not play a role here since it is too quickly consumed by bromide. Alternatively, for the NE-F water, bromate formation does not depend on the temperature (Fig. S8a, ESI†). In this case, the shorter lifetime of HOBr at the higher temperature is compensated by the higher rate of the further oxidation of OBr−, its conjugated base, to bromate (reactions (2) and (3)), which is possible due to a longer lifetime of ozone. At the lower temperature, both HOBr and ozone concentrations are higher, which compensates for the slower bromate formation through reactions (2) and (3).
The effect of temperature (22 ± 2 °C and 4 ± 2 °C) on CHBr3, DBAA, and TBAA formation was also evaluated for all three oxidants for the NE and NOR waters. CHBr3 was only evaluated in the three NE waters (NE-F, NE-B, and NE-S) and DBAA and TBAA were only evaluated for the three NOR waters (NOR-F, NOR-B, and NOR-S). This does not include the effect of temperature on CHBr3 formation during ozonation since its formation at 4 ± 2 °C was not evaluated. In addition, results for NOR-F for all three oxidants and results for NOR-S for ozonation and chlorination will not be further discussed here since either Br-DBPs were <d.l. or only very low Br-DBP formation was observed at either temperature (Fig. 7). For the other waters, the results indicated that a decrease in temperature slightly lowered or had no effect on Br-DBP formation but this depends highly on the specific Br-DBP and oxidant applied (Fig. 7). Three major observations can be summarized from these results: (i) DBAA formation decreased by up to a factor of 2 when the temperature was lowered from 22 ± 2 °C to 4 ± 2 °C after 5 days (120 h) for the majority of water–oxidant combinations (Fig. 7a, d, g). (ii) The decrease in temperature during PAA treatment slightly lowered CHBr3, DBAA, and TBAA formation for the NOR-B water or either slightly lowered or did not affect the DBP formation for the NOR-S water (Fig. 7f–h). One exception was CHBr3 formation in which the effect of temperature was not conclusive since the 4 ± 2 °C temperature results were highly variable over 5 days (120 h) (Fig. 7f). (iii) No effect of temperature on TBAA formation was observed during ozonation (Fig. 7b) and for CHBr3 and TBAA formation during chlorination (Fig. 7c and e). To better understand these results, the data for both 22 ± 2 °C and 4 ± 2 °C were plotted against HOBr exposure (Fig. S9, ESI†) in order to compensate for the higher HOBr stability over 5 days (120 h) when the temperature was decreased (22 ± 2 °C to 4 ± 2 °C (Fig. S10, ESI†)). This was done for the waters treated with ozone and chlorine but could not be done for those treated with PAA since HOBr formation was not measured in these experiments (Fig. S10, ESI†). Results indicate similar trends in Br-DBP formation with temperature when plotting the data against HOBr exposure (Fig. S9, ESI†) as when plotting them against time (Fig. 7). CHBr3 and TBAA formation are not affected by temperature whereas DBAA formation decreases with decreasing temperature over the same HOBr exposures (Fig. S9, ESI†). This implies two important findings that (i) a decrease in temperature does not affect the formation rates of CHBr3 and TBAA but does affect the formation rates of DBAA and (ii) this again demonstrates that the pathways for CHBr3 and TBAA formation are coupled and dissimilar to DBAA formation, as suggested above and further supported by previously proposed reaction pathways55 that are provided in Scheme S1 (ESI†).
Conclusions
As ocean-going ships begin to implement chemical disinfection strategies to treat ballast water, the aim of this study was to identify the key water quality parameters and treatment scenarios responsible for driving disinfection by-product (DBP) formation in these systems. This was especially necessary since ballast waters are typically saline, and most of the previous research on this topic has focused on DBP formation in freshwater systems. The results from this study indicate that multiple factors influence DBP formation in saline waters![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salinity (especially the bromide concentration), DOM type/concentration, and oxidant type/dose. Alternatively, temperature has a varied effect. CHBr3 and TBAA formation was not affected by temperature while DBAA and bromate formation decreased by up to a factor of 2 and 3, respectively, following a temperature decrease from 22 ± 2 °C to 4 ± 2 °C. For DBAA, this difference was not attributed to the change in HOBr exposure but likely driven by the slower kinetics of DBAA formation at a lower temperature. The effect of temperature on bromate formation, especially in the brackish and seawaters, was likely driven by the effect of temperature on ozone stability and its ability to react with bromine.
salinity (especially the bromide concentration), DOM type/concentration, and oxidant type/dose. Alternatively, temperature has a varied effect. CHBr3 and TBAA formation was not affected by temperature while DBAA and bromate formation decreased by up to a factor of 2 and 3, respectively, following a temperature decrease from 22 ± 2 °C to 4 ± 2 °C. For DBAA, this difference was not attributed to the change in HOBr exposure but likely driven by the slower kinetics of DBAA formation at a lower temperature. The effect of temperature on bromate formation, especially in the brackish and seawaters, was likely driven by the effect of temperature on ozone stability and its ability to react with bromine.
Moreover, several findings from this study indicate that much of our understanding behind how DBPs are formed in freshwaters can be qualitatively transferred to saline waters. This is supported through a series of results: (i) bromate formation in DOM-free waters containing bromide concentrations representative of saline waters (38–67 mg L−1 bromide) can be well predicted using a simplified kinetic model that has been developed for freshwaters containing low levels of bromide (ca. 100 μg L−1). (ii) Similar to freshwaters, high bromide concentrations as encountered in brackish waters and seawater can lead to elevated formation of mixed Br-Cl-DBPs and Br-DBPs during ozonation, chlorination, and PAA treatment. This was especially clear during chlorination where the increased bromide concentration triggered a transition from Cl-DBPs to mixed Br-Cl-DBPs/Br-DBPs in these waters. (iii) The DOM concentration and type, especially characterized by SUVA, which is a proxy for DBP formation used in freshwaters, was also a suitable indicator for DBP formation in saline waters. (iv) In agreement with previous findings in fresh waters, CHBr3 and TBAA formation well correlated with each other whereas DBAA formation did correlate with either Br-DBP but to a lesser extent. This was demonstrated when cross-correlating Br-DBP formation at different oxidant doses for all three oxidants. The oxidant type influenced DBP formation in the order chlorine > ozone ≈ PAA for CHBr3 and TBAA and PAA > chlorine > ozone for DBAA. Overall, these results indicate that Br-Cl-DBPs and Br-DBPs formation depends on the bromide concentration and SUVA content of the ballast water and the oxidant dose. The type of oxidant also plays a role, whereas the effect of ballast water temperature seems to have a lesser influence. By evaluating these parameters, BWMSs can optimize their disinfection strategies to minimize DBP formation and discharge.
Acknowledgements
The authors would like to thank Marcel Veldhuis and Norbert Theobald for providing the NE and DE waters, respectively. We would also like to thank Stephanie Delacroix for providing the NOR waters and for analyzing the DOC level for the nine natural waters (NE, NOR, and DE). Zheng-Qian Liu gratefully acknowledges the China Scholarship Council (CSC201208420333) for financial support. This work has been co-founded by the North Sea Region Programme under the European Regional Development Fund of the European Union.References
- J. J. Rook, Water Treat. Exam., 1974, 23, 234–243 Search PubMed
.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Mutat. Res., Rev. Mutat. Res., 2007, 636, 178–242 CrossRef CAS PubMed
.
- D. B. Jones, A. Saglam, H. Song and T. Karanfil, Water Res., 2012, 46, 11–20 CrossRef CAS PubMed
.
- J. Criquet, S. Allard, E. Salhi, C. A. Joll, A. Heitz and U. von Gunten, Environ. Sci. Technol., 2012, 46, 7350–7357 CrossRef CAS PubMed
.
-
P. Westerhoff, M. Siddiqui, J. Debroux, W. Zhai, K. Ozekin and G. Amy, in Critical Issues in Water and Wastewater Treatment: Proceedings of the 1994 National Conference on Environmental Engineering, ed. J. N. Ryan and M. Edwards, American Society of Chemical Engineers, New York, 1994, pp. 670–677 Search PubMed
.
- Y. Bichsel and U. von Gunten, Water Res., 2000, 34, 3197–3203 CrossRef CAS
.
- J. Parinet, S. Tabaries, B. Coulomb, L. Vassalo and J. Boudenne, Water Res., 2012, 46, 828–836 CrossRef CAS PubMed
.
- S. Chowdhury, K. Alhooshani and T. Karanfil, Water Res., 2014, 53, 68–109 CrossRef CAS PubMed
.
- E. Agus, N. Voutchkov and D. L. Sedlak, Desalination, 2009, 237, 214–237 CrossRef CAS PubMed
.
- E. Agus and D. L. Sedlak, Water Res., 2010, 44, 1616–1626 CrossRef CAS PubMed
.
- A. S. Allonier, M. Khalanski, V. Camel and A. Bermond, Mar. Pollut. Bull., 1999, 38, 1232–1241 CrossRef CAS
.
- H. A. Jenner, C. J. L. Taylor, M. vanDonk and M. Khalanski, Mar. Environ. Res., 1997, 43, 279–293 CrossRef CAS
.
- L. Heller-Grossman, J. Manka, B. Limonirelis and M. Rebhun, Water Res., 1993, 27, 1323–1331 CrossRef CAS
.
- L. Heller-Grossman, A. Idin, B. Limoni-Relis and M. Rebhun, Environ. Sci. Technol., 1999, 33, 932–937 CrossRef CAS
.
- M. Y. Ali and J. P. Riley, Water Res., 1989, 23, 1099–1106 CrossRef CAS
.
- A. M. S. El Din, R. A. Arain and A. A. Hammoud, Desalination, 1991, 85, 13–32 CrossRef CAS
.
- A. G. I. Dalvi, R. Al-Rasheed and M. A. Javeed, Desalination, 2000, 129, 261–271 CrossRef CAS
.
- B. Werschkun, S. Banerji, O. C. Basurko, M. David, F. Fuhr, S. Gollasch, T. Grummt, M. Haarich, A. N. Jha, S. Kacan, A. Kehrer, J. Linders, E. Mesbahi, D. Pughiuc, S. D. Richardson, B. Schwarz-Schulz, A. D. Shah, N. Theobald, U. von Gunten, S. Wieck and T. Höfer, Chemosphere, 2014, 112, 256–266 CrossRef CAS PubMed
.
- B. Werschkun, Y. Sommer and S. Banerji, Water Res., 2012, 46, 4884–4901 CrossRef CAS PubMed
.
-
S. N. Davis and R. J. M. DeWiest, Hydrogeology, Wiley, New York, 1966 Search PubMed
.
-
H. D. Holland, Chemistry of the atmosphere and oceans, Wiley, New York, 1978 Search PubMed
.
-
R. Benner, in Biogeochemistry of Marine Dissolved Organic Matter, ed. D. A. Hansell and C. A. Carlson, Academic Press, Amsterdam, 2002, vol. 3, pp. 59–90 Search PubMed
.
-
W. Stumm and J. J. Morgan, Aquatic chemistry, John Wiley & Sons, 1970 Search PubMed
.
- D. A. Reckhow, P. C. Singer and R. L. Malcolm, Environ. Sci. Technol., 1990, 24, 1655–1664 CrossRef CAS
.
- J. de Laat, N. Merlet and M. Dore, Water Res., 1982, 16, 1437–1450 CrossRef CAS
.
- M. B. Heeb, J. Criquet, S. G. Zimmermann-Steffens and U. von Gunten, Water Res., 2014, 48, 15–42 CrossRef CAS PubMed
.
- A. D. Shah, Z.-Q. Liu, E. Salhi, T. Höfer and U. von Gunten, Environ. Sci. Technol., 2015, 49, 1698–1705 CrossRef CAS PubMed
.
- H. Bader and J. Hoigne, Water Res., 1981, 15, 449–456 CrossRef CAS
.
-
C. von Sonntag and U. von Gunten, Chemistry of ozone in water and wastewater treatment: From basic principles to applications, IWA Publishing, London, 2012 Search PubMed
.
- T. H. Chen, Anal. Chem., 1967, 39, 804–813 CrossRef CAS
.
- D. M. Davies and M. E. Deary, Analyst, 1988, 113, 1477–1479 RSC
.
-
J. C. Ianni, in Kintecus, Windows Version 5.2, 2014, http://www.kintecus.com Search PubMed
.
-
L. S. Clesceri and A. P. H. Association, Standard methods for the examination of water and wastewater, American Public Health Association, Washington, D.C., 1989 Search PubMed
.
- E. Salhi and U. von Gunten, Water Res., 1999, 33, 3239–3244 CrossRef CAS
.
- A. Leitzke, E. Reisz, R. Flyunt and C. von Sonntag, J. Chem. Soc., Perkin Trans. 2, 2001, 2, 793–797 RSC
.
-
A. D. Zaffiro, M. Zimmerman, B. V. Pepich, R. W. Slingsby, R. F. Jack, C. A. Pohl and D. J. Munch, EPA Methods, 2009, pp. 1–42 Search PubMed
.
- U. von Gunten and J. Hoigné, Environ. Sci. Technol., 1994, 28, 1234–1242 CrossRef CAS PubMed
.
- R. G. Song, C. Donohoe, R. Minear, P. Westerhoff, K. Ozekin and G. Amy, Water Res., 1996, 30, 1161–1168 CrossRef CAS
.
- B. Legube, B. Parinet, K. Gelinet, F. Berne and J. P. Croue, Water Res., 2004, 38, 2185–2195 CrossRef CAS PubMed
.
- U. von Gunten, Water Res., 2003, 37, 1469–1487 CrossRef CAS
.
- G. Grguric, J. H. Trefry and J. J. Keaffaber, Water Res., 1994, 28, 1087–1094 CrossRef CAS
.
- R. P. Herwig, J. R. Cordell, J. C. Perrins, P. A. Dinnel, R. W. Gensemer, W. A. Stubblefield, G. M. Ruiz, J. A. Kopp, M. L. House and W. J. Cooper, Mar. Ecol.: Prog. Ser., 2006, 324, 37–55 CrossRef CAS
.
- U. von Gunten and J. Hoigné, J. Water Supply: Res. Technol.--AQUA, 1992, 41, 299–304 CAS
.
- W. R. Haag and J. Hoigné, Environ. Sci. Technol., 1983, 17, 261–267 CrossRef CAS
.
- Q. Liu, L. M. Schurter, C. E. Muller, S. Aloisio, J. S. Francisco and D. W. Margerum, Inorg. Chem., 2001, 40, 4436–4442 CrossRef CAS PubMed
.
- S. D. Richardson, A. D. Thruston, T. V. Caughran, P. H. Chen, T. W. Collette and T. L. Floyd, Environ. Sci. Technol., 1999, 33, 3378–3383 CrossRef CAS
.
- J. E. Grebel, J. J. Pignatello and W. A. Mitch, Environ.
Sci. Technol., 2010, 44, 6822–6828 CrossRef CAS PubMed
.
- H. Gallard and U. von Gunten, Water Res., 2002, 36, 65–74 CrossRef CAS
.
- K. Kumar and D. W. Margerum, Inorg. Chem., 1987, 26, 2706–2711 CrossRef CAS
.
- M. Fabbricino and G. V. Korshin, Desalination, 2005, 176, 57–69 CrossRef CAS PubMed
.
- H. A. De Wit, J. Mulder, A. Hindar and L. Hole, Environ. Sci. Technol., 2007, 41, 7706–7713 CrossRef CAS
.
- D. Hongve, G. Riise and J. F. Kristiansen, Aquat. Sci., 2004, 66, 231–238 CrossRef CAS PubMed
.
- J. Wenk, M. Aeschbacher, E. Salhi, S. Canonica, U. von Gunten and M. Sander, Environ. Sci. Technol., 2013, 47, 11147–11156 CrossRef CAS PubMed
.
- G. A. Cowman and P. C. Singer, Environ. Sci. Technol., 1996, 30, 16–24 CrossRef CAS
.
- G. H. Hua and D. A. Reckhow, J. - Am. Water Works Assoc., 2008, 100, 82–95 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00061k |
| ‡ Current address: School of Civil Engineering and Division of Environmental and Ecological Engineering, Purdue University, West Lafayette, Indiana 47907 |
| This journal is © The Royal Society of Chemistry 2015 |