Evaluation of optical surrogates for the characterization of DOM removal by coagulation†
Julie A.
Korak
*,
Fernando L.
Rosario-Ortiz
and
R.
Scott Summers
Department of Civil, Environmental and Architectural Engineering, 428 UCB, University of Colorado Boulder, Boulder, CO 80309, USA. E-mail: Julie.Korak@colorado.edu
First published on 19th May 2015
Abstract
Optical surrogates (i.e., absorbance and fluorescence) are of interest to monitor drinking water treatment processes due to their potential for implementation as online sensors. This study compares the use of different optical surrogates to model dissolved organic carbon (DOC) removal by coagulation with aluminum sulfate in the dose range of 5 to 120 mg L−1 in 22 source waters with a wide range of water qualities (specific UV absorbance (SUVA254) – 1.0 to 4.0 L mgC−1 m−1). Linear regressions were developed relating percent DOC removal to percent fluorescence decrease at discrete wavelength pairs in an excitation-emission matrix (2029 wavelength pairings). DOC removal was modeled with an average 95% prediction interval between 10.5–15% at all emission wavelengths greater than 375 nm, suggesting that wavelength selection for use in fluorescence monitoring is relatively unimportant. Fluorescence data without inner filter corrections led to a decrease in model performance, but prediction intervals were still between 12–15% at emission wavelengths greater than 400 nm. By comparison, the average prediction interval for an analogous model with UV absorbance at 254 nm was 10.5% (DOCr% = 0.7 UVr%, R2 = 0.91), performing the same as the best possible fluorescence wavelength combinations. Additional modeling found that tracking multiple optical surrogates in tandem does not improve model performance due to correlated independent variables. Raw water optical properties (SUVA254 and fluorescence ratios) modeled DOC removal at a single coagulant dose (~40 mg L−1), but fluorescence indicators did not significantly outperform SUVA254. A PARAFAC model built with 112 fluorescence samples with six validated components demonstrated the range of fluorescence behaviors in the dataset and guided the selection of fluorescence ratios to predict removal based on raw water characteristics. These results demonstrated that fluorescence wavelength is relatively unimportant for online monitoring approaches that measure both raw and clarified samples, but emission wavelength is a driving factor for predicting removal based solely on raw water characteristics.
Water impactThe use of optical measurements (absorbance and fluorescence) to understand and model organic matter removal by coagulation is of interest due to its potential for online monitoring applications. Contemporary research has focused on the use of multivariate statistical models (i.e., parallel factor analysis (PARAFAC)) and peak picking approaches to identify fluorescence wavelengths suitable for applied applications. This study demonstrates that when both raw and clarified samples are measured, the fluorescence wavelength selection is relatively unimportant. For predictive models based solely on raw water optical properties, strong models spanning diverse source waters exist, and fluorescence emission wavelength is the driving factor. UV and fluorescence based surrogates perform equally to each other. These results provide guidance for the design and implementation of online monitoring systems for drinking water treatment. |
1. Introduction
The removal of dissolved organic matter (DOM) is an important treatment objective for drinking water utilities in order to meet disinfection byproduct (DBP) regulations, as DOM serves as a DBP precursor.1 As a heterogeneous mixture of organic molecules, the characterization and quantification of DOM is commonly achieved through surrogate measurements of bulk characteristics. Some example surrogates that have been used include dissolved organic carbon (DOC), UV absorbance at 254 nm (UV254),2 and more recently fluorescence. DOC is the most direct measure of DOM by quantifying its mass in the form of oxidizable carbon; DOM is roughly 50% carbon.3 Unlike absorbance or fluorescence, the DOC measurement does not depend on DOM chemical composition or its ability to absorb or emit light.The use of UV254 as a surrogate for DOM removal by coagulation is well established.2,4,5 Fluorescence has recently been proposed as a useful surrogate for evaluating DOC removal by coagulation.6–12 Absorbance and fluorescence both measure the fraction of the DOM that is optically active. Generally, the optical activity of DOM has been associated with aromatic content and sp2 hybridized functional groups, although non-bonding electron pairs can also produce optical transitions in the region of interest.13 Absorbance (most commonly used in the UV-Vis range) measures the capacity of a chromophore to absorb light and produce an excited state as a function of wavelength, or energy level. Fluorescence is a phenomenon where a portion of the absorbed energy is emitted in the form of light, the remaining energy being released via other non-radiative processes. Fluorescence data is often measured as excitation-emission matrices (EEMs), which are contour plots representing the intensity of light emitted as a function of wavelength absorbed (excitation wavelength) and wavelength emitted, as illustrated in Fig. 1 for one of the waters examined in this study.
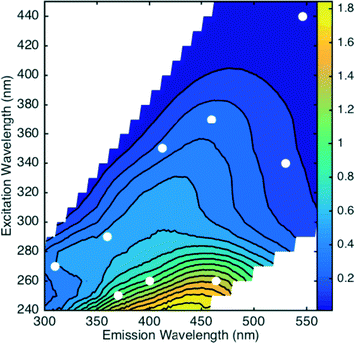 | ||
| Fig. 1 Excitation-emission matrix of water source 8. Markers indicate the nine excitation–emission wavelength combinations for the exploratory analysis. | ||
The application of DOM optical properties as a process-monitoring tool for drinking water treatment is of interest, because analysis can be performed rapidly on bench-top instruments or incorporated into sensors for real-time data collection. There are commercially available sensors that measure either UV absorbance spectra or fluorescence intensities.14–16 Currently, fluorescence sensors are limited to measuring across a range of wavelengths due to large bandpasses (>30 nm).16 Many bench-top spectrofluorometers offer full EEM analysis in less than 20 minutes.
While both absorbance and fluorescence measure fractions of DOM that could be similar in nature, it is important to note that there are some differences in the material that absorbs light efficiently compared to material that fluoresces efficiently. DOM with greater aromaticity absorbs more light per unit mass, which is commonly quantified by the normalization of absorbance at 254 nm to the DOC, specific UV absorbance (SUVA254).17 Higher SUVA254 values have been associated with greater molecular weight fractions,18–21 higher DBP formation2,17 and higher affinity for removal by coagulation.17,22 Fluorescence, on the other hand, measures a subset of UV-vis absorbing moieties that return to the ground state via a radiative process, but this phenomenon tends to occur more efficiently in lower molecular weight fractions on a per unit absorbance basis.23–27 The fraction of emitted light (i.e., fluorescence quantum yield) is estimated to be less than 5% of the light absorbed.23,24,28–30 Since efficient absorbing and fluorescing moieties could be dominant in different DOM pools (with some overlap), it is hypothesized that the capacity of each optical surrogate to predict DOM removal would be different.
Since fluorescence EEMs are multi-dimensional with thousands of data points per sample, several methods have been proposed for best utilizing three-dimensional fluorescence data.31–33 Peak picking approaches often identify four regions across an EEM that have been associated with different chemical characteristics. Peaks A (Ex 240–260 nm, Em 380–460 nm) and C (Ex 300–340 nm, Em 400–480 nm) are associated with humic substances. Peaks B (Ex 270–280 nm, Em 300–310 nm) and T (Ex 270–280 nm, Em 330–370 nm) are associated with protein-like or polyphenolic compounds, because phenols and indoles fluoresce in these regions.31,34,35 The peak intensity is often determined by extracting the maximum intensity within the bound region. Another approach for utilizing fluorescence data is to develop parallel factor analysis (PARAFAC) models. PARAFAC statistically decomposes fluorescence EEMs within a diverse dataset into mathematically derived components that represent groups of fluorescing compounds with similar spectra.33,36
Following a peak picking approach, previous coagulation work has demonstrated that there is a strong linear relationship between the relative decrease in peak C intensity and the relative decrease in DOC.37,38 Few studies have explored relationships outside this peak region.39 Further investigation is necessary for fluorescence online monitoring applications. Most commercial fluorescence dissolved organic matter (FDOM) sensors (Ex 370 nm, Em 460) measure fluorescence outside the peak C region,16 but newer light emitting diode (LED) sensors can monitor the peak C region.14 If these quantitative relationships relating fluorescence removal to DOC removal are confined to limited wavelength combinations, then online sensors would have to be developed adhering to these constraints. If not, then predictive models could be developed utilizing existing fluorescence sensor technology.
Several studies have built PARAFAC models for coagulation treatment processes and have evaluated how the distribution of fluorescence components change due to coagulation.6–11 From an online monitoring perspective, the use of PARAFAC presents a technological barrier. Building a robust model requires the collection of many EEMs and a non-trivial time investment to develop a model. To monitor changes in PARAFAC components in real-time would require the spectroscopic capability and computing power to measure an EEM, correct it for spectral bias and absorbance effects, and run an existing PARAFAC model at short time intervals (e.g., minutes). To overcome such barriers, some have suggested using PARAFAC component results to strategically pick single wavelength combinations to monitor in real-time.10,40 Once again, the question arises as to whether or not such wavelength selectivity derived from PARAFAC models is necessary for monitoring DOM removal in a water treatment plant.
The objectives of this study are to 1) evaluate the effect of fluorescence wavelength selection to model DOC removal, 2) quantify the change in model accuracy if fluorescence data is not corrected for absorbance effects, 3) quantitatively compare UV254 and fluorescence intensities as surrogates for DOC removal, 4) evaluate the possibility of improving model performance through the measurement of multiple optical surrogates in tandem, and 5) evaluate optical properties of solely the raw water to model DOC removal at a constant coagulant dose.
2. Experimental methods
2.1 Source waters
Seventeen different source waters were sampled, yielding 22 water samples that represent a wide range of water qualities and DOM character as listed in Table 1. Five samples were collected at locations outside the State of Colorado, USA, and the remainder came from sources within Colorado. Samples 1–4 were a time series, sampled from the Boulder-Lakewood influent to the City of Boulder, Colorado Betasso Water Treatment Plant. Sampling occurred over the course of two months to capture the effects of spring run-off where DOM is terrestrially dominated with minimal reservoir attenuation. Boulder Reservoir was sampled three times throughout the summer and was experiencing a taste and odor episode during the final sampling (source 7). Barr Lake and Jackson Reservoir are in the South Platte River watershed in eastern Colorado and are impacted by point sources of nutrients. The sampling location at Barr Lake was covered by an algal mat. Sources 10–14 were sampled from water utilities around the USA to expand the dataset to include a wider variety of water qualities. Sources 15–22 are from water sources all associated with municipal water utilities across Colorado, USA.| No. | Source | DOC (mgC L−1) | UV254 (cm−1) | SUVA254 (L mgC−1 m−1) | FI | Alkalinity (mg L−1 as CaCO3) | pH | Turbidity (NTU) | No. of coagulant doses |
|---|---|---|---|---|---|---|---|---|---|
| a Indicates wastewater impacted sources. Sources 1–4 and 15–22 are from Colorado. Sources 1–4 were sampled at four different times between June–July 2011 capturing the spring run-off. Sources 5–7 were collected at different times between June–September 2010. | |||||||||
| 1 | Betasso WTP | 7.1 | 0.28 | 4.0 | 1.36 | — | 6.52 | 1.5 | 6 |
| 2 | Betasso WTP | 7.7 | 0.30 | 4.0 | 1.34 | 17 | 5.67 | 1.5 | 6 |
| 3 | Betasso WTP | 2.7 | 0.10 | 3.6 | 1.34 | — | 7.21 | 0.9 | 6 |
| 4 | Betasso WTP | 2.3 | 0.08 | 3.5 | 1.38 | 57 | 7.32 | 1.7 | 6 |
| 5 | Boulder Reservoir | 3.5 | 0.07 | 2.0 | 1.40 | 40 | 7.8 | 23 | 6 |
| 6 | Boulder Reservoir | 3.8 | 0.07 | 1.9 | 1.45 | 70 | 7.72 | 20 | 6 |
| 7 | Boulder Reservoir | 3.7 | 0.07 | 2.0 | 1.46 | 45 | 7.58 | 13 | 3 |
| 8 | Barr Lakea | 6.2 | 0.11 | 1.8 | 1.83 | 94 | 8.68 | 50 | 6 |
| 9 | Jackson Reservoira | 8.1 | 0.11 | 1.3 | 1.67 | 90 | 7.77 | 9 | 6 |
| 10 | Lake Mead, NV | 2.6 | 0.05 | 1.9 | 1.51 | 134 | 7.8 | 0.2 | 1 |
| 11 | Danville, KY | 3.1 | 0.08 | 2.6 | 1.56 | 84 | 7.2 | 4.6 | 6 |
| 12 | Red River, ND | 10.1 | 0.32 | 3.2 | 1.49 | 160 | 7.6 | 92 | 4 |
| 13 | Red Lake, MN | 12.4 | 0.42 | 3.4 | 1.45 | 95 | 7.52 | 51 | 5 |
| 14 | Lake Erie, OH | 2.2 | 0.02 | 1.0 | 1.58 | 63 | 8.02 | 1.1 | 1 |
| 15 | Arvada | 3.0 | 0.05 | 1.5 | 1.46 | 48 | 7.88 | 7.1 | 1 |
| 16 | Boulder | 3.2 | 0.06 | 2.0 | 1.45 | 80 | 8.2 | 7.6 | 1 |
| 17 | Evergreen | 3.7 | 0.14 | 3.7 | 1.40 | 28 | 7.62 | 39 | 1 |
| 18 | Fort Collins | 4.0 | 0.11 | 2.6 | 1.39 | 30 | 7.85 | 3.6 | 1 |
| 19 | Grand Junction | 2.6 | 0.06 | 2.2 | 1.44 | 69 | 8.23 | 2.5 | 1 |
| 20 | Greely-Loveland | 5.6 | 0.13 | 2.3 | 1.43 | 46 | 7.95 | 5.6 | 1 |
| 21 | Greely-Seaman | 7.1 | 0.17 | 2.4 | 1.42 | 80 | 8.23 | 1.3 | 1 |
| 22 | Pueblo | 2.6 | 0.06 | 2.2 | 1.46 | 80 | 8.15 | 5.0 | 1 |
2.2 Coagulation methods
Jar tests were performed using a 6-jar programmable jar tester (Phipps&Bird model 7790-901) with 2 liter jars (Phipps&Bird B-KER2). Aluminum sulfate, alum, (Al2(SO4)3·16H2O, Mallinckrodt Chemicals, 3208-04) was used as the coagulant. Mixing conditions included a rapid mix phase (1 minute, 290 rpm), two flocculation phases (10 minutes at 55 rpm and 10 minutes at 20 rpm) and a sedimentation period (30 minute with no mixing). Turbidity and pH measurements were recorded directly after sedimentation. The supernatant was filtered through muffled (550 °C for 4 hours) and rinsed 0.7 μm filters (Whatman GF/F) to isolate the dissolved fraction for DOC, UV absorbance, and fluorescence analyses. A GF/F filter is commonly used for fluorescence analyses, because organic-based filters can leach fluorescent material.41 Samples were coagulated and analyzed within a week. Raw water samples were also analyzed for the same water quality parameters (Table 1). Samples were stored in muffled amber bottles in the dark at 4 °C.The number of alum doses utilized varied between water sources depending on the quantity of water available and is listed in Table 1. For readily available source waters, a series of jar tests were performed targeting the point of diminishing coagulant returns. For water sources with limited volume, only one alum dose could be applied, and the dose was estimated using the EPA Water Treatment Plant Model to meet the Stage 1 Disinfection Byproduct Rule total organic carbon removal requirement.42
2.3 Analytical methods
Initial water quality conditions are listed in Table 1. Alkalinity of all source waters was determined using a Hach digital titrator (model 16900-01) and the manufacturer's standard method. pH measurements were collected using a Fisher Scientific Accumet AB15 for all raw water and coagulated samples prior to filtration. A Hach 2100 N turbidimeter was used for all turbidity measurements on raw and coagulated samples, which are reported in nephelometric units (NTUs). DOC was measured with a Shimadzu TOC-VCSH with autosampler using a non-purgeable organic carbon method. The accuracy and precision of a 5 mgC L−1 potassium hydrogen phthalate standard were both within 5% throughout the study. UV-Vis absorbance from 200 to 600 nm was measured using a spectrophotometer (Varian Cary Bio 100, Agilent Technologies, CA) with a 1 cm path length quartz cell. SUVA254 was calculated by dividing the UV254 value by the DOC concentration and reported in units of L mgC−1 m−1.Fluorescence EEMs were measured for all raw and coagulated samples (Fluoromax-4, Horiba, Edison, NJ) yielding a total of 98 unique samples. Excitation wavelengths ranged from 240 nm to 450 nm in 10 nm increments with emission scans collected from 300 to 560 nm in 2 nm increments. The bandpass for both excitation and emission monochromators was set at 5 nm, and the integration time was 0.25 s. Unless explicitly stated (section 3.3), fluorescence data were corrected following published methods43 and are presented in Raman Units (RU). Briefly, measured intensities incorporated instrument-specific correction factors, were corrected for primary and secondary inner filter effects, blank subtracted and normalized to the Raman area of lab-grade water at an excitation of 350 nm. The fluorescence index (FI) was calculated as the ratio of emission intensities (470 nm/520 nm) at excitation 370 nm.44,45 Method development work on this instrument has determined that the analytical error of the fluorescence metrics used in this study are less than 3% for fluorescence intensities in the peak A and C regions and less than 2% for FI. Any sample with a UV254 greater than 0.2 cm−1 was diluted prior to analysis to minimize concentration-based effects.32
2.4 Regression and statistical analysis
Several regression models were built to predict relative DOC removal by coagulation. Percent DOC removal (%DOCr) was calculated according to eqn (1), where DOC0 is the DOC concentration of the raw water and DOCf is the DOC concentration after coagulation. The relative decrease in fluorescence intensity (%Fluor) at discrete wavelength combinations in an EEM (λex, λem) was calculated in a similar fashion (eqn (2)). The percent decrease in absorbance at 254 nm (%UV254r) was calculated according to eqn (3). Finally, the percent increase in fluorescence index (%FIincr) after coagulation was calculated according to eqn (4).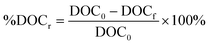 | (1) |
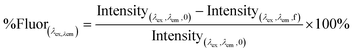 | (2) |
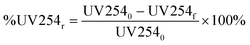 | (3) |
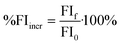 | (4) |
Regression analyses were performed using the Statistics Toolbox in Matlab® (2014b). All tests were performed at a 95% significance level (α = 0.05). For each model, p values for each model parameter were evaluated for term significance. The Kolmogorov-Smirnov (KS) test was used to evaluate the residual distribution. The R2 predicted (R2pred) values were calculated based on the prediction error sum of squares (PRESS). This parameter is more conservative than R2 and penalizes for model overfitting. The 95% prediction interval was also calculated to assess the uncertainty associated with predicting future observations. Finally, model residuals plotted against optical and DOC data were inspected to identify systematic bias in the models.
A PARAFAC model was built using the drEEM toolbox.33 Full details regarding model development and validation are presented in the ESI.† Briefly, the original model included 113 EEMs of raw and alum coagulated water samples, including duplicates. One outlier was identified and removed from the dataset, yielding 112 EEMs in the final model. Sample EEMs were normalized prior to modeling. Models containing 2 to 9 components were investigated, and a 6-component model selected for further investigation and validation based on an analysis of sum-squared error (Fig. S1 and S2†). A random initialization procedure of the 6-component model identified a minimum-error solution with a core consistency value of 80.6%. The 6-component model was further validated by split half analysis (Fig. S3†) following the S4C4T2 approach as described in Murphy et al. (2013). Model residuals were also inspected visually and did not reveal significant, non-random residuals.
3 Results
3.1 Range of source waters and coagulation behaviors.
This study examined twenty-two waters with a wide range of water quality characteristics, listed in Table 1, especially for SUVA254 and alkalinity. For source waters with total organic carbon (TOC) values greater than 2 mgC L−1, the Stage 1 DBP Rule dictates required TOC removal based on a 3 × 3 matrix according to raw water TOC and alkalinity.42 Seven of the nine possible bins were covered with this data set, based on DOC values, including both extremes (i.e., low TOC-low alkalinity and high TOC-high alkalinity).The differences in water quality characteristics led to a wide range of coagulation behaviors as depicted in Fig. 2. Alum doses of 5 to 120 mg L−1 were used resulting in DOC removals between 5 to 70%. The observed DOC removal agreed with those predicted by the coagulation model published by Edwards (1997) using the “General Low DOC” parameters.22 For 85% of the coagulated waters, the predicted DOC removal based on SUVA254 and final pH was within 15% of the experimental data (Fig. S5†). The model under predicted the DOC removal for the high SUVA254, low alkalinity waters by about 20% (sources 1–3). The general agreement between the dataset in this study and the model helps substantiate that this data not only covers a wide range of coagulation behaviors, but also conforms to the observed coagulation behaviors reported elsewhere.
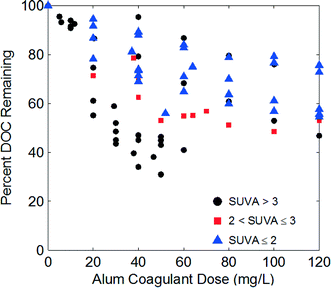 | ||
| Fig. 2 Relative DOC removal as a function of alum dose for all waters. Markers indicate raw water SUVA254. | ||
In addition to SUVA254, other optical properties demonstrate that the modeling approach in this study is based on a dataset with a wide range in water quality characteristics. The 6-components identified in the PARAFAC model exhibit a wide range of fluorescence emission characteristics (Table 2, Fig. S4†). The distribution of validated components span emission wavelengths from 320 to 516 nm demonstrating a wide range of fluorescence behaviors between raw waters. Red-shifted fluorescence (longer emission wavelengths) is often associated with terrestrially-dominated sources where organic matter is more aromatic, highly conjugated and exhibits a greater Stoke's shift.32,46,47 Other studies have found the most red-shifted component (C3) with emission peaks greater than 500 nm is present in terrestrially dominated systems.48 Blue-shifted components occurring at shorter emission wavelengths (e.g., C1 and C5) are often associated with microbial-influenced sources.48 Each validated component in this study is in good agreement with components found in at least one other published coagulation study. Comparing the relative abundance of each PARAFAC component (Fmax/∑Fmax) in the source waters, components 1 and 2 were the most abundant but the contribution ranged between source waters (Table 2). For example, the abundance of component 1 in the source waters ranged from 21–37%, which is greater than the range observed for a similar component in a recent coagulation study by Sanchez et al. (2013). The relative abundance of components 3 to 6 also varied between sources (Table 2). These results demonstrate that the dataset used in this study is based on source waters with a wide variety of fluorescence behaviors.
| Comp. | Excitation maxima (nm) | Emission maxima (nm) | Relative abundance in source waters | Component removal (37–40 mg L−1 alum) | Similar reported components (ref.) |
|---|---|---|---|---|---|
| C1 | <240, 320 | 416 | 21–37% | 7–68% | 6, 8–11, 48 |
| C2 | <240, 360 | 460 | 10–23% | 7–74% | 8,9,10,11 |
| C3 | <240, 400 | 516 | 8–25% | 12–84% | 48 |
| C4 | 250 | 436 | 8–20% | 14–91% | 10,48 |
| C5 | <240, 290 | 360 | 6–23% | −5–22% | 9,10 |
| C6 | <240, 270 | 320 | 3–15% | −12–51 | 10,11 |
PARAFAC is one method for identifying the variability in fluorescence behaviors. Other fluorescence-based compositional indicators also exhibited a wide range of behaviors in the source waters (Table S1†). FI, an indicator of relative aromaticity44 and differences in peak emission (Stoke's shift),32 ranged from 1.34 to 1.83. Peak C emission wavelength, a parameter correlated with aromaticity, bond conjugation and hydrophobicity,46,49 ranged from 408 to 440 nm. Specific peak C intensity, an indicator of fluorescence intensity per unit carbon, ranged from 0.03 to 0.18 RU L mgC−1, which is greater than the range observed for common end-member organic matter isolates.32 Finally, peak C intensity divided by the absorbance at the same excitation wavelength (peak C/UV), a surrogate for fluorescence efficiency per unit absorbance,26,49 varied by more than a factor of 2. Spearman rank-order correlation coefficients between fluorescence compositional indicators of the raw water samples (Table S2†) show that many optical parameters are significantly correlated to each other. In particular, peak C emission wavelength is correlated with SUVA254, relative abundances of all 6 PARAFAC components, FI, Specific peak C intensity and peak C/UV. These results suggest that a PARAFAC analysis is not necessary to judge the statistical variation of fluorescence behaviors in a dataset, and a range of other optical parameters could suffice.
In addition to DOC, a wide range of fluorescence behaviors upon coagulation was observed. Sixteen of the 22 source waters were coagulated with an alum dose between 37–40 mg L−1. By comparing changes in fluorescence at a constant coagulant dose, the range of coagulation behaviors are further exemplified. Table 2 shows that for a similar alum dose, the decrease of each PARAFAC component (Fi,coag/Fi,raw) varied greatly. For example, removal of component 1 (one of the more abundant) ranged from 7–68% at the same dose. Some components (5 and 6) exhibited a small increase in Fmax values in the coagulated waters, but these components were of low abundance in the source water (likely not a significant change). These results demonstrate that the dataset used in this study investigates a wide range of source water qualities with a wide range of fluorescence and coagulation behaviors. Thus, the results can be considered broadly applicable to other surface water sources.
3.2 Relationship between fluorescence and DOC removal
%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOCr = a DOCr = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) · ·![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) % %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fluor(λex,λem) + b Fluor(λex,λem) + b | (5) |
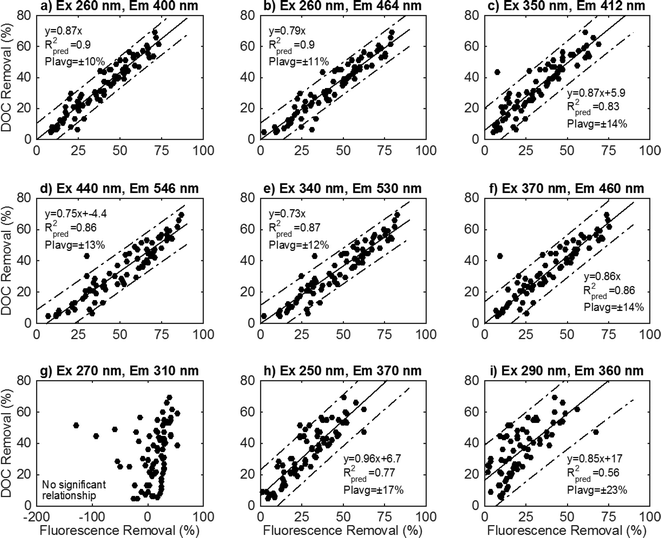
To illustrate the data analysis process, nine wavelength pairings within each EEM were analyzed in an exploratory analysis (Fig. 1). These preliminary locations were selected to represent a range of initial fluorescence intensities across an EEM yet avoid the peak A and C regions that have been analyzed previously.37–39 The fitted regressions and diagnostic results from these nine locations are illustrated in Fig. 3.
Linear regression models at 6 screening locations exhibited strong linear relationships according to eqn (5) (Fig. 3a–f). The slope terms for these six screening locations are significant (p < 0.05). A statistically significant intercept term was only found for two of these six wavelength combinations (Fig. 3c, d). The best-fit model removes the intercept term when not significant (Fig. 3a, b, e, f). At each of these locations, the residuals are random and presumed normally distributed (KS test p > 0.1). The 95% prediction intervals show little variation between different treatment levels (lack of curvature), which is due to the error variance term dominating the prediction interval calculation. Since there is less than a 1% variation in the prediction interval at these wavelength combinations, the average prediction interval (PIavg) percentage is reported and used to compare models at different wavelength combinations. The R2pred values vary from 0.83–0.9, and PIavg ranges from 10–14% between wavelength combinations. This preliminary analysis demonstrates that multiple wavelength combinations across an EEM can be used to develop satisfactory models that predict DOC removal.
The remaining three selected wavelength combinations did not exhibit robust linear regressions with DOC removal. In the traditionally defined peak B region (Fig. 3g), there is no significant relationship between relative fluorescence intensity decrease and DOC removal (slope parameter p = 0.12). In some samples, intensity in this region increased with DOC removal. Two screening locations exhibited a significant relationship between fluorescence intensity decrease and DOC removal (Fig. 3h, i), but exhibited worse model diagnostic parameters. The prediction interval is greater than other locations (17–23%). The residuals are not normally distributed for the wavelength combination Ex 250/Em 370 (KS test p < 0.05), and the model may benefit from a variance stabilizing transformation to better model the data.
Model parameters were screened and evaluated for significance at each wavelength combination (Fig. 4). The slope coefficient (a) in eqn (5) was found to be dependent primarily on the emission wavelength rather than excitation wavelength, as shown by predominantly vertical contour lines in Fig. 4a. The relative unimportance of excitation wavelength agrees well with the PARAFAC components having dual excitation peaks. When the Fmax intensity of component decreases due to coagulation, intensity in the EEM decreases by the same relative amount across multiple excitation wavelengths. The slope parameter approaches a value of 1 near emission values of 375 nm. As the emission wavelengths increase above 375 nm, the a value decreases to about 0.75. At emission wavelengths less than 375 nm, the a value rapidly decreases to less than 0.1. This boundary at 375 nm coincides with the transition between C5 (Em = 360 nm) and C1 (Em = 416 nm). The slope coefficient in each regression is significant at all EEM locations except for the peak B region (Ex 270 nm, Em 310 nm), where the a value falls below 0.1 (parameter p > 0.1) as shown in Fig. 4b. The region where no significant relationship is found neatly coincides with fluorescence associated with phenolic functional groups (peak B, C6)34,35 and blue-shifted PARAFAC components (e.g., C5) often associated with microbial systems. The peak B region is often associated with moieties that can be more hydrophilic in nature and less amenable to removal by coagulation than material that fluoresces at higher emission wavelengths.9,11 A lack of relationship in this region demonstrates that the moieties responsible for fluorescence in this region do not track well with bulk DOC removal during coagulation and that the emission wavelength where this modeling approach is not adequate coincides with the underlying fluorescence behavior, as identified by PARAFAC decomposition.
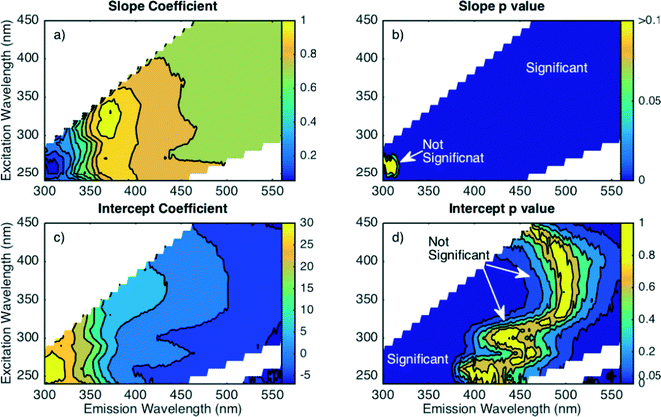 | ||
| Fig. 4 Parameter screening for linear regression (eqn (5)) fit to each wavelength combination for fully corrected fluorescence data as: a) slope parameter estimate, b) slope parameter p value (significance), c) intercept parameter estimate, and d) intercept parameter p value (significance). | ||
The intercept parameter (b in eqn (5)) was also screened for trends in coefficient value and statistical significance. Like the slope, the intercept coefficient value depends largely on the emission wavelength with predominantly vertical contour lines (Fig. 4c). At wavelength combinations where the coefficient value is approximately ±5, the intercept is not statistically significant (Fig. 4d). From a practical standpoint, an intercept value is indicative of a fraction of DOM that can be removed with no associated change in fluorescence at the given wavelength combination. An insignificant intercept indicates that DOC removal is always accompanied by a change in fluorescence intensity at a given wavelength combination. The EEM region with insignificant intercept terms occurs at longer emissions wavelengths (450–500 nm), where PARAFAC models have identified fluorescence components that are most amenable to removal.6,8,9 In this study, PARAFAC components C3 and C4 coincide with this region and these two components were the most amenable to removal (greatest percent decrease) in 73 of 77 coagulated samples. On the contrary, regions with a significant intercept term occur at shorter emission wavelengths (<400 nm), which is associated with more hydrophilic DOM that is less amenable to coagulation.49 These results demonstrate that the form of regression model depends on the selected monitoring wavelengths, and the inclusion of an intercept term suggests a fundamental connection between the fluorescent DOM character and its affinity for removal by coagulation.
After screening for parameter significance, regression models were developed following the best-fit model. At wavelength combinations with insignificant intercept terms, a model was developed only containing the slope coefficient (a). Residual plots were examined visually every 10 nm at each excitation wavelength (Fig. S6†) and no systematic behavior was found. The KS test determined that the there are only two regions where the residuals have a non-normal distribution (Fig. S7†). One region is systematic and appears in a region surrounding Ex 260 nm/Em 370 nm near component C5. Although this region found a statistically significant regression and a moderate prediction interval (15–25%), the linear modeling approach proposed is better suited for other wavelengths combinations where residuals are normally distributed. There are also sporadic areas with non-normal residuals at wavelengths that fall between 1st order Rayleigh and 1st order Raman scattering of the solvent. From a practical online monitoring standpoint, this region of the EEM would not be very conducive to monitor with online sensors, because blank subtraction with inner filter corrected data, as presented here, is necessary to minimize scatter effects.
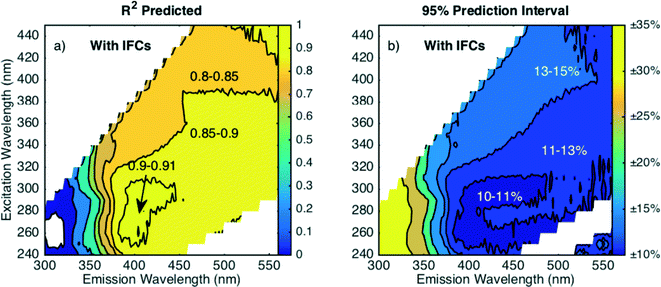
Regression diagnostic statistics (R2pred and PIavg) were calculated for the best-fit models to assess goodness-of-fit and are shown in Fig. 5. At emission wavelengths greater than 375 nm, there was little variation in R2pred with all values greater than 0.8 (Fig. 5a). There is a small region around excitation 250–300 nm and emission 400 nm where R2pred values are maximized with values between 0.9–0.91. The wavelengths where R2pred is maximized roughly coincides with the location of component C1, one of the most abundant components in the raw waters (Table S1†). Other studies have found PARAFAC components with a peak near excitation 300 nm and emission 400 nm,6,8–11,40,48,50 although this component is often not the most amenable to removal by coagulation6,8,9 as is also the case here. In this study, components C3 and C4 were preferentially removed compared to the more abundant C1 and C2 components in most samples. In the region where R2pred is maximized, PIavg is minimized with values between 10–11% (Fig. 5b). In regions where R2pred ranged from 0.8–0.9, PIavg ranged from 11–15%. Following the relative removal of PARAFAC components rather than individual intensities does not improve model performance, as the best R2pred and PIavg values for analogous models are about 0.85 and 15%, respectively (Fig. S8†). While one EEM region did slightly outperform others with a local PIavg minimum, these differences may not be important from a practical monitoring perspective. Any wavelength combination selected at emission wavelengths greater than 375 nm can model the percent DOC removal within 15%. Where most commercial FDOM sensors monitor (Ex 370 nm, Em 460 nm), the average prediction interval is 13.6%, which is good from a practical standpoint. This prediction interval is interpreted as good considering the propagated error in calculating %DOCr based on two independent DOC measurements each with an analytical error of 5% is about 7%.51 These results also suggest that building more complex statistical models, such as PARAFAC, to refine wavelength selection is unnecessary as there is little difference in model performance across most EEM wavelengths. The approach presented here can optimize wavelength selection for online monitoring in a fraction of the time required to build a PARAFAC model.
3.3 Relationship between fluorescence and DOC removal without IFCs
The effect of inner filter corrections (IFCs) on each regression was determined by applying the model in eqn (5) to fluorescence data that had not been corrected for absorbance effects. Correcting for absorbance is standard procedure for analyzing EEMs on bench-top spectrofluorometers,43 but in situ fluorescence sensors do not simultaneously monitor and correct for sample absorbance. In the case of coagulation, the measured fluorescence of the raw water before coagulation will be attenuated more due to absorbance than the treated water, leading to a bias in fluorescence measurements. Reanalyzing uncorrected data will evaluate the potential incremental change in model performance that may be encountered from in situ sensors where data is not corrected for absorbance.Compared to the corrected data, the parameter estimates for uncorrected data change slightly but still exhibit the same general patterns (Fig. S9†). The region where the regression is insignificant increases in the peak B region, and new regions of insignificance emerge along the 1st order Raman scattering line due to imperfect blank subtraction. At emission wavelengths greater than 400 nm, the slope coefficient changes slightly (less than 0.08). The wavelength pairs where the intercept term is insignificant shift to longer emission wavelengths (Fig. S9d†) compared to corrected data (Fig. 4d). The residuals are presumed normally distributed at most wavelength combinations (KS test p > 0.1) except for isolated areas (Fig. S10†). Therefore, the same general model form can be used, but separate models should be built for monitoring applications with and without inner filter corrections.
Model performance decreased at lower excitation and emission wavelengths when fluorescence intensities are not corrected for absorbance effects as shown in Fig. 6. The difference is most apparent at excitation wavelengths less than 280 nm where R2pred values decrease (Fig. 6a) and PIavg values increase (Fig. 6b) compared to corrected data (Fig. 5). Without inner filter corrections, the wavelengths where PIavg exhibits a local minimum shifts. Fig. 6b shows that PIavg is minimized at emission wavelengths greater than 450 nm (excitation 300–350 nm). At excitation wavelengths greater than 350 nm, there is little difference in prediction intervals between corrected and uncorrected data. These trends are supported by theory, because absorbance preferentially attenuates measured fluorescence intensities at lower wavelengths due to the monotonic increase of DOM absorbance with decreasing wavelength. Models based on fluorescence data at lower wavelengths will have greater bias due to absorbance than those based on longer wavelengths.
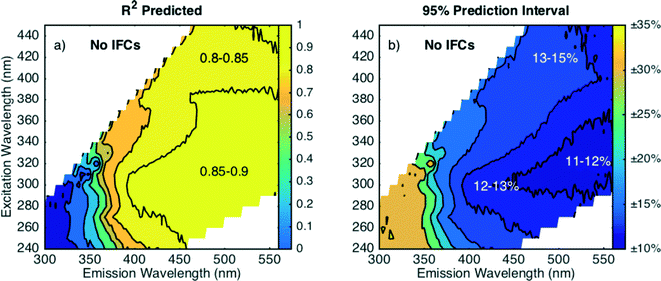 | ||
| Fig. 6 Regression diagnostic criteria a) R2pred and b) average 95% prediction interval for fluorescence data without inner filter corrections. | ||
Even though there is a measurable decrease in model performance, DOC removal can still be modeled within 15% at emission wavelengths greater than 400 nm without inner filter corrections, which is promising for the potential application of in situ fluorescence sensors in water treatment plants. Commercial FDOM sensors monitor fluorescence at longer wavelengths (Ex 370 nm, Em 460 nm), and the prediction interval is practically unaffected by the lack of inner filter corrections with an increase in PIavg from 13.6 to 13.7% (for best-fit models).
3.4 Comparison to UV absorbance at 254 nm
The performance of fluorescence surrogates to predict DOC removal was compared to the performance of UV254. Regression models in the form of eqn (6) were developed, and regression diagnostics (R2pred and PIavg) were assessed. An intercept term was found to be insignificant (parameter p > 0.1, Fig. S11†). Other wavelengths besides 254 nm were investigated, but there was little difference in model performance for absorbance wavelengths between 250–280 nm (Fig. S11†).%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOCr = a DOCr = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) · ·![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) % %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) UV254r UV254r | (6) |
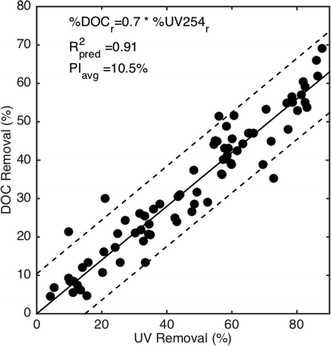
The relative removal of UV254 absorbance can model DOC removal within 10.5% as shown in Fig. 7 with a slope coefficient value of 0.7. A slope value less than 1 aligns with past work demonstrating that UV absorbance is removed preferentially compared to DOC.52,53 The relative removal of DOC is always less than the relative UV254 removal. The slope value agrees with other studies. Cheng et al. (2004) reported slope values between 0.68 and 0.78 when regressions are rearranged to match the form in eqn (6). A reported metadata analysis evaluated SUVA254 as a predictor for both %DOCr and %UV254r for coagulation processes targeting either the maximum organic matter removal or the point of diminishing returns.54 When the empirical models are combined and rearranged to the form of eqn (6), the slope value is 0.67. Therefore, UV254 as a predictor performs as well as the best performing fluorescence region with inner filter corrections.
3.5 Expanded linear regression models
Multiple linear regression models were explored to determine if model performance could be improved by monitoring multiple optical surrogates simultaneously. Multiple linear regression models were built with the relative change in UV254 and fluorescence intensity as independent variables (Table 3, model 1). Both parameter coefficients (a and b) were dependent on the fluorescence excitation and emission wavelengths, which is an artifact of a model with collinearity between independent variables (Pearson correlation ρ > 0.9). There was no decrease in the prediction interval by measuring the change in both UV254 and fluorescence compared to UV254 alone as the PIavg ranged from 9.5–11.5% depending on the fluorescence wavelengths selected.| Model no. | Model | R 2pred | PI range | Pearson correlation of independent variables |
|---|---|---|---|---|
| All models built with fully corrected fluorescence data. All coefficients in natural units (not standardized).a Invalid model. FIfinal term not significant (p = 0.12). | ||||
| 1 | %DOCr = a %UV254r + b %Fluor(λem,λex) | 0.88–0.92 | 9.5–11.5% | ρ = 0.9–0.96 (p < 0.01) |
| 2 | %DOCr = 2.9 %FIincr | 0.85 | 13.7–14.0% | — |
| 3 | %DOCr = 0.51 %UV254r + 0.75 %FIincr | 0.91 | 9.9–11.0% | ρ = 0.925 (p < 0.001) |
| 4a | %DOCr = a %UV254r + b FIfinal + c | — | — | — |
| 5 | %DOCr = 0.64 %UV254r − 3.5 pHfinal + 27 | 0.91 | 10.1–11.2% | ρ = −0.55 (p < 0.001) |
Relationships incorporating FI were investigated, because this intensity ratio is less impacted by inner filter effects than single fluorescence intensities.32 FI increased after DOM removal by coagulation as the more hydrophobic and aromatic fraction is removed.55 A significant linear regression was found between the relative increase in FI (%FIincr) and the relative decrease in DOC (Table 3, model 2 and Fig. S12†). The prediction interval ranged from 13.7–14% across treatment levels and does not outperform the UV254 model (PIavg = 10.5%). A multiple linear regression was developed with %UV254 and %FIincr as independent variables (Table 3, model 3 and Fig. S13†). Since the scale of the independent variables varied, the data was standardized prior to model fitting to evaluate term significance and effects. The model performance did not improve, and the independent variables were strongly correlated (Pearson ρ = 0.925). A third multiple linear regression model was evaluated using %UV254 and the FI of the filtered water after coagulation (FIfinal) as independent variables (Table 3, model 4). Even after standardizing the independent variables, a preliminary screening found that the FIfinal term was not statistically significant (p = 0.12). Finally, a model incorporating %UV254 and the pH after coagulation (pHfinal) was investigated because DOM coagulation and adsorption are dependent on pHfinal.22 Although these independent variables are less correlated, model performance was not improved (Table 3, model 5, Fig. S14†). Each of these models suggests that monitoring absorbance and fluorescence in tandem do not offer improved model performance for DOC removal, because the independent variables are strongly correlated with one another. As such, the simplest models following the relative change in either UV254 or fluorescence (within the optimal region) offer the best simulation of DOC removal.
3.6 Predictive removal based on raw water characteristics
The modeling approaches investigated thus far require information both before and after coagulation. The models presented in sections 3.2–3.5 determine DOC removal based on optical measurements of the raw and coagulated water. The other coagulation model applied, developed by Edwards (1997), predicts the final DOC given the raw water SUVA254 and pH after coagulation. It would be advantageous to develop a model that predicts DOC removal based on the raw water optical characteristics alone. To investigate such predictive models, a subset of this dataset where the alum dose was constant between waters (37–40 mg L−1) was evaluated. Relationships relating raw water characteristics to DOC removal are only meaningful under similar coagulation conditions, such as the same coagulant dose or the point of diminishing returns.54 Source waters 12 and 13 (Red River and Red Lake) were removed from these statistical relationships, because there were outliers in all tested relationships. They were likely outliers, because based on their distribution of PARAFAC components, the ratio of C4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6 components in the raw water was much greater than the other raw waters (Fig. S15n†). These waters were not outliers in the modeling approaches taken in sections 3.2–3.5.
C6 components in the raw water was much greater than the other raw waters (Fig. S15n†). These waters were not outliers in the modeling approaches taken in sections 3.2–3.5.
Several predictive relationships were investigated based on compositional optical properties, but none greatly outperformed another. Following the approach by Shutova et al. (2014), the ratio of raw water component scores as a predictor of DOC removal was investigated, and while many had statistically significant relationships, the component ratio with the smallest PIavg (±13.7%) for the regression was C1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C3 (Fig. S15b†). Increases in the intensity of the blue-shifted component (C1) relative to the most red-shifted component (C3) led to a decrease in DOC removal at the same alum dose (~40 mg L−1). Significant relationships were also identified between the relative abundance of PARAFAC components in the raw water and DOC removal at an alum dose 40 mg L−1 (Fig. S16†). The strongest relationships with the smallest PIavg (16%) were found for components C3 and C5.
C3 (Fig. S15b†). Increases in the intensity of the blue-shifted component (C1) relative to the most red-shifted component (C3) led to a decrease in DOC removal at the same alum dose (~40 mg L−1). Significant relationships were also identified between the relative abundance of PARAFAC components in the raw water and DOC removal at an alum dose 40 mg L−1 (Fig. S16†). The strongest relationships with the smallest PIavg (16%) were found for components C3 and C5.
All of these relationships suggest that the relative contribution of the more blue-shifted components (C1 and C5) that occur at peak emission wavelengths less 420 nm compared to the most red-shifted component C3 (peak emission 516 nm) is an in important factor dictating coagulation efficiency. This trend aligns with other observations that DOM sources with greater aromaticity, molecular size and/or hydrophobicity exhibit greater relative fluorescence at longer emission wavelengths (i.e., red-shifted),24,32,47,49 and these characteristics (i.e., aromaticity, molecular size and hydrophobicity) also make DOM more amenable to removal by coagulation.56
Based on these trends, ratios based on single wavelength pairings corresponding to PARAFAC components C1, C3 and C5 were evaluated as predictors for DOC removal at a single coagulant dose (alum ~ 40 mg L−1). Each ratio consists of the intensity of different emission wavelengths collected at the same excitation wavelength to compare the relative intensities at longer and shorter emission wavelengths. To mimic the ratio of C3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
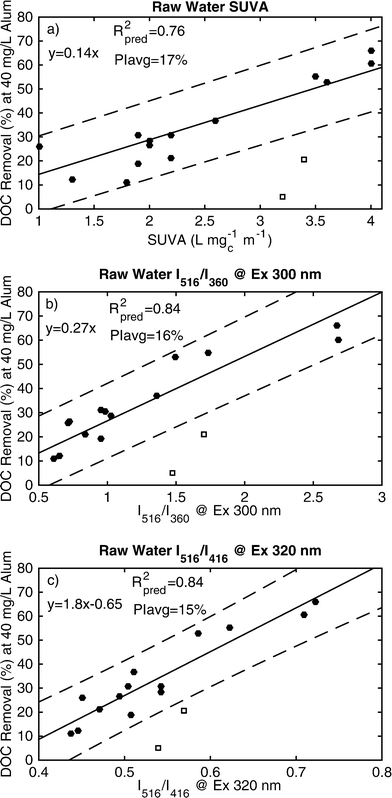
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C1, the ratio of intensities at emission wavelengths 516 and 416 nm (I516/I416) at excitation 320 nm were calculated for each raw water and models DOC removal with a PIavg of ±15% (Fig. 8c). A ratio based on the relative abundances of C3 and C5 was defined as the ratio of emission intensities at 516 nm and 360 nm (I516/I360) at excitation 300 nm. This ratio also modeled DOC removal with a PIavg of ±16% (Fig. 8b). FI of the raw water was a poor indicator of coagulation efficiency (PIavg = ±30%), because the ratio spans too small a difference in emission wavelengths (520 vs. 470) and does not capture the differences of the relevant PARAFAC components (C3 vs. C5 or C1). SUVA254 of the raw water modeled DOC removal with a PIavg of ±17% (Fig. 8a). These differences in model performance (15% compared to 17%) likely does not have practical implications, especially considering the uncertainty of a single DOC measurement is typically ±2–5%. It is important to note that these general trends will likely hold at other coagulant doses, but the quantitative models (i.e., slope and intercept) will change.
C1, the ratio of intensities at emission wavelengths 516 and 416 nm (I516/I416) at excitation 320 nm were calculated for each raw water and models DOC removal with a PIavg of ±15% (Fig. 8c). A ratio based on the relative abundances of C3 and C5 was defined as the ratio of emission intensities at 516 nm and 360 nm (I516/I360) at excitation 300 nm. This ratio also modeled DOC removal with a PIavg of ±16% (Fig. 8b). FI of the raw water was a poor indicator of coagulation efficiency (PIavg = ±30%), because the ratio spans too small a difference in emission wavelengths (520 vs. 470) and does not capture the differences of the relevant PARAFAC components (C3 vs. C5 or C1). SUVA254 of the raw water modeled DOC removal with a PIavg of ±17% (Fig. 8a). These differences in model performance (15% compared to 17%) likely does not have practical implications, especially considering the uncertainty of a single DOC measurement is typically ±2–5%. It is important to note that these general trends will likely hold at other coagulant doses, but the quantitative models (i.e., slope and intercept) will change.
3.7 Applicability to in situ sensors
These models developed from bench-top spectrometers and fluorometers may represent the best-case scenarios for online monitoring based on optical properties. The comparisons between modeling approaches (i.e., UV vs. fluorescence, application inner filter corrections) determine the incremental difference associated with following different optical phenomena (e.g., absorbance vs. fluorescence), different fluorescence wavelengths and different data correction procedures (i.e., inner filter corrections). These models represent best-case scenarios, because all samples are filtered and analyzed at room temperature on highly accurate instruments. The prediction accuracy associated with in situ sensors have yet to be determined and may differ due differences in instrument accuracy, sensitivity and the impact of other water quality characteristics (e.g., turbidity and temperature).16Even though there is little difference in the prediction interval between absorbance and fluorescence surrogates, online sensors based on absorbance may be operationally simpler to implement. To match the performance of a UV254 surrogate, fluorescence data must be corrected for absorbance inner filter effects. This step requires measurement of absorbance in tandem with fluorescence, at which point simply measuring UV254 as the primary optical parameter suffices. One exception would be if other water quality parameters affect the optical surrogate measurements differently. For example, both UV254 and fluorescence measurements are affected by turbidity, which scatters light along the optical path length. Turbidity effects are quite important in this application, because raw water entering a drinking water treatment plant will have greater turbidity than filtered water post-coagulation. Yoo et al. (2014) suggests that UV-vis absorbance is more affected by turbidity at levels less than 50 NTU than FDOM fluorescence. Downing et al. (2012) showed that the attenuation due to turbidity depends on a fluorescence sensor path configuration. These differences may be more important considerations for selecting fluorescence monitoring over UV-vis absorbance than wavelength selection. The practical application of absorbance or fluorescence for online monitoring of DOC removal will likely rely more on building models that can accurately compensate optical data for other system variables (i.e., turbidity) rather than on selecting monitoring wavelengths through complex fluorescence modeling.
4 Conclusions
Regressions were developed to model the relative DOC removal by coagulation by following the relative change in UV and fluorescence based optical surrogates. A subset of samples coagulated at the same coagulant dose (~40 mg L−1) were analyzed to develop models relating raw water composition based on UV and fluorescence data to DOC removal. The study found the following.• The relative decrease in fluorescence intensity modeled DOC removal within 10.5–15% at emission wavelengths greater than 375 nm. Wavelength selection was relatively unimportant within this range.
• Lack of inner filter corrections decreased model performance at excitation wavelengths less 350 nm, but model prediction intervals were within 11–15%.
• The relative decrease in UV254 modeled DOC removal within 10.5%, matching the performance of the best performing fluorescence wavelength combinations.
• Monitoring multiple optical surrogates in tandem did not improve the prediction interval for DOC removal due to correlated independent variables.
• The relative contribution of fluorescence at longer emission wavelengths (516 nm) to shorter (<416 nm) is a dominant factor in modeling coagulation efficiency at a given coagulant dose. Fluorescence based proxies do not outperform SUVA254 as a predictor from a practical standpoint.
Acknowledgements
The authors would like to acknowledge Jennifer Moutinho, Amanda Hohner and Katherine Dowdell for their assistance in the laboratory analyses. The authors also thank Dr. David Clough for the statistical guidance. The authors would like to thank all of the utilities that participated by providing raw water for the study and Colorado Department of Public Health and Environment for partial funding. JAK acknowledges the National Science Foundation Graduate Research Fellowship program for financial support (DGE 1144083).References
- USEPA, National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule, 40 CFR Parts 9, 141, and 142, 2006, vol. 71 Search PubMed.
- J. Edzwald, W. C. Becker and K. L. Wattier, J. - Am. Water Works Assoc., 1985, 77, 122–132 CAS.
- E. M. Perdue and J. D. Ritchie, in Treatise on Geochemistry, ed. H. D. Holland and K. K. Turekian, Elsevier, 2009, pp. 1–46 Search PubMed.
- E. S. Hall and R. F. Packham, J. - Am. Water Works Assoc., 1965, 57, 1149–1166 CAS.
- J. Edzwald, Water-1978 (AICHE Symposium Series), 1978, vol. 75, pp. 54–62 Search PubMed.
- S. A. Baghoth, S. K. Sharma and G. L. Amy, Water Res., 2011, 45, 797–809 CrossRef CAS PubMed.
- D. W. Johnstone, N. P. Sanchez and C. M. Miller, Environ. Eng. Sci., 2009, 26, 1551–1559 CrossRef CAS.
- N. P. Sanchez, A. T. Skeriotis and C. Miller, Environ. Sci. Technol., 2014, 48, 1582–1591 CrossRef CAS PubMed.
- N. P. Sanchez, A. T. Skeriotis and C. M. Miller, Water Res., 2013, 47, 1679–1690 CrossRef CAS PubMed.
- Y. Shutova, A. Baker, J. Bridgeman and R. Henderson, Water Res., 2014, 54, 159–169 CrossRef CAS PubMed.
- K. M. H. Beggs and R. S. Summers, Environ. Sci. Technol., 2011, 45, 5717–5724 CrossRef CAS PubMed.
- W. Cheng, F. Chi and R. Yu, Environ. Monit. Assess., 2004, 98, 421–431 CrossRef CAS.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley Interscience, 1970 Search PubMed.
- G. Y. Yoo, Y. Jeong, E. J. Lee, J. H. Park and N. H. Oh, Biogeosci. Discuss., 2014, 11, 16855–16876 CrossRef.
- J. F. Saraceno, B. A. Pellerin, B. D. Downing, E. Boss, P. M. Bachand and B. A. Bergamaschi, J. Geophys. Res.: Biogeosci., 2009, 114, G00F09 Search PubMed.
- B. D. Downing, B. A. Pellerin, B. A. Bergamaschi, J. F. Saraceno and T. E. C. Kraus, Limnol. Oceanogr.: Methods, 2012, 10, 767–775 CrossRef CAS.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS.
- L. Cavani, S. Halladja, A. ter Halle, G. Guyot, G. Corrado, C. Ciavatta, A. Boulkamh and C. Richard, Environ. Sci. Technol., 2009, 43, 4348–4354 CrossRef CAS.
- C. Belzile and L. Guo, Mar. Chem., 2006, 98, 183–196 CrossRef CAS PubMed.
- Y.-P. Chin, G. R. Aiken and E. O'Loughlin, Environ. Sci. Technol., 1994, 28, 1853–1858 CrossRef CAS PubMed.
- S. J. Traina, J. Novak and N. E. Smeck, J. Environ. Qual., 1990, 19, 151–153 CrossRef CAS.
- M. Edwards, J. - Am. Water Works Assoc., 1997, 89, 78–89 CAS.
- E. S. Boyle, N. Guerriero, A. Thiallet, R. D. Vecchio and N. V. Blough, Environ. Sci. Technol., 2009, 43, 2262–2268 CrossRef CAS.
- S. Mostafa, J. A. Korak, K. Shimabuku, C. M. Glover and F. L. Rosario-Ortiz, in Advances in the Physiochemical Characterization of Organic Matter, ed. F. L. Rosario-Ortiz, American Chemical Society Symposium Series 1160, Washington DC, 2014, pp. 159–179 Search PubMed.
- Z.-D. Wang, B. C. Pant and C. H. Langford, Anal. Chim. Acta, 1990, 232, 43–49 CrossRef CAS.
- A. J. Stewart and R. G. Wetzel, Limnol. Oceanogr., 1981, 26, 590–597 CrossRef.
- C. Richard, O. Trubetskaya, O. Trubetskoj, O. Reznikova, G. Afanas'eva, J. P. Aguer and G. Guyot, Environ. Sci. Technol., 2004, 38, 2052–2057 CrossRef CAS.
- J. F. Power and C. H. Langford, Anal. Chem., 1988, 60, 842–846 CrossRef CAS.
- A. G. Bruccoleri, B. C. Pant, D. K. Sharma and C. H. Langford, Environ. Sci. Technol., 1993, 27, 889–894 CrossRef CAS.
- R. Del Vecchio and N. V. Blough, Environ. Sci. Technol., 2004, 38, 3885–3891 CrossRef CAS.
- J. B. Fellman, E. Hood and R. G. M. Spencer, Limnol. Oceanogr., 2010, 55, 2452–2462 CrossRef CAS.
- J. A. Korak, A. D. Dotson, R. S. Summers and F. L. Rosario-Ortiz, Water Res., 2014, 49, 327–338 CrossRef CAS PubMed.
- K. R. Murphy, C. A. Stedmon, D. Graeber and R. Bro, Anal. Methods, 2013, 5, 6557–6566 RSC.
- P. G. Coble, Mar. Chem., 1996, 51, 325–346 CrossRef CAS.
- N. Maie, N. M. Scully, O. Pisani and R. Jaffe, Water Res., 2007, 41, 563–570 CrossRef CAS PubMed.
- C. A. Stedmon and R. Bro, Limnol. Oceanogr.: Methods, 2008, 6, 572–579 CrossRef CAS.
- M. Bieroza, A. Baker and J. Bridgeman, Sci. Total Environ., 2009, 407, 1765–1774 CrossRef CAS PubMed.
- M. Bieroza, A. Baker and J. Bridgeman, J. Environ. Eng., 2011, 137, 596–601 CrossRef CAS.
- D. L. Gone, J.-L. Seidel, C. Batiot, K. Bamory, R. Ligban and J. Biemi, J. Hazard. Mater., 2009, 172, 693–699 CrossRef CAS PubMed.
- K. R. Murphy, A. Hambly, S. Singh, R. Henderson, A. Baker, R. Stuetz and S. J. Khan, Environ. Sci. Technol., 2011, 45, 2909–2916 CrossRef CAS PubMed.
- Aquatic Organic Matter Fluorescence, ed. P. G. Coble, J. Lead, A. Baker, D. M. Reynolds and R. G. M. Spencer, Cambridge University Press, New York, 2014 Search PubMed.
- USEPA, National Primary Drinking Water Regulations: Disinfectants and Disinfection Byproducts, 1998, vol. 63 Search PubMed.
- K. R. Murphy, K. D. Butler, R. G. M. Spencer, C. A. Stedmon, J. R. Boehme and G. R. Aiken, Environ. Sci. Technol., 2010, 44, 9405–9412 CrossRef CAS PubMed.
- D. M. McKnight, E. W. Boyer, P. Westerhoff, P. Doran, T. Kulbe and D. Andersen, Limnol. Oceanogr., 2001, 46, 38–48 CrossRef CAS.
- R. M. Cory, M. P. Miller, D. M. McKnight, J. J. Guerard and P. L. Miller, Limnol. Oceanogr.: Methods, 2010, 8, 67–78 CrossRef CAS.
- J. J. Alberts and M. Takács, Org. Geochem., 2004, 35, 243–256 CrossRef CAS PubMed.
- N. Senesi, T. M. Miano, M. R. Provenzano and G. Brunetti, Soil Sci., 1991, 152, 259–271 CrossRef CAS PubMed.
- S. K. Ishii and T. H. Boyer, Environ. Sci. Technol., 2012, 46, 2006–2017 CrossRef CAS PubMed.
- A. Baker, E. Tipping, S. A. Thacker and D. Gondar, Chemosphere, 2008, 73, 1765–1772 CrossRef CAS PubMed.
- A. D. Pifer and J. L. Fairey, Environ. Eng. Sci., 2014, 31, 117–126 CrossRef CAS PubMed.
- J. R. Taylor, An Introduction to Error Analysis, University Science Books, 2nd edn, 1997 Search PubMed.
- D. B. Babcock and P. C. Singer, J. - Am. Water Works Assoc., 1979, 71, 149–152 CAS.
- A. D. Archer and P. C. Singer, J. - Am. Water Works Assoc., 2006, 98, 110–123 CAS.
- Water Quality & Treatment, ed. J. Edzwald, McGraw Hill, Denver, 6th edn, 2011 Search PubMed.
- J. A. Korak, F. L. Rosario-Ortiz and R. S. Summers, in Advances in the Physiochemical Characterization of Organic Matter, ed. F. L. Rosario-Ortiz, American Chemical Society Symposium Series 1160, Washington DC, 2014, pp. 281–300 Search PubMed.
- S. J. Randtke, J. - Am. Water Works Assoc., 1988, 80, 40–56 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The supplemental information contains 16 figures and 2 tables. See DOI: 10.1039/c5ew00024f |
| This journal is © The Royal Society of Chemistry 2015 |