Phytoscreening for perchlorate: rapid analysis of tree sap
Matt A.
Limmer
*a,
Danielle M.
West
b,
Ruipu
Mu
b,
Honglan
Shi
b,
Kim
Whitlock†
c and
Joel G.
Burken
a
aDepartment of Civil, Architectural and Environmental Engineering, Missouri S&T, Rolla, MO, USA. E-mail: LimmerM@mst.edu
bDepartment of Chemistry, Missouri S&T, Rolla, MO, USA
cTechLaw, Inc., Chicago, IL, USA
First published on 4th February 2015
Abstract
Perchlorate presents an environmental health risk due to its widespread use, high solubility in water, and ability to interfere with thyroid function in humans. Delineating plumes of mobile contaminants, such as perchlorate, is difficult and time consuming, particularly in remote or forested areas. Phytoscreening, the analysis of contaminants in tree tissues for plume delineation, has been previously applied to shallow chlorinated solvent groundwater plumes and provides a promising alternative to traditional delineation techniques. To test the potential of phytoscreening for perchlorate, a sensitive freeze centrifugation sampling method coupled with ultra-fast ion exchange chromatography tandem mass spectrometry (UFIC-MS/MS) detection was developed. An initial hydroponic greenhouse test using willow cuttings demonstrated concentrations of perchlorate in tree sap were proportional to the perchlorate exposure concentration. Eighty-six tree cores obtained in the field contained measureable amounts of perchlorate, and the distribution of perchlorate in trees reflected the distribution of perchlorate in the groundwater. Perchlorate concentrations in the tree cores were loosely correlated with the groundwater as demonstrated by cross-covariograms and linear regression. Correlations between tree and groundwater perchlorate concentrations were similar in magnitude to tree and groundwater trichloroethylene (TCE) concentrations, implying a similar level of performance between perchlorate and TCE phytoscreening at this site. Phytoscreening of perchlorate was sufficiently accurate to be used as a screening tool to delineate perchlorate contaminated groundwater.
Water impactPerchlorate is an emerging contaminant with properties that lead to large plumes in groundwater, potentially impacting drinking water supplies. This study demonstrates that phytoscreening, the sampling of trees to delineate groundwater contaminants, is able to delineate a perchlorate groundwater plume. Phytoscreening has been used previously to delineate chlorinated solvent plumes, but has been minimally investigated for delineation of inorganic contaminants. This finding widens the domain of phytoscreening applicability, a sustainable tool for investigating shallow, contaminated groundwater. |
Introduction
Perchlorate (ClO4−) is an oxyanion historically used in the manufacturing of explosives and rocket propellants for the defense and aerospace industries. Perchlorate and its salts have also been used in various industrial applications such as manufacturing of matches, airbag inflators, safety flares and fireworks.1 Because perchlorate is readily soluble in water and recalcitrant, perchlorate can be transported vast distances in groundwater once released into the environment.Recently, the U.S. Environmental Protection Agency (EPA) conducted occurrence studies (Unregulated Contaminant Monitoring Regulation 1 program) and found perchlorate contamination in both groundwater and surface waters serving as drinking water sources for more than 16 million people in at least 26 states.2 Perchlorate has been detected in over 4% of public water systems nationally at concentrations above 4 μg L−1.2 Due to perchlorate's link with decreased thyroid hormone output,1 the USEPA is preparing a national primary drinking water regulation for perchlorate.3 The states of California and Massachusetts have set a maximum contaminant level for perchlorate in drinking water of 6 μg L−1 and 2 μg L−1, respectively.4,5
Interest in perchlorate environmental occurrence and fate has led to several publications that assessed human perchlorate exposure potential resulting from uptake by plants, recently reviewed by Seyfferth and Parker.6 Perchlorate has been found in above-ground plant tissues of many plants growing at contaminated sites. At such field sites, concentrations of perchlorate in stems have been reported as high as 6 mg kg−1.7,8 Concentrations of perchlorate are often much higher in the leaves, with concentrations reported in the range of 190–5557 mg kg−1 for several weed species.8,9 For comparison, average leaf perchlorate concentrations have been reported as high as 38.8 mg kg−1 at a site with naturally elevated levels of perchlorate in the subsurface.10 Numerous laboratory studies have also demonstrated the potential for phytoremediation of perchlorate through direct uptake or rhizodegradation under anoxic conditions.6
Uptake of perchlorate by plants is thought to occur through nitrate transporters. Experiments with lettuce (Lactuca sativa) and varying anions showed reduced uptake of perchlorate when nitrate concentrations increased from 4 to 12 mM (~250 mg L−1 to 750 mg L−1), although the degree of competition depended on the variety of lettuce.11 The presence of sulfate (1–10 mM) and chloride (5–15 mM) did not affect perchlorate uptake. Increasing pH or bicarbonate ion also reduced uptake of perchlorate, implicating ClO4−/H+ cotransport was occurring. Another group of researchers working with lettuce also demonstrated competitive uptake of perchlorate with nitrate, again with differences between varieties.12 Competition with chloride was also demonstrated.12
Phytoscreening is a phytoforensic tool that delineates areas of contaminated groundwater by measuring chemical concentrations in plant tissues.13 Phytoscreening has been widely employed at sites contaminated with chlorinated solvents,14–17 but application of phytoscreening for inorganics is lesser studied. Phytoscreening of cadmium, copper, nickel and zinc at one field site showed significant correlations between soil and wood metal concentrations for 4 of 8 combinations of metal and plant genus.18 For example, willow zinc concentrations were positively correlated to zinc in the soil (R2 = 0.725, n = 7), but poplar zinc concentrations were not (R2 = 0.007, n = 15). A phytoscreening effort for arsenic, cadmium, chromium, copper, nickel and zinc found differences in tree core metal concentrations between tree species were greater than differences between a background site and a contaminated site.19
Phytoscreening of chlorinated solvents appears to be independent of plant species when plants have similar access to the contaminated horizon, likely due to the passive nature of uptake of these pollutants.20–22 For metal uptake by plants, active transport is likely required, leading to distinct species–metal interactions. Such regulated interactions likely hinder the applicability of phytoscreening for many metals. However, for perchlorate, the nitrate concentration shown to compete with perchlorate uptake is typically far greater than the nitrate concentration observed in groundwater. Additionally hindering phytoscreening of metals are the variety of plant unavailable metal forms found in the subsurface, resulting in a poor correlation between total metals in soil and metals measured in plant tissues. Perchlorate differs in this regard, as perchlorate is largely plant available, making perchlorate a promising inorganic for phytoscreening.
In this research, a greenhouse study was designed to test the feasibility of phytoscreening for perchlorate. Previous research investigating leaf perchlorate concentrations has shown substantial seasonal variations,23 raising questions about the quantitative use of leaves for perchlorate phytoscreening. Hydroponic testing of wetland plants has shown stem perchlorate concentrations to be proportional to dosing concentration, although the tests were performed at high concentrations (20–500 mg L−1) and some growth inhibition was present at these elevated levels.24 To test phytoscreening in the lab, willow cuttings were grown hydroponically and dosed with a range of perchlorate concentrations. To analyze perchlorate in tree sap, a rapid, sensitive analytical method was developed. Phytoscreening was applied at a field site with extensive perchlorate and chlorinated solvent groundwater contamination to compare perchlorate phytoscreening to the established chlorinated solvent phytoscreening.
Methods
Tissue sampling and analysis
To rapidly analyze tree sap perchlorate concentrations for phytoscreening, a centrifugation method was developed. Tree xylem sections (cores or de-barked cuttings) were placed into a 1.5 mL centrifuge tube and then frozen for two hours. Upon thawing, the tubes were centrifuged at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g to remove xylem tissue liquid, hereafter ‘sap’. A 25 μL aliquot of sap was taken and diluted 4× with mobile phase and spiked with isotopically labeled perchlorate (NaCl18O4) as the internal standard. The liquid was then filtered through a 4 mm diameter, 0.2 μm pore nylon filter.
000 g to remove xylem tissue liquid, hereafter ‘sap’. A 25 μL aliquot of sap was taken and diluted 4× with mobile phase and spiked with isotopically labeled perchlorate (NaCl18O4) as the internal standard. The liquid was then filtered through a 4 mm diameter, 0.2 μm pore nylon filter.
To detect perchlorate in sap and dosing solution, an ultra-fast ion exchange liquid chromatography tandem mass spectrometry (UFIC-MS/MS) method was used.25 A 2 × 50 mm Dionex IonPac AG21 guard column, 2 × 250 mm Dionex IonPac AS21 analytical column, and Shimadzu UFLC system were used for the separation. The sample injection volume was 20 μL. The mobile phase was 200 mM methylamine in water with a flow rate of 500 μL min−1. Detection was performed using a 4000 Q Trap MS/MS system operated in a multiple-reaction monitoring mode (MRM) with ESI-negative ionization. The quantification ion pair was m/z 98.7/82.9 amu and m/z 100.9/84.8 amu was used as the confirmation ion pair. The ion pair for isotope labeled perchlorate was 106.9/89 amu. The method performed optimally using the following parameters: ion source temperature 500 °C, ion spray voltage −4500 V, auxiliary gas 30 psi, nebulizer gas 40 psi, curtain gas 25 psi, dwell time 150 ms, DP (V) −5, EP (V) −10, CE (V) −38, CXP (V) −15. Using these parameters, the instrument calibration was linear from 0.2 to 200 μg L−1 with a method detection limit of 0.1 μg L−1 in water.
Tree cores obtained from the field site were also analyzed for chlorinated solvents using a previously published method.14 Briefly, cores were placed into 20 mL Teflon-capped vials. The vial headspace was sampled for five minutes using a 100 μm polydimethylsiloxane solid-phase microextraction (SPME) fiber. The fiber was then desorbed in the gas chromatograph inlet and compounds were separated on a 10 m VOCOL column and detected using an electron capture detector.
Greenhouse study
A greenhouse study was performed to test the viability of perchlorate phytoscreening under ideal conditions. Laurel-leaf willow (Salix pentandra) cuttings were obtained from locally grown clones. All cuttings were ~20 cm in length and between 7.5 and 9 mm in diameter to fit into 1.5 mL centrifuge tubes for later sap centrifugation. The willows were grown in 10% modified Hoagland's solution (87 mg L−1 NO3−) for 30 days to allow shoot and root development. To encourage shoot development near the top of the cutting, the upper 5 cm of the cutting was brushed with a 10 mg L−1 solution of indole-3-butyric acid.Established cuttings were placed into 1 L jars of 10% modified Hoagland's solution dosed with differing concentrations of perchlorate. Jars were covered with aluminum foil to minimize light exposure to the roots. Plants received natural daylight in greenhouse with day lengths of approximately 14 hours. Every two to three days the transpiration rate was assessed volumetrically and the dosing solution was replaced. No effort was made to sterilize the solution, and no degradation was observed in solution. Conversely, the final concentrations in solution were higher than the initial concentration due to exclusion of perchlorate by the root membrane.
The experimental design included two factors: perchlorate concentration and exposure duration. Perchlorate concentration had nominal levels of 10 μg L−1, 100 μg L−1, 1 mg L−1 and 10 mg L−1. Exposure duration had levels of 7 days, 14 days and 21 days. Each treatment combination was performed in triplicate and controls without perchlorate were also grown. Control perchlorate concentrations remained below the detection limit throughout the experiment. During harvest, the willow trunks were sectioned into three 2 cm long pieces to increase the amount of sap collected. The three portions of sap were combined, diluted, spiked with internal standard and filtered prior to injection on the UFIC-MS/MS. To measure tree water content, tree sections were weighed prior to centrifugation and after oven drying at 100 °C overnight. When harvested, perchlorate concentration in the dosing solution was measured. This final measured concentration was averaged with the initial measured solution concentration to obtain the typical exposure concentration.
Field site
The field site was the Longhorn Army Ammunition Plant (LHAAP) in Karnack, Texas.8 This former military base historically manufactured trinitrotoluene, rocket motor propellants, pyrotechnics and ammunition. The site was placed on the USEPA National Priorities List in 1990 due to groundwater contamination by various compounds such as chlorinated solvents and perchlorate. The areas of interest for this study were area 16, a former landfill, and area 18/24, a former burning ground and unlined evaporation pond (Fig. 1). In both areas, a Record of Decision was signed in 1995 resulting in removal of contaminated soil and/or water, capping and extraction of groundwater.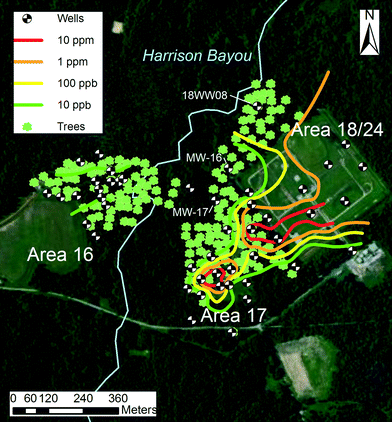 | ||
| Fig. 1 LHAAP site map with sampled trees and groundwater perchlorate isoconcentration contours krigged from the depicted shallow groundwater wells. | ||
LHAAP is situated above the Wilcox Group, which consists of fine- to medium-grained sands interbedded with considerable clay and lignite.26 Groundwater at the site is unconfined with levels that fluctuate seasonally. The topography is relatively flat and groundwater flow is generally towards the Harrison Bayou, but seasonal fluctuations occur. In area 18/24 groundwater is generally at depths of 5–20 feet (1.5–6 meters), and groundwater in area 16 is generally 5–10 feet (1.5–3 meters) below ground surface. The southeastern edge of the former landfill in area 16 sits in the 100 year floodplain.26
Tree cores were taken in the vicinity of areas 16, 17, 18/24 during June 18–28, 2012 (Fig. 1). Trees were selected from predetermined 30 × 30 meter grid nodes. The sampled tree near the node was required to have a diameter at breast height greater than 10 cm. Preference was given to hardwoods over softwoods for consistency. The location, diameter and genus were recorded for all 183 trees. Nine different genera were sampled, although Quercus represented approximately half of the tree sampled. Liquidambar comprised ~1/4 of trees sampled, while all other genera represented <10% of the total trees sampled. Cores were taken using a 5 mm increment borer to a depth of 8 cm following published methods.27 At each tree, two cores were taken, one for analysis of perchlorate and another for analysis of chlorinated solvents. The increment borer was rinsed with deionized water after each tree coring. Field duplicates were collected every ten samples. The samples were shipped overnight on ice for analysis at Missouri S&T.
Historical groundwater perchlorate and chlorinated solvent concentrations were available from 50 wells screened in the shallow aquifer (Fig. 1). Because the wells were sampled at varying frequencies, 226 measured perchlorate concentrations were averaged over the period 2007–2012. Wells frequently sampled over this period showed no discernible trend in perchlorate concentrations. The most commonly detected chlorinated solvent, TCE, was measured in 291 samples over the same time period.
Data analysis
All data were log10 transformed prior to statistical analysis to improve normality and homoscedasticity. Pearson correlation coefficients and Spearman rank correlation coefficients were calculated by SAS 9.2 using proc CORR. ANOVA (proc GLM) and regression (proc REG) analysis of the data were also performed in SAS 9.2. Pairwise comparisons were performed using LSMEANS with a Tukey adjustment. Errors-in-variables regression followed the approach of Fuller,28 using the modified estimator 3.1.20. Geospatial mapping and ordinary kriging were performed in ArcGIS 10.1. A spherical semivariogram was used for all kriging with a lag size of 10 meters. A nugget was included to explain measurement error of approximately one order of magnitude for perchlorate in groundwater. The model was parameterized through iterative cross-validation. Cross covariograms were fit by the package gstat in R 3.1.1.Results and discussion
Analytical method
The centrifugation method performed adequately to remove sap from the wood specimens. On average, 19% (standard deviation of 7%) of the water contained in tree samples was removed by centrifugation. Dilution and filtration of the sap resulted in a method detection limit of 1 μg L−1. Spike recoveries showed sufficient analytical accuracy (Table 1).| Spike (μg L−1) | n | Average recovery (range) | |
|---|---|---|---|
| Willow cuttings | |||
| 5 | 2 | 86.4% (84.4–88.5%) | |
| 10 | 2 | 100% (92.9–108%) | |
| Field site tree cores | |||
| 2.5 | 3 | 84.0% (79.5–92.9%) | |
| 125 | 4 | 101% (99.5–102%) | |
Duplicate tree core samples from LHAAP showed modest variability. Perchlorate was below detection limits in both samples for half of the 18 duplicate tree cores taken. Only six duplicates resulted in detections for both samples, yielding a relative percent difference (RPD) of 51%. To examine potential sources of variability, duplicate injections of sap were performed, resulting in an RPD of 3.2% (n = 6). Duplicate centrifugations of another tree core segment from the same tree core were also performed, resulting in an RPD of 25.2% (n = 8), suggesting much of the variability results from heterogeneities in perchlorate concentrations in the tree core. Such heterogeneities have been demonstrated in tree core VOC concentrations for trees growing above heterogeneous contaminant plumes.29
Greenhouse study
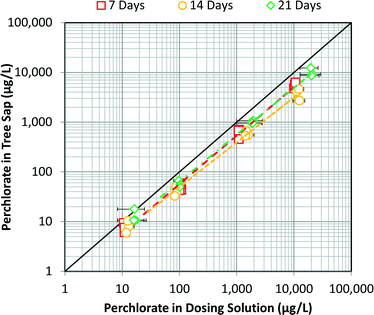
No signs of toxicity were observed during the three-week experiment. At harvest, trees contained 49% water with a standard deviation of 4%. Over the experimental period, the transpiration rate increased from 30 mL per day to 100 mL per day as the plants grew.Concentrations of perchlorate in tree sap correlated well to the dosing perchlorate concentration (Fig. 2). ANOVA revealed both the dosing concentration (p < 0.0001) and the day of harvest (p = 0.0043) significantly affected perchlorate concentrations in tree sap. Trees harvested after 14 days of exposure had significantly reduced concentrations as compared to 7 and 21 days of exposure (p = 0.037 and p = 0.0042, respectively). Adding a flag for harvest after 14 days to a regression model provided minimal additional explanatory power, with an adjusted R2 of 0.993, improved from 0.990 with dosing solution as the sole explanatory variable. This indicates exposure duration of 7 days was sufficient to reach a steady state condition.
The overall ordinary least squares (OLS) fit for the data shown in Fig. 2 demonstrates that trees can serve as biosensors of perchlorate contamination in groundwater. The data do not indicate accumulation of perchlorate in stems over time, as has been shown for leaves.23 The regression fit yielded a slope near unity, indicating a nearly proportional response. Using an errors-in-variables regression approach to incorporate uncertainty in the dosing concentration the slope of the regression fit increased slightly to 0.946. The standard error of this slope estimate increased to 0.0303, resulting in a confidence interval of (0.885, 1.01).
log10Csap = 0.941(0.0160)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10Cwater − 0.133(0.0456) log10Cwater − 0.133(0.0456) |
Where:
C sap is the sap perchlorate concentration (μg L−1).
C water is the water perchlorate concentration (μg L−1).
Values in parenthesis represent OLS standard errors.
The negative intercept is indicative of exclusion of perchlorate by the root membrane. The small magnitude of the intercept indicates minimal rejection. Assuming a slope of unity, the intercept can be interpreted as the average fraction of perchlorate transported across the membrane, which is 74%, or a rejection of 26%. The fraction rejected, as measured by the fractional increase in dosing concentration over the experiment, showed no dependence on the concentration.
The greenhouse data are in agreement with another experimental dataset involving hydroponic dosing of wetland plants. He et al.24 found concentrations of perchlorate in stem tissue were correlated with perchlorate in dosing solution. Their data fall above the extrapolated regression line in this study, suggesting species dependency in uptake efficiency.
Field site
From the 201 tree cores collected at LHAAP, 86 trees had detectable levels of perchlorate. The maximum perchlorate concentration measured was 1.6 mg L−1 in a 13 cm diameter oak tree. Qualitatively, concentrations of perchlorate found in trees resided in regions of groundwater contaminated with perchlorate, particularly outside of area 16 (Fig. 3). Two “outlier” trees had very high levels of perchlorate and were adjacent to monitoring wells. The “outlier” tree to the north, near 18WW08 is in an area of uncertain groundwater contamination. Groundwater perchlorate concentrations in 18WW08 fluctuated erratically from <2.5 to 2750 μg L−1 during measurements taken between 2007 and 2012 (n = 15). MW-17, near another “outlier” tree, had consistently low levels of perchlorate, but was only sampled three times from 2007–2012. The nearby MW-16 showed large fluctuations in perchlorate concentrations, ranging from <0.1 to 5600 μg L−1 from 2007 to 2012 (n = 17). The largely random variation in concentration trends for wells 18WW08 and MW-16 may indicate the presence of small, dynamic regions of contaminated groundwater to the north and west of area 18/24. The sporadic, moderate levels of perchlorate observed in trees in this area support this interpretation.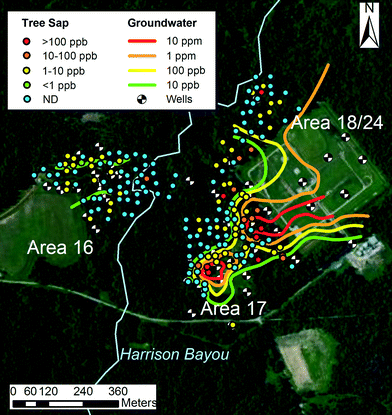 | ||
| Fig. 3 Measurements of tree perchlorate overlaid on groundwater perchlorate isoconcentration contours. | ||
A krigged tree sap perchlorate plume also resembled the krigged groundwater perchlorate plume (Fig. 4). The general features of the tree sap plume remain similar to the groundwater perchlorate plume, particularly for the hotspot in area 17. The plume in area 17 shows less connection to the plume emanating from areas 18/24, and another hotspot is evident northwest of the main area of contamination.
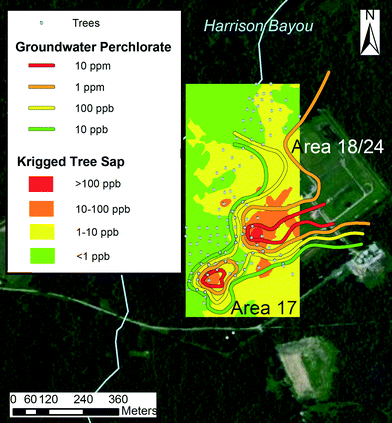 | ||
| Fig. 4 Comparison of tree sap and groundwater isoconcentration contours. Area 16 was not considered due to the low levels of perchlorate measured in the groundwater. | ||
Concentrations of TCE in groundwater followed a similar distribution to that of perchlorate. The most notable difference is in area 16, where concentrations of TCE are comparable to the TCE concentrations found in area 18/24. Tree concentrations of TCE qualitatively appear to correlate with groundwater data, with the highest concentrations of TCE in trees nearest the contaminated groundwater. When the tree TCE data are krigged, the plume in areas 16 and 18/24 is well captured by the tree data. However, the trees fail to capture the plume in area 17, perhaps due to the lower concentration of TCE in the groundwater.
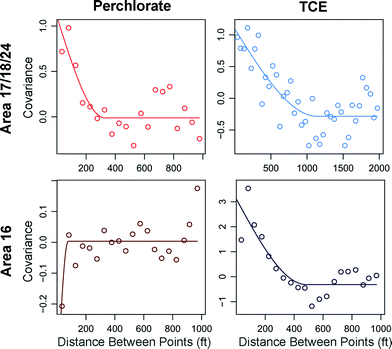
Cokriging is a theoretically useful technique for combining two spatial datasets that share a common spatial distribution.30 If tree contaminant concentrations are related to groundwater contaminant concentrations, then the spatial covariance between the two variables will be observable on the cross-covariogram. Fig. 5 demonstrates the spatial correlation between groundwater and tree sap concentrations as distances between trees and monitoring wells increases for both perchlorate and TCE. In area 17/18/24, the magnitude of positive covariance at close distances is similar for both perchlorate and TCE. Conversely, in area 16, where TCE phytoscreening appeared well-correlated to groundwater contours, the covariance between groundwater and trees is larger while the covariance between groundwater and trees for perchlorate is effectively zero. In area 17/18/24, where the groundwater plumes of TCE and perchlorate are similar in intensity and spread, the cross-covariograms indicate phytoscreening performs similarly well for both compounds. However, few data pairs are available at close distances, resulting in a sparse data cloud. When relatively few points are available for cokriging, accurate parameterization of the semivariograms is difficult.31 Nevertheless, cokriging can be used to generate a more detailed plume map due to the additional sample data.
An alternative to cokriging is to transform the secondary data (i.e., tree perchlorate concentrations) into primary data (i.e., groundwater perchlorate concentrations) via a regression equation.31,32 To create the regression equation, wells were paired with all trees within a 30 m radius, which was the distance which maximized correlation. Paired data revealed that tree perchlorate concentrations in area 16 were not correlated with groundwater perchlorate concentrations. The lack of significant correlation may be due the limited range of the explanatory variable in area 16. In areas 17/18/24, tree perchlorate concentrations were weakly correlated with groundwater perchlorate concentrations (Pearson: r = 0.47, p = 0.036; Spearman: r = 0.44, p = 0.050). For comparison, tree TCE concentrations were weakly correlated to groundwater TCE concentrations in areas 17/18/24 (Pearson: r = 0.54, p = 0.013; Spearman: r = 0.44, p = 0.055), but highly correlated in area 16 (Pearson: r = 0.75, p = 0.0007; Spearman: r = 0.71, p = 0.002). As in the cross covariograms, these data indicate that phytoscreening accuracy for perchlorate is likely similar to that of TCE in area 17/18/24.
While the correlation between tree sap and groundwater perchlorate is statistically significant at this site, the correlation is relatively low. The greenhouse experiment suggests this variability is not due to concentration-dependent uptake or temporal variability. The potential competition of perchlorate with nitrate is unlikely to be occurring at this site, with the concentration of nitrate in the groundwater at ~0.5 mg L−1, lower than demonstrated for competition.11 Other plant-environment interactions are likely reducing the strength of the correlation, as has been demonstrated for phytoscreening of VOCs. The fraction of groundwater consumed by the trees would likely have a substantial effect on tree concentrations of perchlorate, particularly with the deeper groundwater in area 17/18/24. Redox conditions in the shallow aquifer may allow for microbial degradation of perchlorate prior to entering the plant, as has been shown for benzene.33 Perchlorate can be used as an electron acceptor under anoxic conditions, preventing phytoscreening from being an effective technique in reduced aquifers.34 Differential uptake of clean rainwater between trees may be particularly problematic, which has been occasionally demonstrated for trichloroethene (TCE).35–37 For TCE, much of the contaminant is partitioned to lignin,38 providing a reservoir of contaminant not likely present for perchlorate. Additionally, trees may metabolize perchlorate at varying rates,39,40 although the relative importance of this pathway is debatable given the numerous observations of perchlorate in plants. Other factors such as rooting depth, depth to groundwater, and redox conditions will strongly affect the applicability of perchlorate phytoscreening at other field sites.
The use of secondary data, such as tree cores, in site investigations can provide additional site detail and reduce the overall uncertainty in contaminant distribution. As the true distribution of contaminants at a site is never completely known, investigators can only attempt to reduce uncertainty in a cost effective manner. The approach of tree coring offers mobility and sampling speed, particularly in heavily forested areas such as LHAAP. At this field site, phytoscreening was capable of resolving the general shape and intensity of the groundwater perchlorate plume in a rapid (<3 weeks), non-invasive fashion. The observation that phytoscreening performed equally well for TCE and perchlorate indicates phytoscreening can likely be applied to other water soluble contaminants in shallow groundwater.
Acknowledgements
This project was funded through TechLaw and the US EPA (grant 00038833) and included support from the Environmental Research Center at Missouri S&T. The authors thank Dr. Yuan Yuan and Amanda Holmes for assisting with sample preparation and Rich Mayer of US EPA for support at the field site.References
- E. T. Urbansky, Perchlorate Chemistry: Implications for Analysis and Remediation, Biorem. J., 1998, 2(2), 81–95 CrossRef CAS.
- USEPA, Drinking Water: Regulatory Determination on Perchlorate. 76FR7762, 2011.
- USEPA Final Regulatory Determination for Perchlorate in Drinking Water, http://water.epa.gov/drink/contaminants/unregulated/perchlorate.cfm, (Accessed 4/1/ 2014).
- CDPH Perchlorate in Drinking Water, http://www.cdph.ca.gov/certlic/drinkingwater/Pages/perchlorate.aspx, (Accessed 4/1/14).
- MassDEP Current Regulatory Limit: Perchlorate, http://www.mass.gov/eea/agencies/massdep/water/drinking/standards/perchlorate.html, (Accessed 4/1/2014).
- A. L. Seyfferth and D. R. Parker, Uptake and Fate of Perchlorate in Higher Plants, in Advances in Agronomy, ed. L. S. Donald, Academic Press, 2008, vol. 99, pp. 101–123 Search PubMed.
- E. T. Urbansky, M. L. Magnuson, C. A. Kelty and S. K. Brown, Perchlorate Uptake by Salt Cedar (Tamarix ramosissima) in the Las Vegas Wash Riparian Ecosystem, Sci. Total Environ., 2000, 256(2–3), 227–232 CrossRef CAS.
- P. N. Smith, C. W. Theodorakis, T. A. Anderson and R. J. Kendall, Preliminary Assessment of Perchlorate in Ecological Receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas, Ecotoxicology, 2001, 10(5), 305–313 CrossRef CAS.
- P. N. Smith, L. Yu, S. T. McMurry and T. A. Anderson, Perchlorate in Water, Soil, Vegetation, and Rodents Collected from the Las Vegas Wash, Nevada USA, Environ. Pollut., 2004, 132(1), 121–127 CrossRef CAS PubMed.
- B. J. Andraski, W. A. Jackson, T. L. Welborn, J. K. Böhlke, R. Sevanthi and D. A. Stonestrom, Soil, Plant, and Terrain Effects on Natural Perchlorate Distribution in a Desert Landscape, J. Environ. Qual., 2014, 43, 980–994 CrossRef CAS PubMed.
- A. Seyfferth, M. Henderson and D. Parker, Effects of Common Soil Anions and pH on the Uptake and Accumulation of Perchlorate in Lettuce, Plant Soil, 2008, 302(1–2), 139–148 CrossRef CAS.
- W. Ha, D. L. Suarez and S. M. Lesch, Predicting Perchlorate Uptake in Greenhouse Lettuce from Perchlorate, Nitrate, and Chloride Irrigation Water Concentrations, J. Environ. Qual., 2013, 42(1), 208–218 CrossRef CAS PubMed.
- J. G. Burken, D. A. Vroblesky and J. C. Balouet, Phytoforensics, Dendrochemistry, and Phytoscreening: New Green Tools for Delineating Contaminants from Past and Present, Environ. Sci. Technol., 2011, 45(15), 6218–6226 CrossRef CAS PubMed.
- M. A. Limmer, J.-C. Balouet, F. Karg, D. A. Vroblesky and J. G. Burken, Phytoscreening for Chlorinated Solvents Using Rapid in Vitro SPME Sampling: Application to Urban Plume in Verl, Germany, Environ. Sci. Technol., 2011, 45(19), 8276–8282 CrossRef CAS PubMed.
- M. Limmer, G. Martin, C. Watson, C. Martinez and J. G. Burken, Phytoscreening: A Comparison of In planta Portable GC-MS and In vitro Analyses, Ground Water Monit. Rem., 2014, 34(1), 49–56 CAS.
- A. Sorek, N. Atzmon, O. Dahan, Z. Gerstl, L. Kushisin, Y. Laor, U. Mingelgrin, A. Nasser, D. Ronen, L. Tsechansky, N. Weisbrod and E. R. Graber, Phytoscreening: The Use of Trees for Discovering Subsurface Contamination by VOCs, Environ. Sci. Technol., 2008, 42(2), 536–542 CrossRef CAS.
- A. Wahyudi, P. Bogaert, S. Trapp and J. Macháčková, Pollutant plume delineation from tree core sampling using standardized ranks, Environ. Pollut., 2012, 162, 120–128 CrossRef CAS PubMed.
- M. Algreen, S. Trapp and A. Rein, Phytoscreening and phytoextraction of heavy metals at Danish polluted sites using willow and poplar trees, Environ. Sci. Pollut. Res., 2014, 21, 8992–9001 CrossRef CAS PubMed.
- M. Algreen, A. Rein, C. N. Legind, C. E. Amundsen, U. G. Karlson and S. Trapp, Test of Tree Core Sampling for Screening of Toxic Elements in Soils from a Norwegian Site, Int. J. Phytorem., 2011, 14(4), 305–319 CrossRef PubMed.
- J. G. Burken and J. L. Schnoor, Predictive Relationships for Uptake of Organic Contaminants by Hybrid Poplar Trees, Environ. Sci. Technol., 1998, 32(21), 3379–3385 CrossRef CAS.
- M. A. Limmer and J. G. Burken, Plant Translocation of Organic Compounds: Molecular and Physicochemical Predictors, Environ. Sci. Technol. Lett., 2014, 1(2), 156–161 CrossRef CAS.
- M. Limmer and J. G. Burken, Phytoscreening with SPME: Analysis of Variability, in review.
- K. Tan, T. A. Anderson, M. W. Jones, P. N. Smith and W. A. Jackson, Accumulation of Perchlorate in Aquatic and Terrestrial Plants at a Field Scale, J. Environ. Qual., 2004, 33(5), 1638–1646 CrossRef CAS.
- H. He, H. Gao, G. Chen, H. Li, H. Lin and Z. Shu, Effects of Perchlorate on Growth of Four Wetland Plants and its Accumulation in Plant Tissues, Environ. Sci. Pollut. Res., 2013, 20(10), 7301–7308 CrossRef CAS PubMed.
- D. M. West, R. Mu, S. Gamagedara, Y. Ma, C. D. Adams, T. Eichholz, J. G. Burken and H. Shi, Simultaneous Detection of Perchlorate and Bromate Using Rapid High-Performance Ion Exchange Chromatography–Tandem Mass Spectrometry and Perchlorate Removal in Drinking Water, Environ. Sci. Pollut. Res., 2015 DOI:10.1007/s11356-014-4028-8 , in press.
- Complete Environmental Service, First Five-Year Review Report for Longhorn Army Ammunition Plant Sites 18, 24, 16 and 12, 2002 Search PubMed.
- D. A. Vroblesky, User's Guide to the Collection and Analysis of Tree Cores to Assess the Distribution of Subsurface Volatile Organic Compounds. USGS Report 2008-5088, p. 72 Search PubMed.
- W. A. Fuller, Measurement Error Models, John Wiley & Sons, 1987, p. 440 Search PubMed.
- M. Limmer, M. Shetty, S. Markus, R. Kroeker, B. L. Parker, C. Martinez and J. G. Burken, Directional Phytoscreening: Contaminant Gradients in Trees for Plume Delineation, Environ. Sci. Technol., 2013, 47(16), 9069–9076 CrossRef CAS PubMed.
- M. Vauclin, S. R. Vieira, G. Vachaud and D. R. Nielsen, The Use of Cokriging with Limited Field Soil Observations, Soil Sci. Soc. Am. J., 1983, 47(2), 175–184 CrossRef.
- K. C. Abbaspour, R. Schulin, M. T. van Genuchten and E. Schläppi, An Alternative to Cokriging for Situations with Small Sample Sizes, Math. Geol., 1998, 30(3), 259–274 CrossRef.
- G. Gopalakrishnan, B. S. Minsker and A. J. Valocchi, Monitoring Network Design for Phytoremediation Systems Using Primary and Secondary Data Sources, Environ. Sci. Technol., 2011, 45(11), 4846–4853 CrossRef CAS PubMed.
- J. Wilson, R. Bartz, M. Limmer and J. Burken, Plants as Bio-Indicators of Subsurface Conditions: Impact of Groundwater Level on BTEX Concentrations in Trees, Int. J. Phytorem., 2013, 15(3), 257–267 CrossRef CAS PubMed.
- V. A. Nzengung, H. Penning and W. O'Niell, Mechanistic Changes During Phytoremediation of Perchlorate Under Different Root-Zone Conditions, Int. J. Phytorem., 2004, 6(1), 63–83 CrossRef CAS PubMed.
- D. A. Vroblesky, B. D. Clinton, J. M. Vose, C. C. Casey, G. J. Harvey and P. M. Bradley, Ground Water Chlorinated Ethenes in Tree Trunks: Case Studies, Influence of Recharge, and Potential Degradation Mechanism, Ground Water Monit. Rem., 2004, 24(3), 124–138 CrossRef PubMed.
- M. A. Limmer, A. J. Holmes and J. G. Burken, Phytomonitoring of Chlorinated Ethenes in Trees: A Four-Year Study of Seasonal Chemodynamics in Planta, Environ. Sci. Technol., 2014, 48(18), 10634–10640 CrossRef CAS PubMed.
- O. Holm and W. Rotard, Effect of Radial Directional Dependences and Rainwater Influence on CVOC Concentrations in Tree Core and Birch Sap Samples Taken for Phytoscreening Using HS-SPME-GC/MS, Environ. Sci. Technol., 2011, 45(22), 9604–9610 CrossRef CAS PubMed.
- A. A. MacKay and P. M. Gschwend, Sorption of Monoaromatic Hydrocarbons to Wood, Environ. Sci. Technol., 2000, 34(5), 839–845 CrossRef CAS.
- S. Susarla, S. T. Bacchus, G. Harvey and S. C. McCutcheon, Uptake and Transformation of Perchlorate by Vascular Plants, Toxicol. Environ. Chem., 2000, 74(1–2), 29–47 CrossRef CAS.
- V. A. Nzengung, C. Wang and G. Harvey, Plant-Mediated Transformation of Perchlorate into Chloride, Environ. Sci. Technol., 1999, 33(9), 1470–1478 CrossRef CAS.
Footnote |
| † Current Affiliation: Pioneer Environmental Services, LLC. Chicago, IL USA. |
| This journal is © The Royal Society of Chemistry 2015 |