Solar thermal decomposition of desalination reject brine for carbon dioxide removal and neutralisation of ocean acidity
P. A.
Davies
Sustainable Environment Research Group, School of Engineering and Applied Science, Aston University, Birmingham B4 7ET, UK. E-mail: p.a.davies@aston.ac.uk
First published on 12th January 2015
Abstract
Desalination plants could become net absorbers (rather than net emitters) of CO2. Thermal decomposition of salts in desalination reject brine can yield MgO which, added to the ocean, would take up CO2 through conversion to bicarbonate. The process proposed here comprises dewatering of brine followed by decomposition in a solar receiver using a heliostat field.
Water impactThe work advances our understanding of the environmental implications of desalination by highlighting the opportunity, not only of reducing CO2 emissions, but also of making net emissions negative. Whereas earlier works tended to emphasise the use of solar energy to power the desalination process and thus substitute fossil fuels, this work explores the use of solar energy to treat desalination reject brine. By the proposed method, reject brine rich in MgO could absorb CO2 already dispersed in the oceans and atmosphere, and not just CO2 from point emitters. The brine would also help counter ocean acidification. This could mean that the reject brine provides benefits, and not only hazards, for the environment. |
Desalination currently provides some 30 billion m3 per year of freshwater worldwide and this output is growing at a rate of about 10% per year.1 Within a decade we can expect a doubling of output, resulting in additional electrical energy requirement of 10 GW and CO2 emissions of 0.045 GtCO2 per year (assuming state-of-the-art reverse osmosis [RO] technology with specific energy consumption of 3 kW h m−3 and electricity generated with carbon intensity 0.5 kgCO2 kW−1 h−1). By 2030 a shortfall in renewable freshwater of 2700 billion m3 per year is forecast, and this could drive an even more rapid growth in desalination capacity with associated energy demand and emissions.2
One way to offset the emission of CO2 from a seawater desalination plant is to take advantage of the magnesium in the concentrated brine rejected by the plant. This could be converted to magnesium oxide which, discharged to the oceans, would gradually absorb ambient CO2 through conversion to magnesium carbonate or bicarbonate (Fig. 1). Each m3 of seawater contains 1.32 kg of Mg which is retained in the reject brine and could in principle absorb up to 4.76 kg m−3 of CO2 if converted fully to Mg(HCO3)2. Fed to a desalination plant, 1 m3 of seawater typically provides 0.5 m3 of freshwater with CO2 emission of 0.75 kg associated with electricity usage of the plant (based on assumptions above). Thus the maximum CO2 absorption potential of the reject brine exceeds the CO2 emission from desalination by a factor of >6, resulting in a theoretical net absorption capacity per freshwater output of 8 kg CO2 m−3.
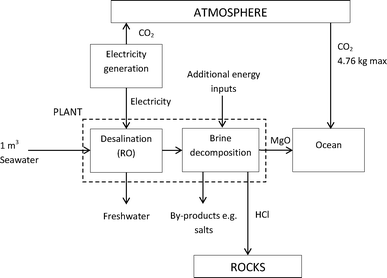 | ||
| Fig. 1 Concept of proposed process using desalination reject brine to absorb carbon dioxide from the atmosphere. | ||
It is worth noting that the ocean provides a very large stock of magnesium for CO2 absorption. The top 100 m of the ocean is generally considered to be well mixed providing gaseous exchange with the atmosphere. Based on the maximum absorption capacity of 4.76 CO2 m−3, this top layer could absorb up to 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GtCO2 – greatly exceeding the current excess stock of 900 GtCO2 in the atmosphere.3
000 GtCO2 – greatly exceeding the current excess stock of 900 GtCO2 in the atmosphere.3
Reject brine is usually considered a harmful aspect of desalination because of its impact on the marine environment.4 To address this issue, researchers have investigated ways to manage and add value to this waste stream – including carbonation for CO2 sequestration. Ferrini et al.,5 for example, showed that addition of carbon dioxide to magnesium chloride solution in the presence of ammonia leads to formation of magnesium carbonate with ammonium chloride as a by-product. This method is interesting for the absorption of CO2 from point-source emitters.
The alternative investigated in this study is to decompose reject brine such that the magnesium chloride is hydrolysed to magnesium oxide according to the endothermic reaction:
| MgCl2 + H2O → MgO + 2HCl | (1) |
It is proposed that brine decomposition be carried out using solar thermal energy in the post-treatment stage of desalination (Fig. 1). The MgO will be incorporated in the plant reject stream and returned to the ocean. This process may be considered a variant of the geothermal technology of ‘ocean liming’ which, as well as removing CO2, helps to neutralise ocean acidity.6 But instead of adding crushed minerals to the sea, the neutralising substance (MgO) will be obtained from the seawater itself. The by-product of HCl can be sequestered by injecting hydrochloric acid into silicate rocks. The aim of this study is to investigate the feasibility of this proposition at the preliminary level. The study investigates experimentally the yield of MgO from thermal decomposition of seawater. It then presents and models a system for brine thermal decomposition (BTD) using a heliostat field. This includes estimates of the energy penalties involved and the land area needed. Finally, geological disposal of the hydrogen chloride is discussed. For the purpose of illustrating the results, we consider two examples: (i) a desalination plant of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day freshwater output equipped with BTD; (ii) scale up to give additional global desalination output of 30 billion m3 per year, corresponding to a doubling in current capacity.
000 m3 per day freshwater output equipped with BTD; (ii) scale up to give additional global desalination output of 30 billion m3 per year, corresponding to a doubling in current capacity.
Yield of magnesium oxide from seawater
Samples (100 ml) of seawater were thermally decomposed, first by evaporation in a convection oven at 200 °C, and then by heating in a furnace at 450 °C until hydrogen chloride was no longer evolved. The dry residue was dispersed in distilled water to make up the same initial volume of 100 ml giving a milky white solution of pH 9.86. Titration against HCl showed 78% conversion of magnesium to MgO.† The 22% shortfall against stoichiometric yield can be explained by likely precipitation of magnesium sulphate (instead of chloride) which does not readily decompose.To confirm presence of MgO in the suspension and its potential to absorb CO2, accelerated carbonation was carried out by subjecting the suspension to pressurised CO2 at 4 bar for 17 h at room temperature. The liquid became substantially clear indicating conversion to soluble magnesium bicarbonate (Fig. 2).
 | ||
| Fig. 2 Thermal treatment of seawater yields MgO which absorbs CO2: (1) raw seawater, (2) suspension of salts after thermal decomposition, (3) solubilised salts after subjecting to CO2 gas. | ||
Process design and modelling
The hydrolysis process of eqn (1) is preceded by dehydration of magnesium chloride. The overall reaction and its energy requirement may be summarised:7| MgCl26H2O → MgO + 2HCl(g) + 5H2O(l) ΔH0 = −283 kJ mol−1 | (2) |
However, due to the low concentration of MgCl2, the enthalpy needed to evaporate all the water in the reject brine is 100 times larger than the above, making direct thermal decomposition of the brine impractical. The brine must be dewatered prior to thermal treatment. Methods of dewatering could include: (i) solar evaporation ponds, which precipitate NaCl salt leaving a supernatant brine rich in MgCl2. Harvesting such brine is already standard practice in salt works and the salt by-product would have economic value.8 However, the salt production from desalination at the scale discussed here would be huge. For example, the scenario of additional 30 billion m3 per year desalination capacity with salt recovery would result in salt production of 1 billion t per year which is 4 times current global production.9 The land required for the ponds would also be large. (ii) Seawater greenhouses to evaporate water from the brine, thus providing cooling for protected cultivation of crops. The crop production would be economically valuable and make efficient use of desalinated water. The main limitation would be that seawater greenhouses would not be suitable for concentrating brine by a factor of more than 2–3 (depending on ambient humidity) beyond which hygroscopicity of the brine would hinder evaporation and cooling.10 (iii) Nanofiltration (NF) would remove sodium chloride from the brine while concentrating the divalent ions i.e. magnesium and calcium. Nanofiltration like reverse osmosis requires work input for the high-pressure pumping. It is a compact process which could be used (or even retrofitted) with most desalination plant. (iv) Multiple effect distillation (MED) is another compact desalination process. It can handle strong brines and requires thermal energy input. MED will produce a small amount of freshwater additional to the reverse osmosis (RO) output.
These processes could be combined in various ways according to local requirements and land availability. In this study, we select NF and MED, these being the most generally applicable options. The proposed system (Fig. 3) will dewater brine using NF followed by MED, prior to decomposition using concentrated solar thermal energy. The mass-energy balance for the whole system has been modelled using the assumptions of Table 1. Important aspects of each process are as follows.
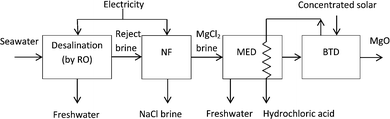 | ||
| Fig. 3 Desalination and downstream processes making up the plant in Fig. 1. Dewatering of reject brine by nanofiltration (NF) and then multiple effect distillation (MED) reduces the energy requirement of the subsequent brine thermal decomposition (BTD). The heat input for the MED is provided by condensing hydrochloric acid vapours from the decomposition process. | ||
| General | |
|---|---|
| a Per area of heliostat field, averaged over year. b Based on this study. | |
| Carbon intensity electricity supply | 0.5 kgCO2 kW−1 h−1 |
| Reverse Osmosis (RO) | |
| Specific energy consumption | 3 kW h m−3 |
| Recovery ratio | 0.5 |
| Nanofiltration (NF) | |
| Number of stages | 2 |
| Recovery ratio each stage | 0.7 |
| Pump efficiency | 85% |
| Operating pressure/osmotic pressure at brine outlet | 120% |
| Energy recovery device efficiency | 95% |
| Multiple Effect Distillation (MED) | |
| Number of effects | 4 |
| Performance ratio | <3 |
| Brine thermal decomposition (BTD) | |
| Gross power to receiver per land areaa | 25 W m−2 |
| Decomposition yieldb | 78% |
| Solar receiver efficiency | 90% |
| Solar receiver temperature | 550 °C |
| Solar receiver pressure | 3 bar abs |
Nanofiltration
It is proposed to use NF to reduce the brine volume by a factor of 10, providing brine with magnesium chloride concentration of 10% (20 times that in raw seawater). Operation in 2 stages with intermediate booster pump and use of an energy recovery device will reduce the amount of energy input required for the process. The osmotic pressure of the magnesium chloride brine determines the energy requirements of pumping, and has been determined from published osmotic coefficients.11Multiple effect distillation
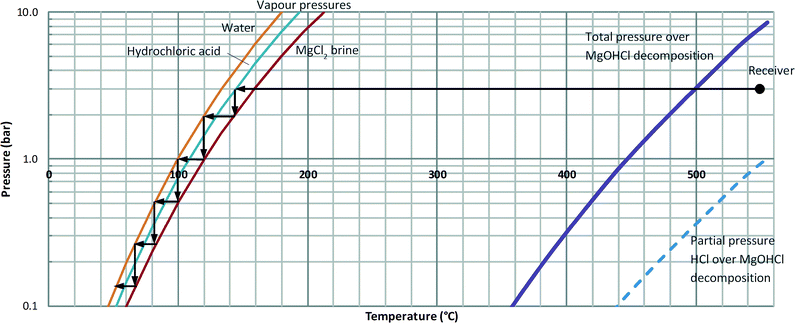
MED will be used to further concentrate the brine to 30%, approaching saturation concentration of 35% (mass solute/mass solution). Use of multiple effects economises the energy input to the distillation process and increases the performance ratio (mass water output/steam input). MED has already been developed for seawater desalination using 12 effects to achieve performance ratio up to 16 with performance further enhanced using thermal vapour compression. For concentrated brine, however, the boiling point elevation limits the number of effects to 4 or 5 as illustrated in the T–P diagram of Fig. 4. It is proposed to drive the MED with condensing hydrochloric acid vapour from the brine thermal decomposition. This means that thermal vapour compression cannot be used, as it would remix the hydrochloric acid with the brine. Consequently the performance ratio is assumed to be limited to below 3.12Condensing hydrochloric acid is highly corrosive presenting challenges for the selection of materials for the MED unit. Specialised Ni–Mo alloys may be acceptable while more expensive Zr alloys have higher corrosion resistance.13 Kim et al. used magnetron sputtering to prepare an amorphous Cr–Zr alloy with 3-fold decreased corrosion rate compared to pure Zr with 6 M HCl.14 This alloy could be used as a protective coating. Note that resistance to HCl is only required in the first effect of the MED unit, as in subsequent effects pure water and not HCl condenses. In all effects there is still a requirement to resist corrosion by concentrated MgCl2 solution, for which purpose more conventional materials such as titanium alloys may be used.13
Brine thermal decomposition
Though the energy requirement of BTD is large, temperatures required are modest (300–600 °C) such that the input can readily be supplied by solar energy. The reaction of eqn (2) occurs in several steps involving dehydration and decomposition of MgCl2 and intermediate magnesium hydroxychloride compounds.15 The last step is decomposition of MgOHCl:7| MgOHCl → MgO + HCl | (3) |
The viable temperature–pressure region for the decomposition is determined by the equilibrium constant of this reaction, which in turn determines the maximum allowable partial pressure of HCl, and hence the allowable total pressure as determined by the molar concentrations of HCl and water in the vapours from decomposition (Fig. 4). The choice of 3 bar as the decomposition pressure means that the temperature can be limited to <600 °C while the saturation temperature of the exiting vapour will remain high enough to drive the MED.
Such temperature requires a point focussing solar concentrator and, because of the large scale needed, it is proposed to carry out the operation using a central receiver system with heliostat field. Concentrated brine will be injected into a solar receiver mounted on a tower, where it will vaporise and substantially decompose to yield steam, HCl and MgO. The design of the receiver has not been attempted in detail, but it is envisaged that – due to the multiphase flow leading to gas-phase output with suspended MgO particles – a volumetric receiver would be selected. Volumetric receivers have been developed to an advanced stage for use with the air Brayton cycle and have achieved efficiencies of 70–80% at temperatures of >1000 °C.16 Losses are dominated by re-radiation which is strongly temperature dependent.17 Therefore, at lower temperatures of 550 °C as used here an efficiency of 90% is assumed. Additional assumptions include: recovery of sufficient heat from the superheated vapours leaving the receiver to preheat the feed brine to saturation temperature; and no heat recovery from the exiting MgO.
It is interesting to note that the presence of MgO particles in the flow also implies similarities with solid particle receivers, a type of receiver that is currently being researched due to its potential to provide energy storage. Particles may aid optical absorption, reducing the need for mesh elements inside the receiver. However, these receivers are still at an early stage of development, so it is difficult to draw conclusions about the achievable performance.17
Results of modelling
The results for a BTD-equipped plant (as in Fig. 3) of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day freshwater output are now compared against an unmodified RO plant of the same output. The additional electrical requirement for the NF increases specific energy consumption of the whole plant by 50% from 3 to 4.5 kW h m−3 (per total volume freshwater output). To supply the thermal input, the size of the heliostat field is 50 ha. Taking into account the increased electricity usage, the plant will absorb net 18.2 ktCO2 per year from the atmosphere, compared to 5.3 ktCO2 per year emissions from the unmodified plant. These results take into account the additional water output from the MED which is only 3.5% of the RO output. The energy penalties of the process (per mass gross sequestered CO2) are: 0.8 GJ/tCO2 and 13 GJ/tCO2 corresponding to electrical and solar thermal energy requirements respectively.
000 m3 per day freshwater output are now compared against an unmodified RO plant of the same output. The additional electrical requirement for the NF increases specific energy consumption of the whole plant by 50% from 3 to 4.5 kW h m−3 (per total volume freshwater output). To supply the thermal input, the size of the heliostat field is 50 ha. Taking into account the increased electricity usage, the plant will absorb net 18.2 ktCO2 per year from the atmosphere, compared to 5.3 ktCO2 per year emissions from the unmodified plant. These results take into account the additional water output from the MED which is only 3.5% of the RO output. The energy penalties of the process (per mass gross sequestered CO2) are: 0.8 GJ/tCO2 and 13 GJ/tCO2 corresponding to electrical and solar thermal energy requirements respectively.
Scale up to 30 billion m3 per year freshwater output would require the equivalent of 8200 such plants, with heliostat fields covering about 4000 km2 of land. In total, these plants would absorb 0.15 GtCO2 per year net which is about 0.4% of current anthropogenic emissions.
Geological disposal of hydrogen chloride
Hydrogen chloride leaves the plant as hydrochloric acid (concentration 21%, approximately azeotropic) which must be sequestered to prevent it from returning to the sea. It can react with silicates to yield chlorides and silica, an example being the reaction with olivine:| Mg2SiO4 + 4HCl → 2MgCl2 + 2H2O + SiO2 | (4) |
The abundance of silicate minerals in the earth's crust greatly exceeds that needed to offset all CO2 emissions.18 However, direct carbonation of silicates has so far been hindered by the slowness of reaction at ordinary temperatures and pressures. Conditions of 150 atm and 185 °C were required for CO2 to react with powdered silicates over timescales of hours.19 In contrast, hydrochloric acid reacted over similar timescales at atmospheric pressure and temperatures of only 30–70 °C. In a comparative study of five acids in reaction with serpentine, hydrochloric acid reacted the second fastest after sulphuric acid.20 It is believed therefore that injection of hydrochloric acid into silicate rocks would provide a permanent route and fast route to in situ acid sequestration.
Silicate minerals from the earth's mantle have been brought to the surface through tectonic and volcanic activities in several locations close to where desalination is needed and employed. Examples include: peridotite of the Samail ophiolite in Oman and of the Troodos ophiolite in Cyprus; and laval outflows of mafic rocks approaching the Red Sea coast near Jeddah. The Samail ophiolite alone is theoretically capable of absorbing more CO2 than present in the entire atmosphere and would thus have similarly large capacity to absorb HCl.21 The energy requirement of transferring hydrochloric acid to sites of disposal has not been considered in this study. Nonetheless, since the process produces only 28 kg of hydrochloric acid for each m3 of freshwater desalinated, it is anticipated that this energy will be small in relation to the energy already used in distribution of the freshwater.
Discussion
Comparison against other options
The proposed BTD process should be evaluated against other options for neutralising ocean acidity or for removing or avoiding CO2. In general, such comparisons may cover issues of effectiveness, capital and operational costs, energy penalties, land usage, time scales, and environmental impacts and risks.22 More broadly, evaluation of geoengineering measures should encompass issues of ethics, governance, and public perception.23 Only very preliminary comparisons are possible here pending more extensive studies. Specifically we compare against: (i) ocean liming via calcination of limestone (instead of via thermal decomposition of MgCl2 in seawater); (ii) avoidance of CO2 using concentrated solar power (CSP) to generate electricity (instead of using similar solar plant to achieve the thermal decomposition).Ocean liming via limestone calcination has been evaluated in depth in a recent study by Renforth et al.6 who predicted electrical and thermal energy penalties of 0.1–1.2 and 0.6–5.6 and GJ/tCO2 respectively. Thus the electrical penalty calculated here for BTD (0.8 GJ/tCO2) is in this range, but the thermal penalty (13 GJ/tCO2) is more than doubled. A breakdown of the thermal energy penalty shows evaporation of water to be the main overhead (Fig. 5). In the proposed system, this energy is only recovered in a limited manner to drive the MED and pre-heat the brine feed to the BTD. Therefore improvements should focus on more effective energy recovery. For example, an organic Rankine cycle could be used to extract useful work, or a mechanical vapour compression cycle may enable the condensing vapours to supply enthalpy for the evaporation of the brine prior to it reaching the solar receiver.
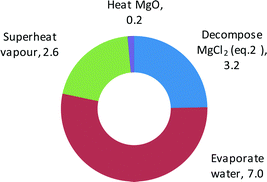 | ||
| Fig. 5 Breakdown of solar thermal energy penalty of brine thermal decomposition (BTD), making up total of 13 GJ/tCO2. | ||
On the other hand, the temperature requirement for BTD is lower than for limestone calcination (550 °C vs. 850 °C) and so the use of solar energy for BTD appears more feasible. Moreover, mining, calcination and transport of limestone to sites for ocean addition will require very substantial planning and investment in infrastructure, and BTD may be advantageous in avoiding these requirements. Further, calcination of limestone liberates CO2 which has to be sequestered somewhere. Recently a new process of Solar Thermal Electrochemical Decomposition (STEP) of limestone has been put forward which produces C or CO rather than CO2.24 The process requires solar thermal energy and electrical energy as inputs. The energy budget and scale up of this process would require further evaluation to enable comparison with BTD.
For comparison against CSP, the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day desalination plant requires a solar field covering 50 ha which is comparable area to the PS10 CSP plant in Spain (land area 55 ha).25 As PS10 has net electrical output of about 20 GW h per year, it avoids an estimated 10 ktCO2 per year (based on consistent assumption of 0.5 kgCO2 kW−1 h−1 carbon intensity of substituted electricity).26 Per land area, this is only half the absorbed amount expected with the BTD process. PS10 is chosen for comparison, because it is a heliostat plant similar to that envisaged for BTD. Note that other types of solar plant may have greater CO2 avoidance per land area. The preferred choice of CSP plant for a given project can depend on a range of criteria in technical, economic and environmental categories.27
000 m3 per day desalination plant requires a solar field covering 50 ha which is comparable area to the PS10 CSP plant in Spain (land area 55 ha).25 As PS10 has net electrical output of about 20 GW h per year, it avoids an estimated 10 ktCO2 per year (based on consistent assumption of 0.5 kgCO2 kW−1 h−1 carbon intensity of substituted electricity).26 Per land area, this is only half the absorbed amount expected with the BTD process. PS10 is chosen for comparison, because it is a heliostat plant similar to that envisaged for BTD. Note that other types of solar plant may have greater CO2 avoidance per land area. The preferred choice of CSP plant for a given project can depend on a range of criteria in technical, economic and environmental categories.27
Valorisation of hydrochloric acid
As an alternative to geological disposal of hydrochloric acid, valorisation may be considered. The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day desalination-BTD plant will produce 100 kt per year of hydrochloric acid at concentration 21% which could be sold commercially. Since conventional manufacture of hydrochloric acid results in emission of about 0.7 kgCO2 kg−1, substitution of 100 kt per year would result in avoidance of 70 kt CO2 per year.‡ This is several times larger than the 18.2 ktCO2 per year potentially absorbed from the atmosphere by MgO. However, scale up to 30 billion m3 per year freshwater output would produce 840 Mt per year of hydrochloric acid containing 176 Mt per year of HCl gas. In contrast, current world production of HCl is reported as only 20 Mt per year, much of which is consumed and produced on site, and open world market size is only 5 Mt per year.28 Therefore production of 176 Mt per year could not be absorbed by current world markets.
000 m3 per day desalination-BTD plant will produce 100 kt per year of hydrochloric acid at concentration 21% which could be sold commercially. Since conventional manufacture of hydrochloric acid results in emission of about 0.7 kgCO2 kg−1, substitution of 100 kt per year would result in avoidance of 70 kt CO2 per year.‡ This is several times larger than the 18.2 ktCO2 per year potentially absorbed from the atmosphere by MgO. However, scale up to 30 billion m3 per year freshwater output would produce 840 Mt per year of hydrochloric acid containing 176 Mt per year of HCl gas. In contrast, current world production of HCl is reported as only 20 Mt per year, much of which is consumed and produced on site, and open world market size is only 5 Mt per year.28 Therefore production of 176 Mt per year could not be absorbed by current world markets.
Local impacts to marine environment
Ocean discharge of alkaline substance would tend to neutralise acidity globally, but may cause undesired local environmental impacts. MgO gradually hydrolyses to form Mg(OH)2 which partially dissolves liberating hydroxide ions. Seawater normally has pH in the range 7.9 to 8.5, lowered by 0.1 units from pre-industrial levels.29 Thus the pH of the MgO suspension prepared here (pH 9.86) is about 1.5 units above natural levels, and pH would be significantly increased in proximity of the ocean outlet. Hansen30 found that growth of phytoplankton slows at high pH. Species C. lineatum, H. triqueta and P. minimum ceased growing at pH 8.8, 9.45 and 9.6 respectively. However, most ecological studies about impacts of pH focus on acidification, whereas studies concerning local impacts of alkalinity are lacking.As the MgO would be discharged together with NaCl brines, salinity would also be increased, as it is near standard desalination plant. Concerns about impacts of salinity, temperature and chemicals near outlets have prompted detailed modelling and experimental studies.31–33 Rapid dispersion and dilution of brines is favoured by long discharge pipes, multiport diffusers, and avoidance of lagoons and shallow waters.32 The knowledge and modelling tools already developed in this area could in the future be applied to effective dispersion and mixing of alkaline discharge.
Timescale of absorption of CO2
Ocean liming is generally considered a long term option for CO2 removal as, due to slowness of gas exchange between the atmosphere and oceans, it could take >100 years to lower atmospheric CO2 levels.34 However, by the same argument, direct atmospheric CO2 reduction or mitigation would be slow to remediate the oceans.Studies of mineral carbonation have shown that Mg(OH)2 reacts more readily with CO2 than does MgO.35 Since the enthalpy of formation of Mg(OH)2 is lower than that of MgO, it could be more energy efficient to use a conversion pathway that produces Mg(OH)2 directly from brine [rather than via MgO which is then hydrolysed to Mg(OH)2]. Future studies of absorption kinetics and timescales could build on the existing models of brine outlets, and would require additional information about the size and dissolution rates of MgO or Mg(OH)2 particles dispersed in seawater.
Conclusions
This preliminary study of brine thermal decomposition (BTD) by solar energy has shown that this process could be an interesting variant of ocean liming to help combat ocean acidification and remove atmospheric carbon dioxide. The BTD process has been studied for application to desalination plant reject. The main findings are:- Decomposition of MgCl2 in seawater is readily achieved at temperatures <600 °C, well within the capabilities of solar volumetric receivers heated by heliostat fields.
- The electrical requirement of the desalination plant is increased by about 50%, but this is more than offset by the absorptive capacity of the MgO produced through BTD.
- Electrical energy penalty is in the range estimated for that of ocean liming based on limestone calcination; whereas thermal energy penalty is more than double.
- A doubling of current world desalination capacity could, if equipped with BTD, remove 0.4% of current anthropogenic CO2 emissions, requiring heliostat fields covering 4000 km2 of land.
- The land usage per mass of CO2 avoided are competitive against those for equivalent avoidance by means of CSP plants using heliostat fields to generate electricity.
- To reduce thermal energy penalties and land requirements, research is needed into lowering the energy needed to evaporate water from the MgCl2 brine (or recovering more useful energy from condensing HCl vapours).
In principle the BTD process could be used anywhere with sea access; however, use with desalination has advantages: the infrastructure for intake and discharge already exists; and the seawater is already partially dewatered as reject brine, providing a more suitable feed to the process than raw seawater.
Notes and references
- Global Water Intelligence, Market Data, 2012.
- 2030 Water Resources Group, Charting our Water Future, 2009.
- IPCC, Fifth Assessment Report: Summary for Policymakers, 2014.
- J. Zhou, V. W. C. Chang and A. G. Fane, Desalination, 2013, 308, 233–241 CrossRef CAS.
- V. Ferrini, C. De Vito and S. Mignardi, J. Hazard. Mater., 2009, 168, 832–837 CrossRef CAS PubMed.
- P. Renforth, B. G. Jenkins and T. Kruger, Energy, 2013, 60, 442–452 CrossRef CAS.
- K. K. Kelley, Technical Paper 676, US Dept of the Interior, 1945 Search PubMed.
- G. Lychnos, R. Amdouni and P. A. Davies, RSC Adv., 2012, 2, 7978–7982 RSC.
- US Geological Survey, Minerals Information, 2014.
- P. A. Davies, K. Turner and C. Paton, in International Engineering Conference, Mutah, 2004, pp. 523–540 Search PubMed.
- R. Robinson and R. Stokes, Electrolyte solutions, Butterworths, London, 1959 Search PubMed.
- H. Ettouney, in Seawater Desalination, ed. A. Cipollina, G. Micale and L. Rizutti, Springer, 2009 Search PubMed.
- P. A. Schweitzer, Corrosion Resistance Tables, Marcel Dekker, 2004 Search PubMed.
- J. H. Kim, H. Habazaki, A. Kawashima, K. Asami and K. Hashimuto, Corros. Sci., 1993, 34, 1817–1827 CrossRef CAS.
- J. S. C. de Bakker, The recovery of magnesium oxide and magnesium hydroxide from magnesium chloride brines and molten salt hydrates, PhD thesis, Queen's University, Ontario, 2011 Search PubMed.
- A. L. Ávila-Marín, Sol. Energy, 2011, 85, 891–910 CrossRef.
- C. K. Ho and B. D. Iverson, Renewable Sustainable Energy Rev., 2014, 29, 835–846 CrossRef CAS.
- K. S. Lackner, Annu. Rev. Energy Environ., 2002, 27, 193–232 CrossRef.
- S. J. Gerdemann, W. K. O'Connor, D. C. Dahlin, L. R. Penner and H. Rush, Environ. Sci. Technol., 2007, 41, 2587–2593 CrossRef CAS PubMed.
- S. Teir, H. Revitzer, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Int. J. Miner. Process., 2007, 83, 36–46 CrossRef CAS.
- P. B. Kelemen and J. Matter, Proc. Natl. Acad. Sci. U. S. A., 2008, 45, 17295–17300 CrossRef.
- K. Caldeira, G. Bala and L. Cao, Annu. Rev. Earth Planet. Sci., 2013, 41, 231–256 CrossRef CAS.
- Royal Society, Geoengineering the climate: science, governance and uncertainty, Policy Document 10/2009, London Search PubMed.
- S. Licht, H. Wu and C. Hettige, et al. , Chem. Commun., 2012, 48, 6019–6021 RSC.
- http://www.nrel.gov/csp/solarpaces/project_detail.cfm/projectID=38 [accessed 3 Dec 2014].
- M. Romero, R. Buck and J. E. Pacheo, Trans. ASME, 2002, 124, 98–108 CrossRef.
- J. D. Nixon, P. Dey and P. A. Davies, Energy, 2012, 46, 541–554 CrossRef.
- R. C. Ropp, Encyclopedia of the Alkaline Earth Compounds, Elsevier, 2012p.34 Search PubMed.
- Royal Society, Ocean Acidification due to increasing atmospheric carbon dioxide, Policy Doc 12/2005, London Search PubMed.
- P. Hansen, Aquat. Microb. Ecol., 2002, 28, 279–288 CrossRef.
- I. Alameddine and M. El-Fadel, Desalination, 2007, 214, 241–260 CrossRef CAS.
- A. Loya-Fernández, L. M. Ferrero-Vicente and C. Marco-Méndez, et al. , Desalination, 2012, 217–224 CrossRef.
- S. Al-Sanea, J. Orfi, A. Najib, 2014, DOI:10.1080/19443994.2014.940658, in press.
- L. D. D. Harvey, J. Geophys. Res.: Oceans, 2008, 113, C04028 Search PubMed.
- J. Fagerlund, J. Highfield and R. Zevenhoven, RSC Adv., 2012, 2, 10380–10393 RSC.
Footnotes |
| † Methods note: titration was carried out by first dissolving the MgO in excess 0.5 M HCl then titrating back to the neutral point with 0.5 M NaOH to determine the net HCl needed. Amount of Mg initially present in samples was determined by conductivity measurement and standard salt composition for seawater was assumed. Experiment repeated 3 times. |
| ‡ Emission of 0.7 kgCO2 kg−1 was calculated using Simapro Life Cycle Assessment software (v8.0.2) and Ecoinvent 3 database. Hydrochloric acid (conc. 30%) manufactured in N. America or Europe was analysed using the CML-IA baseline method v3.01. The result was adjusted to the concentration of 21% as used in this study. |
| This journal is © The Royal Society of Chemistry 2015 |