Anaerobic membrane bioreactor treatment of domestic wastewater at psychrophilic temperatures ranging from 15 °C to 3 °C†
A. L.
Smith‡
a,
S. J.
Skerlos
ab and
L.
Raskin
*a
aDepartment of Civil and Environmental Engineering, University of Michigan, 2350 Hayward Road, Ann Arbor, MI 48109, USA. E-mail: raskin@umich.edu
bDepartment of Mechanical Engineering, University of Michigan, 2350 Hayward Road, Ann Arbor, MI 48109, USA
First published on 1st December 2014
Abstract
Anaerobic membrane bioreactor (AnMBR) treatment of a simulated domestic wastewater was evaluated at psychrophilic temperatures of 15, 12, 9, 6, and 3 °C. Chemical oxygen demand (COD) removal >95% was achieved at temperatures as low as 6 °C, but fell to 86% at 3 °C. As temperature decreased, soluble COD in the bioreactor increased suggesting a reduction in suspended biomass activity. The high total COD removal was maintained via biological activity in the membrane biofilm, which resulted in significant dissolved methane oversaturation in the permeate. Sequencing of 16S rRNA suggested that the biofilm's metabolic diversity increased as temperature decreased in response to a greater flux of complex organics into the biofilm due to temperature-based suspended biomass inhibition. Hydrogenotrophic methanogenesis as opposed to aceticlastic methanogenesis was the preferred pathway in the biofilm, but not in the suspended biomass. This research demonstrated that AnMBR treatment of domestic wastewater at very low temperatures is feasible. However, it is important to develop technologies for dissolved methane recovery and to consider strategies to improve suspended biomass activity at low temperatures to decrease the reliance on biofilm treatment thereby decreasing dissolved methane oversaturation.
Water impactThis paper demonstrates the viability of anaerobic membrane bioreactors (AnMBRs) for domestic wastewater treatment at temperatures as low as 6 °C. The key to successful low temperature treatment is the development of an active biofilm on the surface of the membrane. Specifically, we observed that biofilms increasingly contributed to treatment as the operating temperature decreased from 15 °C to 6 °C. This relative increase in biofilm activity led to an increase in dissolved methane oversaturation in the AnMBR effluent. It can therefore be concluded that while low temperature treatment of domestic wastewater can be achieved with AnMBRs, recovering the increased dissolved methane is needed to achieve net positive energy and reduced global warming potential. |
Introduction
With few exceptions, anaerobic biological waste treatment processes to date are operated at mesophilic (30–40 °C (ref. 1)) or thermophilic (50–60 °C (ref. 2)) temperatures by heating reactors using produced biogas. However, maintaining these reactor temperatures for anaerobic treatment of domestic wastewater using biogas heating alone is challenging in most climates due to the low energy content of domestic wastewater.3 Operation at ambient temperatures is one approach to circumvent this challenge and will facilitate deployment of anaerobic systems for domestic wastewater treatment. The majority of the world's population lives in temperate climate zones with moderately variable seasonal temperatures.4 In these regions, wastewater temperatures are higher than the air temperature during most of the year, except during the hottest summer months, which leads to a relatively flat seasonal wastewater temperature profile.5 The annual mean temperature of domestic wastewater in the U.S. varies from approximately 3 °C to 27 °C, with a nationwide average of about 16 °C (ref. 5) suggesting that domestic wastewater temperatures below 20 °C are common, especially in winter months. Anaerobic membrane bioreactor (AnMBR) systems have recently come to the forefront as promising options for mainstream anaerobic treatment of domestic wastewater at various temperatures.6–8 Understanding the lower temperature limits for AnMBR treatment is imperative to determine climate-based barriers to implementation.Despite the importance of assessing AnMBR operation in the psychrophilic temperature range (<20°), only a few studies9–15 have done so and no studies to our knowledge have explored temperatures <8 °C. Chemical oxygen demand (COD) removal >85% has been reported at temperatures as low as 15 °C.9–12,15 However, Chu et al.10 observed COD removals of only 76–81% at 11 °C. A few studies reported an increase in the amount of COD removal across the membrane when the operational temperature decreased9–11 suggesting that the membrane biofilm plays an increasingly important role in COD removal as temperature decreases.
Temperature-based impacts on the distribution of methane between the gas and liquid phase are a concern for anaerobic domestic wastewater treatment at low temperatures. Dissolved methane in the liquid phase increases at lower operational temperatures and impairs the energy balance if lost through the AnMBR permeate. The dissolved methane in the permeate may also be released to the atmosphere, increasing the global warming potential of AnMBR treatment.16 Multiple AnMBR studies have reported dissolved methane oversaturation,12,17,18 exacerbating these concerns. We recently established a positive correlation between dissolved methane oversaturation and methanogenic activity in the membrane biofilm.15 The potential for greater reliance on the membrane biofilm, as opposed to the suspended biomass, for treatment at psychrophilic conditions9–11 as indicated above could further increase dissolved methane oversaturation. Understanding methane fate, especially for low temperature operation, is necessary to gauge the potential energy recovery and environmental impacts of AnMBR systems.
Understanding how psychrophilic temperatures influence treatment performance and methane fate is challenging without evaluating the activity of the diverse microbial populations in the suspended biomass and the membrane biofilm of an AnMBR. In low temperature anaerobic treatment, syntrophic propionate oxidation and methanogenesis are typically considered rate-limiting metabolisms.19,20 In addition, aceticlastic methanogens appear to be more strongly affected by low temperature than their hydrogenotrophic counterparts21,22 and a shift towards hydrogenotrophic methanogenesis at low temperatures has been reported,23–25 possibly due to an increase in hydrogen solubility.1 Given the (1) complexity of anaerobic microbial communities involved in degrading domestic wastewater, (2) challenges specific to AnMBRs due to distinct microbial communities in the suspended biomass and membrane biofilm,15 and (3) temperature-based effects on hydrogen availability and methane distribution, it is necessary to evaluate the response of specific microbial populations to temperature changes to help improve AnMBR performance.
The objective of this study was to evaluate AnMBR operation at decreasing temperatures to assess the potential for AnMBR domestic wastewater treatment in temperate climates. A bench-scale AnMBR with a history of operation with controlled membrane fouling at 15 °C was operated for five to six weeks each at 12, 9, 6, and 3 °C to represent a range of potential temperatures experienced during fall and winter in a domestic wastewater treatment plant in a temperate climate. Illumina sequencing of 16S rRNA genes (rDNA) and 16S rRNAs was applied to evaluate microbial community structure and activity dynamics in the suspended biomass and biofilm in response to the decrease in operational temperature.
Materials and methods
AnMBR operation and chemical assays
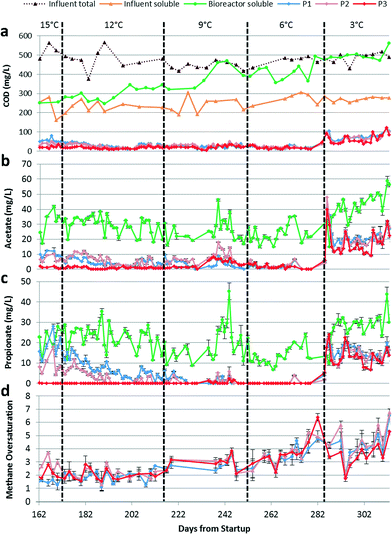
A bench-scale AnMBR15 was operated to evaluate system performance at varying psychrophilic temperatures while treating a simulated domestic wastewater.12,26 The bench-scale AnMBR was previously operated at 15 °C for 172 days.15 The reactor temperature was controlled using a water jacket connected to a Polystat 6-L recirculating water bath (Cole-Parmer, Vernon Hills, IL). The water bath temperature was adjusted based on temperature measurement of a submerged probe located in close proximity to the membrane surface. The bench-scale AnMBR contained three individually controlled membrane housings, designated P1, P2, and P3, and generated three permeate streams.The AnMBR temperature was reduced from 15 °C to 12 °C on day 173, then to 9 °C on day 216, to 6 °C on day 252, and to 3 °C on day 286 (Table 1). The AnMBR was initially operated at an HRT of 17 h corresponding to an organic loading rate (OLR) of 630 mg COD L−1 d−1. However, membrane fouling became more severe throughout the operational period resulting in a reduction in flux (due to pump slippage) and an increase in HRT (Table 1). Biomass was only removed from the AnMBR for sampling purposes, which resulted in a solids retention time (SRT) of approximately 300 days. The biogas sparging flow rate for P3 was 3.0 L min−1 (5.8 m3 h−1 m2) throughout the current study, although P3 had a history of fouling. The biogas sparging flow rates for P1 and P2 were decreased from 3.0 L min−1 to 1.5–2.0 L min−1 from days 173 to 200 to permit biofilm development (described further below) and were increased to 3.0 L min−1 from days 201 to 313. Backflushing was performed for 30 s every 10 min of bioreactor operation initially, but was modified on day 253 in attempt to improve flux and lower the HRT by increasing the duration to 1 min and decreasing the interval time to 5 min.
| Days from startup | Temperature (°C) | HRT (h) | ORL (mg COD L−1 d−1) | Flux (LMH) | ||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | ||||
| 162–172 | 15 | 17 ± 0.79 | 630 | 3.0 ± 0.24 | 1.8 ± 0.28 | 2.5 ± 0.06 |
| 173–215 | 12 | 17 ± 1.0 | 630 | 2.5 ± 0.23 | 2.2 ± 0.21 | 2.5 ± 0.14 |
| 216–251 | 9 | 19 ± 1.3 | 560 | 2.2 ± 0.16 | 2.1 ± 0.15 | 2.1 ± 0.14 |
| 252–285 | 6 | 26 ± 3.5 | 410 | 1.9 ± 0.22 | 1.88 ± 0.21 | 12 ± 0.10 |
| 286–313 | 3 | 29 ± 2.2 | 370 | 1.6 ± 0.16 | 1.5 ± 0.15 | 1.2 ± 0.10 |
Influent, permeate, biogas, and bioreactor content sampling, sample preservation, and storage were performed as described previously.15 COD, total suspended solids (TSS), and volatile suspended solids (VSS) were determined using procedures outlined in Standard Methods.27 Soluble COD was determined by filtering samples through a 0.2 μm filter to replicate the physical removal of the membrane (same pore size). Concentrations of volatile fatty acids (VFAs; formate, acetate, propionate, butyrate, and valerate) and sulfate were determined by ion chromatography (ICS-1600, Dionex, Sunnyvale, CA).15
Biogas methane content and dissolved methane concentration were measured with a gas chromatograph (Gow-Mac, Bethlehem, PA).15 Biogas production was measured by collecting gas in a 1-L Tedlar bag and quantifying the production daily using a wet-type gas meter (Actaris Metering Systems, Dordrecht, The Netherlands).
Nucleic acids extraction and cDNA synthesis
Suspended and biofilm biomass samples from the AnMBR were collected15 on days 215, 251, 285, and 313 at the end of each temperature phase, pelletized by centrifugation at 5000 × g for 5 min at 4 °C, decanted, and immediately stored at −80 °C. Biomass samples for RNA extraction were prepared similarly except for the addition of RNAlater (Qiagen, Valencia, California) prior to storage. DNA and RNA were extracted from pelletized biomass and the quality and quantity were assessed as described previously.15 Reverse transcription to generate single-stranded complementary DNA (cDNA) from RNA extracts was performed using the SuperScript VILO cDNA Synthesis Kit (Life Technologies, Grand Island, NY).16S rDNA and rRNA sequencing
Universal primers targeting the V4 region28 were used to amplify 16S rDNA and rRNA as described previously.15 PCR of 16S rDNA and rRNA from DNA extracts and synthesized cDNA, respectively, taken during reactor operation at 12, 9, and 6 °C was performed by the Host Microbiome Initiative (University of Michigan, Ann Arbor, MI). PCR conditions included 20 μL reactions with the aforementioned primers at 500 nM, 10 μL 2× Accuprime buffer 11, 0.15 μL Accuprime TAQ (Invitrogen, Carlsbad, CA), 0.5 ng template, and nuclease-free water. Thermocycling conditions consisted of an initial 2 min denaturation at 95 °C, followed by 30 cycles of denaturing at 95 °C for 20 s, annealing at 55 °C for 15 s, and extension at 72 °C for 5 min, followed by a final extension at 72 °C for 5 min. DNA and cDNA from 3 °C biomass was amplified using the above described primer sets at 500 nM, 0.3 mg mL−1 bovine serum albumin (BSA), 10 μL 2× Phusion High-Fidelity Master Mix (NEB, Ipswich, MA), 0.5 ng template, and nuclease-free water. Thermocycling conditions consisted of an initial 2 min denaturation at 95 °C, followed by 30 cycles of denaturing at 95 °C for 20 s, annealing at 55 °C for 15 s, and extension at 72 °C for 30 s, followed by a final extension at 72 °C for 5 min. Amplicons were pooled by equal mass using the SequalPrep Normalization Plate Kit (Life Technologies, Grand Island, NY). Multiplexed amplicons were sequenced by the Host Microbiome Initiative via Illumina MiSeq using the MiSeq Reagent Kit V2 (samples from 12, 9, and 6 °C; 2 × 250 bp reads) and V3 (samples from 3 °C; 2 × 300 bp reads). The resulting sequences were processed with mothur29 following the Schloss MiSeq SOP. In brief, sequence data were screened to eliminate sequences containing ambiguous bases and then aligned using the SILVA reference alignment.30 The UCHIME algorithm31 was used to identify and remove chimeras. 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 paired-end reads per sample were generated after quality filtering and subsampling. Sequences were classified using the Ribosomal Database Project32 and further analysed for operational taxonomic unit (OTU)-based clustering (average neighbor algorithm at 3% cutoff) and principle co-ordinate analyses using the θYC index.33
922 paired-end reads per sample were generated after quality filtering and subsampling. Sequences were classified using the Ribosomal Database Project32 and further analysed for operational taxonomic unit (OTU)-based clustering (average neighbor algorithm at 3% cutoff) and principle co-ordinate analyses using the θYC index.33
Results and discussion
COD removal remained excellent when reducing the AnMBR temperature from 15 °C to 6 °C, but was impacted at 3 °C
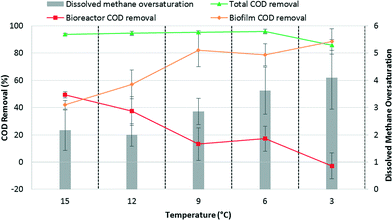
The bench-scale AnMBR had previously been operated for 172 days at 15 °C.15 During days 100–138 of the previous study, P1, P2, and P3 were operated under varying levels of membrane fouling by adjusting biogas sparging flow rates to evaluate the effect of differential fouling levels on biofilm treatment.15 We observed a decrease in permeate COD, primarily due to acetate and propionate removal, when membranes were operated under high fouling conditions. Under those conditions, membrane biofilm treatment allowed the system to achieve COD removal >95%. Further, we observed no negative effect on biofilm treatment after reducing the transmembrane pressure (TMP) to near zero once an active biofilm had been developed. This suggests that we can stimulate biofilm development by reducing fouling control temporarily and can maintain sufficient biofilm activity to obtain excellent treatment performance after returning to low TMP. At the end of the previous study,15 P3 had an active biofilm, P2 was in the process of developing its biofilm activity, and P1 had a history of low fouling and limited biofilm treatment. The corresponding performance results are shown in Fig. 1 for the last 10 days of operation at 15 °C. After changing the temperature to 12 °C on day 173, we elected to also operate P1 under conditions supporting biofilm development to maximize overall treatment performance.Because of the membranes' different histories, the COD levels in the three permeates initially differed but gradually converged as a mature biofilm was formed on each membrane. Despite the initial differences in permeate quality, the overall COD removal averaged 95 ± 1.6% at 12 °C and was maintained as the temperature was reduced, averaging 95 ± 1.1 and 96 ± 1.8% at 9 and 6 °C, respectively. However, a gradual increase in soluble COD in the bioreactor suggested that the suspended biomass activity declined with temperature (Fig. 1a). Biofilm activity was therefore critical to maintain the high total COD removal. At 3 °C, influent total and bioreactor soluble COD concentrations were similar indicating that the suspended biomass was responsible only for particulate COD hydrolysis (Fig. 1a). Effectively, all COD removal occurred in the biofilm at 3 °C (Fig. 2). Permeate COD averaged 70 ± 21 mg L−1 corresponding to a COD removal of 86 ± 4.0% at this temperature. Continued operation for 70 days at 3 °C did not result in improved COD removal (data not reported).
VFA removal was similarly unaffected by temperature decrease from 15 °C to 6 °C. While the acetate and propionate concentrations were still decreasing in P1 and P2 due to the membrane fouling histories described above, all permeates had acetate and propionate concentrations below 10 mg L−1 starting around day 190 (Fig. 1b and c). Other VFAs (formate, butyrate, and valerate) were below their detection limits (Fig. S1 in ESI†). Immediately after the temperature decrease from 6 °C to 3 °C, permeate acetate and propionate concentrations increased sharply and briefly exceeded those in the bioreactor. This observation can be explained if the biofilm was fermenting complex organic compounds and methanogens and syntrophic propionate-oxidizing bacteria in the biofilm had yet to adapt to the temperature decrease. The steady-state concentrations of acetate, propionate, and formate (Fig. 1b and c and S1†) in the permeates were elevated at 3 °C relative to the corresponding concentrations for the other temperatures. However, the amount of acetate and propionate removed by the biofilm remained similar after the change to 3 °C, suggesting that aceticlastic methanogens and syntrophic propionate-oxidizing bacteria in the biofilm were not inhibited by the lower temperature. The increase in acetate and propionate concentrations in the bioreactor suggest inhibition of these populations in the suspended biomass (Fig. 1b and c).
We considered heat release from exothermic reactions (e.g., hydrogenotrophic methanogenesis) as a potential difficulty when trying to maintain low operational temperatures uniformly throughout the AnMBR. Heat release from exothermic reactions could increase temperature in the biofilm relative to the suspended biomass and bias interpretations of observed data. However, we calculated a maximum heat increase of 0.67 °C for a worst-case scenario (i.e., operation at 3 °C assuming hydrogenotrophic methanogenesis in the biofilm in accordance with 7 times dissolved methane oversaturation and reaction enthalpies provided by Westermann et al.34). In reality, some methane is produced in the biofilm by aceticlastic methanogenesis, an endothermic reaction.35 Further, other reactions occurring in the biofilm are also endothermic (e.g., propionate oxidation). Therefore, biological activity in the biofilm likely did not appreciably increase the temperature in the biofilm above the temperature in the suspended biomass.
COD removal was potentially impacted by the increase in HRT as temperature decreased, particularly at 6 and 3 °C (Table 1). A positive correlation between COD removal and HRT in AnMBR has been reported at low temperatures.10 The increase in HRT in this study was due to membrane fouling and the corresponding high TMP, which reduced the flux. For each 3 °C temperature decrease, the TMP increased ~20 kPa over the course of several hours. Specific constituents often linked to membrane fouling such as extracellular polymeric substances (EPS) have been shown to increase as temperature decreases in aerobic MBRs.36 However, the inverse has been shown in AnMBRs in a comparison of mesophilic and psychrophilic temperatures.37 The rapid onset of fouling observed here suggests that the fouling may have been non-biological in nature.
Reliance on the membrane biofilm for treatment led to significant dissolved methane oversaturation
The high observed dissolved methane concentrations in the permeates indicated significant methanogenic activity in the biofilm. During the last 10 days of operation at 15 °C, methane oversaturation averaged 2.2 ± 0.74 (Fig. 2). Methane oversaturation remained similar at 12 °C, but then increased as temperature decreased (Fig. 1d and 2) with an average of 2.0 ± 0.41, 2.9 ± 0.48, 3.6 ± 0.87, and 4.1 ± 1.2 at 12, 9, 6, and 3 °C, respectively. We hypothesize that the higher oversaturation resulted from a greater dependence on the biofilm for treatment, which resulted in more methane production in the biofilm. The majority of methane produced by the biofilm was likely drawn through the membrane leading to methane oversaturation in the permeate. An increase in methane oversaturation variability was observed as the temperature decreased (e.g., between approximately 2 and 7 times oversaturation during operation at 3 °C; Fig. 1d) potentially suggesting less biological stability in the biofilm at the lowest operational temperature. Oversaturation positively correlated with the biofilm's contribution to COD removal as a function of temperature (Fig. 2) indicating a strong link between dependence on the biofilm for COD removal and dissolved methane concentrations in the permeate. Dissolved methane was a major constituent in the COD mass balance at all operational temperatures, comprising on average 31 ± 12%, 37 ± 9.8%, 63 ± 11%, 72 ± 10%, and 52 ± 19% of CODin at 15, 12, 9, 6, and 3 °C, respectively (Fig. S2†).Dissolved methane was significant due to both methane oversaturation and the high methane content in the biogas, >90% at all temperatures. The methane content for low-temperature anaerobic treatment of domestic wastewater is higher than in mesophilic anaerobic digestion of high-strength waste streams due to (1) the lower OLR, which results in less gas production relative to effluent flow, and (2) the lower temperature, which increases carbon dioxide solubility more so than methane solubility. Therefore, the majority of carbon dioxide is removed from the reactor in the dissolved form preventing its accumulation in the biogas and resulting in a higher methane content in the biogas (for more details see calculations in the ESI†). Although the high methane content exacerbates dissolved methane concerns, it also results in generation of a higher quality biogas requiring less purification prior to energy recovery via cogeneration.
Our data suggest that biofilm treatment may be a requirement at low operational temperatures for AnMBR to achieve effluent discharge criteria. However, strategies to improve suspended biomass activity such that biofilm treatment is unnecessary may limit methane oversaturation. We have previously shown that methane oversaturation is minimal in the absence of biofilm treatment.15 Operation at low temperatures for longer time periods may provide sufficient time for temperature adaptation of the suspended biomass. Alternatively, inoculation with psychrophilic biomass may also be beneficial. Currently, psychrophilic anaerobic digesters are rare, as most engineered anaerobic systems are operated in the mesophilic or thermophilic temperature range. This has prompted researchers to investigate seeding anaerobic systems with psychrophilic biomass from the environment,38 which may be a strategy of interest in AnMBR research.
The microbial community activity changed substantially when decreasing AnMBR temperature
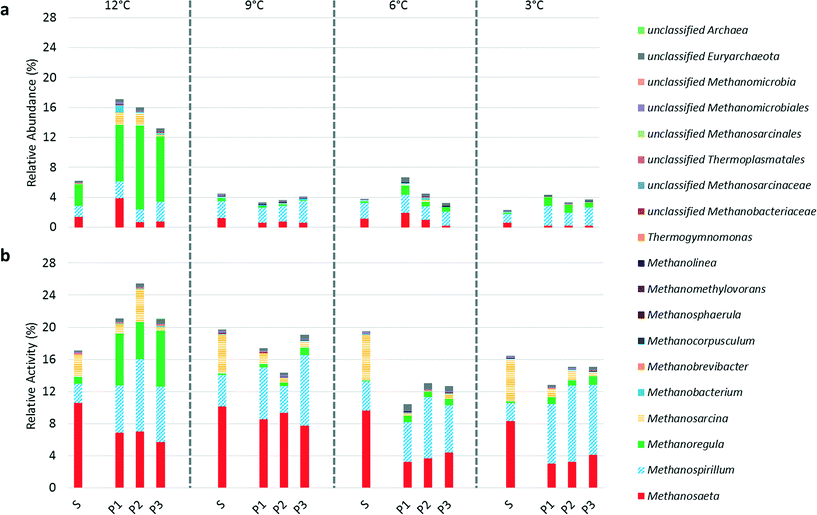
RNA-based methods to assess microbial activity, as opposed to DNA-based methods evaluating microbial community structure, may be particularly valuable in environments with low microbial growth and high biomass retention, such as in psychrophilic AnMBRs. DNA-based approaches can only accurately characterize the active microbial populations in such systems after sufficient operational time, which is a limitation in bench- or pilot-scale studies. Further, DNA-based approaches also include extracellular DNA and/or DNA from inactive community members. We previously studied an AnMBR operated at 15 °C using a microbial characterization approach that combined DNA- and RNA-based methods and observed significant differences between 16S rDNA and 16S rRNA relative sequence abundances, especially for methanogens and syntrophic fatty acid-oxidizing bacteria. Although 16S rRNA sequencing to infer microbial activity has a number of limitations,39 we believe that the temporal and comparative evaluation of suspended biomass and biofilm using the combination of 16S rDNA and 16S rRNA data presented here is much more informative than 16S rDNA sequencing alone. Quantifying functional gene expression may be a more accurate method to determine activity of specific populations. However, this approach is challenging given the high diversity in anaerobic microbial communities and the lack of suitable functional gene targets for several of the trophic groups. We previously reported an excellent correlation between methanogenic activity based on 16S rRNA sequencing and expression of the methyl coenzyme-M reductase (mcrA) gene in an AnMBR operated at 15 °C,15 suggesting that 16S rRNA sequencing is a valid tool to study psychrophilic AnMBR communities. Below we use the terms “relative abundance” and “relative activity” for DNA and RNA-based sequencing, respectively.A decline in the relative activity of methanogens was observed in the biofilm as temperature decreased (Fig. 3). However, temperature and dissolved methane concentrations in the permeates were inversely correlated (Fig. 2 and S2†), suggesting higher methanogenic activity in the biofilm in response to the increase in soluble COD in the bioreactor (i.e., an increase in COD flux to the biofilm) (Fig. 1) as temperature decreased. The combined interpretation of these sequence and performance data suggests that the absolute activity of methanogens in the biofilm increased in parallel with an increase in activity of other populations (e.g., fermenters) as the temperature decreased. Therefore, the observed decline in relative activity of methanogens signifies an increase in the range of microbial metabolisms in the biofilm rather than a reduction in absolute methanogenic activity. We hypothesize that biofilm biomass is more resilient to temperature drops compared to suspended biomass due to a greater spatial organization of the microbial community in the biofilm enhancing syntrophy and/or reduced mass-transport limitations resulting in increased substrate availability.
A limitation of this study is the lack of absolute abundance and activity sequence data. Quantitative nucleic acid extraction from biomass is challenging particularly when matrix differences such as those between the suspended biomass and biofilm are unavoidable. Constituents such as EPS, which we previously reported as higher in biofilms from fouled membranes,12 can decrease extraction efficiency and nucleic acid quality, which makes quantitative characterization difficult. Constituents such as EPS and other microbial products may also vary as a function of temperature36 creating additional complications when temperature is varied. Therefore, we relied on relative molecular characterization and process performance data to make inferences regarding absolute activity.
Although 16S rRNA sequencing may be more representative of community activity, 16S rDNA sequencing revealed changes in the methanogenic community structure as temperature decreased. Methanoregula spp., mesophilic hydrogenotrophic methanogens,40 were the dominant methanogens in terms of abundance at 12 °C comprising 46% and 60 ± 14% of Archaea in suspended biomass and biofilm biomass, respectively. However, at 9 °C Methanoregula spp. comprised only 9.0% of Archaea in the suspended biomass and <8.0% in the biofilm biomass suggesting abrupt inhibition when switching from 12 °C to 9 °C. 16S rRNA sequencing supported this observation, although the relative activity of Methanoregula spp. was much lower than their relative abundance. Two studies that cultivated Methanoregula spp. observed growth at temperatures as low as 10 °C but not at 4 °C.40,41 The shifts in relative activity of other methanogens were less severe as temperature decreased (Fig. 3) and, therefore, temperature based inhibition appears to be of lower importance for these methanogens.
A θYC-based33 principal coordinate analysis (PCoA) of bacterial and archaeal 16S rRNA sequences revealed significant changes in the biofilm community activity as temperature decreased (Fig. 4). Relatively high variability (i.e., poor clustering) among P1, P2, and P3 biofilms was observed at 12 and 9 °C, but this variability was reduced at 6 and 3 °C, which is consistent with convergence of the permeate performance data over time (Fig. 1) as each biofilm matured. The suspended biomass community activity was distinct from the biofilm community at all temperatures and remained relatively constant suggesting limited changes in the community's membership or in the relative activity of each member as a function of temperature (Fig. 4). This indicates that the temperature decrease non-specifically reduced activity of the suspended biomass community. The greater shift in the activity of the biofilm communities suggests better adaptation to the changing conditions in the system.
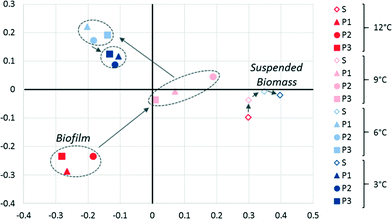 | ||
| Fig. 4 PCoA of the θYC index of the microbial community (Bacteria and Archaea) based on 16S rRNA sequencing of the suspended biomass (S) and biofilm (P1, P2, and P3) at operational temperatures of 12, 9, 6, and 3 °C. The x and y-axes represent 45 and 20% of the variation, respectively. The top 20 classified phylotypes are shown in Fig. S5.† | ||
The dominant type of methanogenic pathway was specific for suspended and biofilm biomass at all temperatures
The hydrogenotrophic methanogenic pathway and the aceticlastic methanogenic pathway were favored in the biofilm and suspended biomass, respectively (Fig. S4†). Higher activity of hydrogenotrophic methanogens relative to their aceticlastic counterparts in the biofilm suggests a metabolic advantage, possibly due to spatial organization of the microbial community supporting syntrophic interactions. It is tempting to speculate that high shear due to biogas sparging interrupted syntrophic interactions in the suspended biomass. We previously reported that the calculated average velocity gradient (g) due to sparging in our system was indeed higher than typically recommended for anaerobic digestion.15 However, no studies to date have conclusively determined how high shear conditions may impact anaerobic microbial communities and this concern has yet to be evaluated in AnMBRs operated at low loading rates.The relative activity of Methanosarcina spp., mixotrophic methanogens capable of metabolizing acetate, hydrogen, and C1 compounds,42 increased in the suspended biomass as temperature decreased (Fig. S4†). Since we cannot determine substrate utilization patterns of Methanosarcina spp. in our system with the available data, we hypothesize that Methanosarcina spp. may have an advantage over other methanogens at low temperatures due to their metabolic flexibility. For example, Methanosarcina spp. might have the capability to transition from aceticlastic to hydrogenotrophic methanogenesis as temperature decreases and the thermodynamics of hydrogenotrophic methanogenesis become more favorable.1 Typically, Methanosarcina spp. are thought to outcompete Methanosaeta spp. when acetate concentrations are high due to their higher growth rate but lower substrate affinity for acetate.43 The high activity of Methanosarcina spp. in suspended biomass in our system is unusual given acetate concentrations were low, which should have favored Methanosaeta spp. based on known growth and substrate utilization characteristics. We therefore suggest that Methanosarcina spp. were primarily hydrogenotrophic here or that competition between Methanosarcina spp. and Methanosaeta spp. for acetate at such low temperatures has different outcomes than expected. Since few studies have monitored microbial interactions in psychrophilic engineered anaerobic environments, it is difficult to derive which selective pressures (temperature or substrate availability) led to the functional methanogenic community observed here.
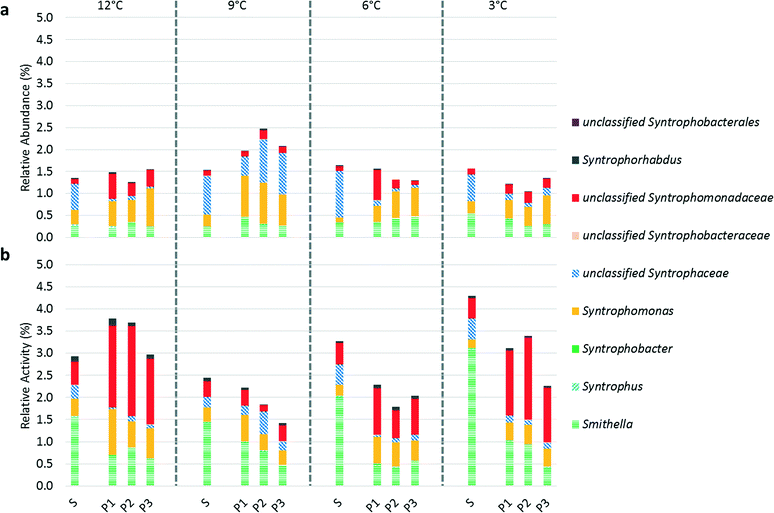
Evaluating syntrophic fatty acid-oxidizing bacterial activity is an alternative approach to gauging methanogenic pathway prevalence as hydrogenotrophic methanogens and syntrophic fatty acid-oxidizing bacteria have coupled metabolisms. The relative activity of syntrophic fatty acid-oxidizing bacteria was higher in the suspended biomass at 9, 6, and 3 °C (Fig. 5), which apparently contradicts our hypothesis regarding enhanced syntrophy in the biofilm (Fig. 5). However, as discussed above, it is difficult to interpret relative activity data without information on the absolute microbial activity. At all operational temperatures, except at 9 °C, an unclassified OTU belonging to family Syntrophomonadaceae was significantly more active in the biofilm than in the suspended biomass. Syntrophomonadaceae typically only oxidize C4 and higher order fatty acids44 and, therefore, its high activity in the biofilm is remarkable given that butyrate and valerate were below our detection limit in the bioreactor. We hypothesize that this unclassified OTU may play a role in a novel pathway in which propionate oxidizing syntrophs of the genus Smithella first dismutate propionate to acetate and butyrate followed by butyrate oxidation by Syntrophomonas spp. via a trophic interaction45 or alternatively that this species possesses a unique capability of C3 oxidation that is unknown in other members of its family.15
Environmental implications
This study assessed the lower temperature limits for AnMBR treatment of domestic wastewater. COD removal >95% was achieved at temperatures as low as 6 °C by operating under conditions supporting membrane biofilm development. We hypothesize that the biofilm microbial community is more resilient than suspended biomass to decreases in operational temperature, possibly because of spatial organization in the biofilm enhancing syntrophy and resulting in a metabolic advantage. This hypothesis was supported by 16S rRNA sequencing in which hydrogenotrophic methanogenesis was the dominant methanogenic pathway in the biofilm, but not in the suspended biomass. Future work could explore this possibility using fluorescence in situ hybridization (FISH) targeting syntrophic fatty acid-oxidizing bacteria and hydrogenotrophic methanogens in the biofilm and suspended biomass to visually investigate spatial juxtapositioning. Alternatively, we hypothesize that the biofilm may have a metabolic advantage relative to suspended biomass due to reduced mass-transport limitations resulting in increased substrate availability. Although biofilm treatment was shown to be an effective strategy here, it introduces concerns regarding (1) long-term and potentially irreversible membrane fouling, (2) the established reliance on chemical cleaning in membrane bioreactor systems, and (3) high methane oversaturation in permeate streams. Low operational temperature is detrimental to the AnMBR energy balance without the development of low-energy dissolved methane recovery technologies. Future work should evaluate different options for dissolved methane recovery and strategies to improve suspended biomass treatment at low temperatures to decrease the reliance on biofilm treatment thereby decreasing dissolved methane oversaturation.Acknowledgements
The authors would like to thank Qaboos Imran and Julie Pierce for their assistance in the lab, and Judy Opp for help with Illumina sequencing. This study was supported by the U.S. National Science Foundation (project CBET 1133793). ALS was partially supported by a Borchardt Fellowship from the University of Michigan.Notes and references
- G. Lettinga, S. Rebac and G. Zeeman, Trends Biotechnol., 2001, 19, 363–370 CrossRef CAS.
- M. Kim, Y.-H. Ahn and R. Speece, Water Res., 2002, 36, 4369–4385 CrossRef CAS.
- I. Martin, M. Pidou, A. Soares, S. Judd and B. Jefferson, Environ. Technol., 2011, 32, 921–932 CrossRef CAS PubMed.
- J. E. Cohen and C. Small, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 14009–14014 CrossRef CAS.
- G. Tchobanoglous, F. L. Burton and H. D. Stensel, Wastewater Engineering: Treatment and Reuse, McGraw-Hill, New York, NY, 4th edn, 2003 Search PubMed.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS PubMed.
- H. Ozgun, R. K. Dereli, M. E. Ersahin, C. Kinaci, H. Spanjers and J. B. van Lier, Sep. Purif. Technol., 2013, 118, 89–104 CrossRef CAS PubMed.
- H. Lin, W. Peng, M. Zhang, J. Chen, H. Hong and Y. Zhang, Desalination, 2013, 314, 169–188 CrossRef CAS PubMed.
- C. Wen, X. Huang and Y. Qian, Process Biochem., 1999, 35, 335–340 CrossRef CAS.
- L. B. Chu, F. L. Yang and X. W. Zhang, Process Biochem., 2005, 40, 1063–1070 CrossRef CAS PubMed.
- J. Ho and S. Sung, Bioresour. Technol., 2010, 101, 2191–2196 CrossRef CAS PubMed.
- A. L. Smith, S. J. Skerlos and L. Raskin, Water Res., 2013, 47, 1655–1665 CrossRef CAS PubMed.
- D. Martinez-Sosa, B. Helmreich and H. Horn, Process Biochem., 2012, 47, 792–798 CrossRef CAS PubMed.
- C. Shin, P. L. McCarty, J. Kim and J. Bae, Bioresour. Technol., 2014, 159, 95–103 CrossRef CAS PubMed.
- A. L. Smith, S. Skerlos and L. Raskin, in review, 2014.
- A. L. Smith, L. B. Stadler, L. Cao, N. G. Love, L. Raskin and S. J. Skerlos, Environ. Sci. Technol., 2014, 48, 5972–5981 CrossRef CAS PubMed.
- J. Kim, K. Kim, H. Ye, E. Lee, C. Shin, P. L. McCarty and J. Bae, Environ. Sci. Technol., 2011, 45, 576–581 CrossRef CAS PubMed.
- H. Yeo and H.-S. Lee, Environ. Technol., 2013, 34, 2105–2112 CrossRef CAS PubMed.
- K. Bialek, D. Cysneiros and V. O'Flaherty, Archaea, 2013, 2013, 346171 CrossRef PubMed.
- S. Rebac, J. Ruskova, S. Gerbens, J. B. Van Lier, A. J. Stams and G. Lettinga, J. Ferment. Bioeng., 1995, 80, 499–506 CrossRef CAS.
- A. N. Nozhevnikova, C. Holliger, A. Ammann and A. J. B. Zehnder, Water Sci. Technol., 1997, 36, 57–64 CrossRef CAS.
- G. Lettinga, S. Rebac, S. Parshina, A. Nozhevnikova, J. B. van Lier and A. J. Stams, Appl. Environ. Microbiol., 1999, 65, 1696–1702 CAS.
- G. Collins, S. McHugh, S. Connaughton, A. M. Enright, A. Kearney, C. Scully, T. Mahony, P. Madden and V. O'Flaherty, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 881–895 CrossRef CAS PubMed.
- S. Connaughton, G. Collins and V. O'Flaherty, Water Res., 2006, 40, 1009–1017 CrossRef CAS PubMed.
- S. McHugh, M. Carton, G. Collins and V. O'Flaherty, FEMS Microbiol. Ecol., 2004, 48, 369–378 CAS.
- S. Aiyuk and W. Verstraete, Bioresour. Technol., 2004, 93, 269–278 CrossRef CAS PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, D.C., 21 edn, 2005 Search PubMed.
- J. G. Caporaso, C. L. Lauber, W. A. Walters, D. Berg-Lyons, C. A. Lozupone, P. J. Turnbaugh, N. Fierer and R. Knight, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4516–4522 CrossRef CAS PubMed.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn and C. F. Weber, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- E. Pruesse, C. Quast, K. Knittel, B. M. Fuchs, W. Ludwig, J. Peplies and F. O. Glöckner, Nucleic Acids Res., 2007, 35, 7188–7196 CrossRef CAS PubMed.
- R. C. Edgar, B. J. Haas, J. C. Clemente, C. Quince and R. Knight, Bioinformatics, 2011, 27, 2194–2200 CrossRef CAS PubMed.
- B. L. Maidak, G. J. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey and C. R. Woese, Nucleic Acids Res., 1997, 25, 109–110 CrossRef CAS PubMed.
- J. C. Yue and M. K. Clayton, Commun. Stat. Theory Methods, 2005, 34, 2123–2131 CrossRef PubMed.
- P. Westermann, B. K. Ahring and R. A. Mah, Appl. Environ. Microbiol., 1989, 55, 514–515 CAS.
- J. S. Liu, I. W. Marison and U. von Stockar, Biotechnol. Bioeng., 2001, 75, 170–180 CrossRef CAS PubMed.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 2504–2512 CrossRef CAS PubMed.
- A. Robles, M. Ruano, J. Ribes and J. Ferrer, Sep. Purif. Technol., 2013, 104, 290–296 CrossRef CAS PubMed.
- E. Petropoulos, J. Dolfing, E. Bowen, R. Davenport and T. Curtis, 13th World Congress on Anaerobic Digestion, Santiago de Compostela, Spain, 2013 Search PubMed.
- S. J. Blazewicz, R. L. Barnard, R. A. Daly and M. K. Firestone, ISME J., 2013, 7, 2061–2068 CrossRef CAS PubMed.
- S. L. Bräuer, H. Cadillo-Quiroz, R. J. Ward, J. B. Yavitt and S. H. Zinder, Int. J. Syst. Evol. Microbiol., 2011, 61, 45–52 CrossRef PubMed.
- Y. Yashiro, S. Sakai, M. Ehara, M. Miyazaki, T. Yamaguchi and H. Imachi, Int. J. Syst. Evol. Microbiol., 2011, 61, 53–59 CrossRef CAS PubMed.
- Z. Mladenovska and B. K. Ahring, Appl. Microbiol. Biotechnol., 1997, 48, 385–388 CrossRef CAS.
- A. Conklin, H. D. Stensel and J. Ferguson, Water Environ. Res., 2006, 78, 486–496 CrossRef CAS.
- A. J. Stams, D. Z. Sousa, R. Kleerebezem and C. M. Plugge, Water Sci. Technol., 2012, 66, 352–362 CrossRef CAS PubMed.
- Y. Gan, Q. Qiu, P. Liu, J. Rui and Y. Lu, Appl. Environ. Microbiol., 2012, 78, 4923–4932 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ew00070f |
| ‡ Current Affiliation: Astani Department of Civil and Environmental Engineering, University of Southern California, 3620 South Vermont Avenue, Los Angeles, CA 90089, USA. |
| This journal is © The Royal Society of Chemistry 2015 |