A dielectrophoresis-assisted adsorption approach significantly facilitates the removal of cadmium species from wastewater
Jing
Hu
a,
Huiying
Chen
*a,
Bihao
Lan
a,
Junfeng
Geng
b,
Hua
Li
a and
Xuan
Xing
a
aCollege of Life and Environmental Science, Minzu University of China, Beijing, 100081, China. E-mail: huiyingrchen@aliyun.com
bInstitute for Materials Research and Innovation, Institute for Renewable Energy and Environmental Technologies, University of Bolton, Bolton, BL3 5AB, UK
First published on 23rd December 2014
Abstract
A newly designed apparatus and process, including dielectrophoresis and adsorption to efficiently remove cadmium species from wastewater, is reported in this study. With this technique, cadmium ions are firstly adsorbed by shell powder particles, which are subsequently trapped and concentrated by a dielectrophoresis process. The factors affecting the metal removal efficiency are systematically investigated, which allows us to determine the optimal operation conditions. Our experimental data indicate that via the adsorption and dielectrophoresis steps, cadmium can be almost completely removed from wastewater as evidenced by the very high removal efficiency of 99.4%. This suggests that our dielectrophoresis-assisted adsorption approach is highly effective for the removal of cadmium, and the technique could be extended to the removal of other heavy metal ions from wastewater as well.
Water impactWe have constructed a new effective apparatus to remove cadmium species from wastewater via the adsorption and dielectrophoresis steps: first, the cadmium ions are adsorbed by the shell powder particles (SPPs), and then the cadmium-adsorbed SPPs are trapped and concentrated by a dielectrophoresis process. After the dielectrophoresis process, the SPPs became smaller and more homogeneous, which increased the surface area of SPPs, and thus increased the sites available for cadmium adsorption such that the cadmium removal rate can be highly improved. The optimal operation conditions are determined through systematic investigation. With the dielectrophoresis process, the removal rate can be increased from 51.27% to 99.44%, and the processing time has been substantially reduced to 4 h from 48 h. |
1. Introduction
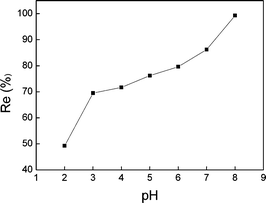
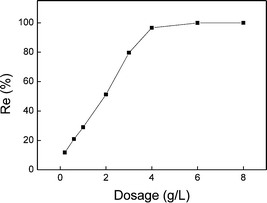
Cadmium (Cd) is a toxic heavy metal of considerable environmental and occupational concerns.1 It has been released into the environment through various sources such as combustion of fossil fuels,2 metal production and application of electroplating,3 use of phosphate fertilizers, and development of screens technology.4 The industrial emission of cadmium has resulted in serious contamination of both soil and water. Cadmium has been classified as a carcinogen, which impacts human kidneys, lungs, liver and reproductive organs.1,5 The standard of the allowed cadmium release is constantly reinforced, and it has been limited to 0.1 mg L−1 by the emission standard of pollutants for mining and mineral processing industry since June 2012.6Various physical and chemical methods have been developed or modified for the removal of cadmium from natural or industrial wastewater. Among these methods, adsorption is widely regarded as an efficient method for the removal of heavy metals including cadmium because of its good efficiency, simplicity and economic feasibility. A variety of adsorbents, including biomass and biopolymer,7 metal oxides,8 microorganisms,9 zeolites10 and activated carbon,11 have been used for the removal of cadmium. Moreover, other cheaper materials, such as clay,2 dry agriculture plants12 and fly ash,13 have also been used as natural absorbents. However, the removal efficiency by adsorption itself needs to be improved. Powdered activated carbon and granular activated carbon have been found to show a removal efficiency of nearly 50% under a certain pH range;7–9,14 the maximum removal of Cd(II) has been 87.15% at pH 8.6 using bio-sorbent wheat bran, with initial Cd(II) concentration of 12.5 mg L−1 (at 20 °C).15 Moreover, the subsequent desorption following the initial adsorption process may lead to secondary pollution. As a consequence, it is necessary to develop a more efficient but also a cost-effective method for the complete removal of cadmium and other heavy metals from wastewater.
Here, we report a method of using dielectrophoresis (DEP) in conjunction with adsorption (ADS) to efficiently remove cadmium from wastewater. This combined ADS/DEP approach has been found to show an exceptionally high removal efficiency of 99.4%. Considering the nature of the DEP technique that can be used to manipulate polarized particles suspended in a fluid in a non-uniform electrical field, we have designed and assembled an apparatus for metal removal purpose. In our method, Cd(II) ions in wastewater were firstly adsorbed by suspended powder particles (SPPs), which were then trapped and concentrated at the electrodes within a DEP vessel. The vessel was sufficiently big to treat an adequate amount of sample, and the flame atomic absorption spectrometry (FAAS) was used to determine the metal concentration in the wastewater.
2. Experimental
2.1 Materials
2.2 Adsorption experiments
Adsorption experiments were carried out by the batch technique due to its simplicity and reliability at room temperature. A given mass of adsorbent was added to a fixed volume of cadmium-containing solution, and the entirety was stirred during the experiment. The samples were carried out at various time intervals and filtered. The filtrate was collected and tested.2.3 Dielectrophoresis experiments
Batch DEP experiments were conducted with a self-made apparatus, as shown in Fig. 1. The vessel (4) was fitted with slots on both sides and electrodes (3) were installed at room temperature. The distance between these electrodes was set at 10 mm. A direct current power device (5) was used to supply voltage to the electrodes. Following an initial adsorption process, the solution mixed with adsorbents was passed through the vessel (4) via pump (2) from the storage tank (1) to the reception tank (6), and during the process the SPPs were trapped on the electrodes. A series of experiments were carried out by applying different voltages ranging from 6 V to 16 V. The analysis of the cadmium concentration was conducted by an atomic absorption spectrophotometer (PERKIN ELMER, A 800) with a wavelength of 228.8 nm and the flame obtained by the mixture of air and acetylene (C2H2). | ||
| Fig. 1 The apparatus layout used in the experiments: 1. Storage tank; 2. pump; 3. electrode; 4. vessel; 5. direct current power source; 6. reception tank. | ||
3. Results and discussion
As is well known, Dielectrophoresis (DEP) is a technique that can be used to manipulate polarized particles suspended in fluid media in a non-uniform electric field.16 In the case of a spherical particle, the dielectrophoretic force FDEP is given as follows:| FDEP = 2πR3εmRe[K(ω)]∇E2 | (1) |
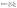 | (2) |
A non-uniform electric field is necessary to induce the DEP forces stated in eqn (1) (otherwise ∇E = 0). The positive values of Re[K(ω)], see eqn (1), denote the induction of a positive DEP force that causes a particle to get trapped within the regions of high electric field gradient. The negative values of Re[K(ω)] denote negative DEP, which means particles move towards the regions of low or no electric field.
As a well-known particle manipulation technique, DEP can be used to efficiently collect and remove particles suspended in wastewater.17 We previously reported that the particles containing Pb2+ may be trapped on the electrodes in a microfluid DEP device.18 However, the removal rates of heavy metals could not be evaluated in the microfluid systems.18,19 We later contemplated whether heavy metals could be removed in a practical process, and thus we designed an apparatus to test and evaluate the real efficiency of the ADS/DEP process for the removal of cadmium from wastewater.
3.1 Effect of the adsorption related parameters
3.2 The effect of DEP process
To determine whether the DEP process can help to remove the SPPs adsorbed with cadmium, an ADS/DEP experiment was conducted at pH = 5, with initial cadmium concentration of 150 mg L−1 and adsorbent dose of 2 g L−1. After stirring for 48 hours, the suspension was pumped through the vessel at a voltage of 8 V and at a flow rate of 0.2595 mL s−1. Fig. 5(a) and (b) show the solutions before and after the DEP process, respectively. The suspension was cloudy before the DEP process and it became clear afterwards. The scanning electron microscopy (SEM) images (Fig. 6) revealed that the SPPs were trapped on the electrodes. This clearly indicates that the ADS/DEP process can be used in the treatment of wastewater containing Cd ions.3.3 The effect of voltage
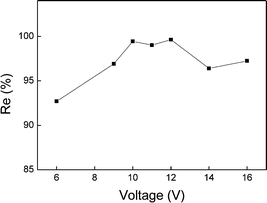
In the ADS/DEP experiments, the voltage applied on the electrodes directly affected the intensity of non-uniform electric field, which is proportional to the DEP force acting on the suspended particles.24 The effect of voltage on cadmium removal rates was investigated at a constant flow rate of 0.1765 mL s−1, and the result are shown in Fig. 7. We can see that the cadmium removal rate increased when the voltage was increased from 6 V to 10 V. However, after 12 V, the cadmium removal rate begins to decrease. Thus, 10 V could be used as a threshold, and in consideration of the energy consumption, experiments afterwards were conducted at 10 V. Compared with using adsorption only, the ADS/DEP substantially improved the cadmium removal rate from 51.27% to 99.64%.3.4 The reduction of adsorption time
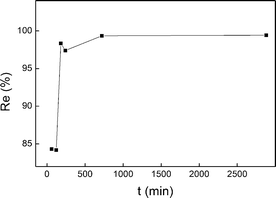
To find out whether the adsorption time could be reduced by combining ADS and DEP, we designed a batch experiment in which adsorption was carried out first for a certain time, and then DEP was carried out at the optimum adsorption condition and voltage. The adsorption time was set from 1 h to 48 h. As shown in Fig. 8, a sharp increase occurred from 84.19% to 98.36%, when the adsorption time increased from 120 min to 180 min. Then, the removal rates became practically constant. The adsorption time could be greatly shortened to 240 min from 2880 min.3.5 Analysis of shell powder by SEM and EDX
Energy dispersive X-ray (EDX) analysis was conducted to evaluate the adsorption of cadmium on SPPs, and the result is summarized in Table 1. The cadmium-containing SPPs samples were collected after the equilibrium adsorption experiment (experimental conditions: pH: 5; the initial cadmium concentration: 150 mg L−1; adsorbent dosage: 2 g L−1). Similarly, the adsorption/DEP SPPs were collected from the vessel when they settled down. The EDX spectrum indicated the presence of oxygen (62.76%), carbon (10.84%) and calcium (38.76%) on the original SPPs but did not show the characteristic signal of cadmium. This EDX result also indicated that cadmium content on SPPs after the ADS/DEP process is higher than that on SPPs by adsorption only. This confirms that the ADS/DEP process facilitated more cadmium ions to be bonded to the SPPs surface.| SPPs | Element | C | O | Ca | Cd |
|---|---|---|---|---|---|
| Original SPPs | % | 10.84 | 50.41 | 38.76 | — |
| Cadmium-adsorbed | % | 12.00 | 47.58 | 35.45 | 4.97 |
| Adsorption/DEP | % | 16.93 | 44.83 | 25.14 | 13.11 |
The morphological analysis of SPPs was performed by SEM. Fig. 9 shows the morphologies of SPPs before and after the DEP. It is interesting to note that the size of the particles became smaller and more homogeneous after DEP. The downsizing of the particles increased the surface area of SPPs, and thus increased the sites available for cadmium adsorption, which explained why the removal rate by the combined adsorption/DEP is considerably higher than that by adsorption only.
4. Conclusions
The results presented in this study show that our combined adsorption/dielectrophoresis process can not only trap and concentrate the adsorbents efficiently, but also significantly improve the cadmium removal rate compared to using the adsorption method alone. Under identical experimental conditions, this removal rate can be increased from 51.27% without dielectrophoresis, to 99.44% with dielectrophoresis. Moreover, the processing time has been substantially reduced to 240 min from 2880 min, as a comparison, which is also significant in wastewater treatment. All these data suggest that the dielectrophoresis-assisted adsorption may be considered as a novel and efficient technique for the removal of cadmium species, and perhaps other heavy metals in general, from wastewater.Acknowledgements
This work has been supported by “the Fundamental Research Funds for the Central Universities”, First-class discipline construction project for the first-class university of Minzu University of China (YLDX01013)and 111 Project B08044.References
- P. W. Micheal, Cadmium carcinogenesis in review, J. Inorg. Biochem., 2000, 79(1), 241–244 Search PubMed.
- Y. C. Sharma, Thermodynamics of removal of cadmium by adsorption on an indigenous clay, Chem. Eng. J., 2008, 145(1), 64–68 CrossRef CAS PubMed.
- B. J. Alloway and E. Steinnes, Anthropogenic additions of cadmium to soils, in Cadmium in soils and plants, Springer, Netherlands, 1999, pp. 97–123 Search PubMed.
- A. B. Pérez-Marín, V. M. Zapata and J. F. Ortuno, Removal of cadmium from aqueous solutions by adsorption onto orange waste, J. Hazard. Mater., 2007, 139(1), 122–131 CrossRef PubMed.
- M. P. Mahalik, W. H. Henry and C. P. Walter, Teratogenic effects and distribution of cadmium (Cd2+) administered via osmotic minipumps to gravid CF-1 mice, Toxicol. Lett., 1995, 76(3), 195–202 CrossRef CAS.
- GB 28661-2012, Emission standard of pollutants for mining and mineral processing industry, Chinese environmental science press, Beijing, 2012 Search PubMed.
- G. Tan and D. Xiao, Adsorption of cadmium ion from aqueous solution by ground wheat stems, J. Hazard. Mater., 2009, 164(2), 1359–1363 CrossRef CAS PubMed.
- Y. T. Meng, Y. M. Zheng and L. M. Zhang, Biogenic Mn oxides for effective adsorption of Cd from aquatic environment, Environ. Pollut., 2009, 157(8), 2577–2583 CrossRef CAS PubMed.
- N. Cihangir and N. Saglam, Removal of cadmium by Pleurotus sajor-caju basidiomycetes, Acta Biotechnol., 1999, 19(2), 171–177 CrossRef CAS.
- R. Cortés-Martínez, V. Martínez-Miranda and M. Solache-Ríos, Evaluation of natural and surfactant-modified zeolites in the removal of cadmium from aqueous solutions, Sep. Sci. Technol., 2004, 39(11), 2711–2730 CrossRef PubMed.
- D. Mohan and K. P. Singh, Single-and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste, Water Res., 2002, 36(9), 2304–2318 CrossRef CAS.
- U. Garg, M. P. Kaur and G. K. Jawa, Removal of cadmium (II) from aqueous solutions by adsorption on agricultural waste biomass, J. Hazard. Mater., 2008, 154(1), 1149–1157 CrossRef CAS PubMed.
- M. Visa, C. Bogatu and A. Duta, Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash, Appl. Surf. Sci., 2010, 256(17), 5486–5491 CrossRef CAS PubMed.
- K. K. Singh, A. K. Singh and S. H. Hasan, Low cost bio-sorbent ‘wheat bran’ for the removal of cadmium from wastewater: kinetic and equilibrium studies, Bioresour. Technol., 2006, 97(8), 994–1001 CrossRef CAS PubMed.
- P. Karuppanna and N. Chinnaiya, Process development for removal and recovery of cadmium from wastewater by a low-cost adsorbent: adsorption rates and equilibrium studies, Ind. Eng. Chem. Res., 1994, 33(2), 317–320 CrossRef.
- M. D. Rodrigo, Microfabrication technologies in dielectrophoresis applications-A review, Electrophoresis, 2012, 33(21), 3110–3132 CrossRef PubMed.
- H. A. Pohl, Dielectrophoresis: the behavior of neutral matter in nonuniform electric fields, Cambridge university press, Cambridge, 1978 Search PubMed.
- H. Chen, H. Zhang and L. Yu, Removing Pb(II) from water using hollow microspheres and dielectrophoresis, Acta Sci. Circumstantiae, 2010, 30(4), 756–761 CAS.
- J. Batton, A. J. Kadaksham and A. Nzihou, Trapping heavy metals by using calcium hydroxyapatite and dielectrophoresis, J. Hazard. Mater., 2007, 139(3), 461–466 CrossRef CAS PubMed.
- N. Azouaou, Z. Sadaoui and A. Djaafri, Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics, J. Hazard. Mater., 2010, 184(1), 126–134 CrossRef CAS PubMed.
- D. Ozdes, A. Gundogdu and B. Kemer, Removal of Pb (II) ions from aqueous solution by a waste mud from copper mine industry: equilibrium, kinetic and thermodynamic study, J. Hazard. Mater., 2009, 166(2), 1480–1487 CrossRef CAS PubMed.
- N. Y. Mezenner and A. Bensmaili, Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste, Chem. Eng. J., 2009, 147(2), 87–96 CrossRef CAS PubMed.
- S. Wang, X. Jin and H. Zhao, Phosphate biosorption characteristics of a submerged macrophyte Hydrilla verticillata, Aquat. Bot., 2008, 89(1), 23–26 CrossRef CAS PubMed.
- L. Semerjian, Equilibrium and kinetics of cadmium adsorption from aqueous solutions using untreated Pinus halepensis saw dust, J. Hazard. Mater., 2010, 173(1), 236–242 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |