Quantification of corrosion inhibitors used in the water industry for steam condensate treatment: the indirect electroanalytical sensing of morpholine and cyclohexylamine†
Athanasios V.
Kolliopoulos
,
Jonathan P.
Metters
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Chemistry and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, Lancs, UK. E-mail: c.banks@mmu.ac.uk; Web: http://www.craigbanksresearch.com Fax: +44 (0)1612476831; Tel: +44 (0)1612471196
First published on 7th November 2014
Abstract
Corrosion inhibitors are widely used in the water industry for steam condensate treatment. Due to their application into the food industry, the Food and Drug Administration (FDA) legislate that such inhibitors are not to exceed 10 mg L−1. Various analytical protocols exist to provide an offsite analysis and a point of site analysis is required. Consequently, the first example has been reported for the indirect electroanalytical determination of cyclohexylamine and morpholine, two important corrosion inhibitors using disposable and economical screen-printed graphite macroelectrodes. These two analytes have been demonstrated to have no measureable direct electrochemical signatures in aqueous solutions using carbon and noble metal based macroelectrodes and we propose, for the first time, an indirect electrochemical sensing protocol which utilises screen-printed graphite macroelectrodes. This indirect approach utilises an EC type-mechanism using the compounds, N,N′-(1,4-phenylene)dibenzenesulfonamide and N-(4-amino-2-methyl-phenyl)-benzenesulfonamide as mediators. In this approach the mediators are electrochemically oxidised which then chemically react with the target analytes, cyclohexylamine and morpholine; the products of these reactions are then electrochemically interrogated and provide an indirect electroanalytical signal with which to quantify cyclohexylamine and morpholine. The two mediators are required for the two analytes as one mediator cannot measure both analytes. It is demonstrated that this indirect sensing protocol allows the electroanalytical detection of cyclohexylamine and morpholine over the range 1 to 10 mg L−1 in pH 10 carbonate buffer utilising cyclic voltammetry. This indirect electroanalytical protocol is found to exhibit a limit of detection (3σ) of 0.9 and 1 mg L−1 for cyclohexylamine and morpholine respectively which are significantly below the FDA sanctioned levels. This indirect electroanalytical protocol utilising screen-printed sensors has the potential to be applied into-the-field enabling the measurement of these two neutralizing amines to ensure they do not exceed FDA guidelines.
Water impactCorrosion inhibitors are widely used in the water industry for steam condensate treatment. Since steam condensate is utilised in the food industry and due to the toxic nature of the corrosion inhibitors, the FDA have strictly limited these to not exceed 10 mg L−1. Currently this is being addressed by laboratory based equipment such as: high-pressure liquid chromatography, ion chromatography, or spectrophotometry. In our paper we report the first electrochemical approach for the rapid and sensitive determination of these inhibitors at FDA levels; the proposed electrochemical technology offers a potentially portable and onsite measurement of the corrosion inhibitors. This paper falls into the remit of measurement of important analytes in water technology. |
1 Introduction
Water treatment for boilers is divided into external (physico-chemical) and internal (chemical) treatments.1 External treatment involves the physico-chemical treatment of the raw water to produce a satisfactory quality boiler feed water such as ion exchange, water softening and filters for suspended solids. Internal treatment comprises the addition of chemicals for the treatment of the boiler feed water and steam condensate. The treatment of the boiler feed water controls mineral scaling, corrosion and general deposition in the boiler drums and tubes. The treatment of the steam condensate controls carbonic acid and dissolved oxygen corrosion of the steam condensate lines by the addition of chemicals either in the steam lines or directly into the boiler.2 The main components to be chemically treated in the steam condensate are carbon dioxide and oxygen. Bicarbonate and carbonate ions in feed water thermally decompose to carbon dioxide gas (CO2) in the boiler water with the majority passing into the steam and forming carbonic acid in the condensate as described below:| CO2 + H2O ↔ H2CO3 | (1) |
| H2CO3 ↔ H+ + HCO3− | (2) |
| HCO3− ↔ H+ + CO32− | (3) |
Consequently, the pH of the condensate can decrease to very low levels, often below pH 3 which at this pH level is of course very corrosive resulting in corrosion and ultimately failure of metal pipelines.3 Additionally in the presence of oxygen, the corrosion rate increases and it is observed usually as localized pitting.4 The cathodic reactions may occur either by the direct reduction of hydrogen ions, or via carbonates:5
| 2H+ + 2e− → H2 | (4) |
| H2CO3 + e− → HCO3− + 1/2H2 | (5) |
| HCO3− → CO32− + 1/2H2 | (6) |
The anodic reaction occurs simply via the oxidation of iron:
| Fe → Fe2+ + 2e− | (7) |
There is experimental evidence for the presence of carbonate (FeCO3) layer formation upon steel surfaces;6 this corrosion layer is formed as described by Glezakou:7
| Fe2+ + CO32− → FeCO3 | (8) |
| Fe2+ + 2HCO3− → Fe(HCO3)2 | (9) |
| Fe(HCO3)2 → FeCO3 + CO2 + H2O | (10) |
Carbonate alkalinity can be minimized via de-alkalization and de-carbonation in the pretreatment step, but in most medium and low pressure boilers this is not routinely undertaken.
In order to prevent acidic corrosion in the condensate lines (see above), carbon dioxide must be neutralized. This is undertaken by adding neutralizing amines (volatile amines) into the feed water; the addition of these amines adjusts the pH of the feed water into the range between 8 to 10, which is optimum for a mixed metallurgy system to avoid corrosion.1 As these added amines pass into the boiler, they are also distributed into the steam, and condense with the steam and carbon dioxide. Apart from the neutralizing amines which inhibit the corrosion by controlling the condensate pH, filming amines are being used in steam condensate treatment. Filming amines form water repellent films upon metal surfaces inhibiting the corrosion process further by preventing the metals from contacting with the corrosive substances, such as oxygen and carbon dioxide.
The most common neutralizing amines utilised to reduce corrosion are cyclohexylamine, morpholine, and ammonia.4,8 Ammonia, with a high concentration distribution ratio in steam to that in condensate (vapour/liquid distribution) transfers into steam easier than into the condensate and is not very effective for preventing the corrosion in the steam line close to the boiler.1 Apart from that, ammonia is not applicable for the boilers employing copper materials in the steam lines.1 The detection of ammonia has been widely reported.9 Cyclohexylamine and morpholine are the amines of interest in this work. The food industry uses steam extensively10 and since the above chemicals are used to reduce/inhibit corrosion, contamination can occur with food products. As such, the Food and Drug Administration (FDA) limits the use of these amines. According to FDA Amine Standards-Title 21 CFR 173.310.d, both cyclohexylamine and morpholine are not to exceed 10 parts per million in steam. If the steam comes in contact with milk and milk products, the two amines are totally excluded from steam.11
Cyclohexylamine and morpholine can be analysed by an array of analytical techniques such as high-pressure liquid chromatography,12 ion chromatography,13 or spectrophotometry14 with detection limits in the required low part-per million (ppm) range. Electroanalytical techniques are sensitive portable and cost-effective especially through the use of screen-printed electrochemical sensors which have scales of economy.15 Such an approach is promising for the measurement of chemicals in water treatment for on-site applications where rapid and accurate results are required.
In this work, an indirect electrochemical sensing protocol for cyclohexylamine and morpholine is described for the first time since they have no direct measurable electrochemical signatures. These two neutralizing amines are used extensively as anticorrosion agents of steam condensate lines and the proposed electrochemical protocol allows their sensing at limits below and close to the FDA legislation. The use of screen-printed sensors, which are disposable and easily portable, enables the sensing protocol to be potentially applied into-the-field.
2 Experimental
All chemicals used were obtained by Sigma Aldrich and used as is. Deionised water of resistivity 18.2 MΩ cm was used for the preparation of all aqueous solutions. Voltammetric measurements were carried out using Ivium Compactstat potentiostat (The Netherlands).Measurements were conducted using a screen-printed three electrode configuration. Screen-printed carbon-based electrodes (denoted as SPGEs) were fabricated in-house with appropriate stencil designs using a microDEK 1760RS screen-printing machine (DEK, Weymouth, UK). Note that this screen-printed electrode design has been previously reported16 “as is” without electrode pre-treatment or modification in various electroanalytical endeavours. For fabrication of the SPGEs, first a carbon ink formulation (product code: C2000802P2; Gwent Electronic Materials Ltd, UK) utilised for the efficient connection of all three electrodes and the electrode material for both the working and counter electrodes was screen-printed onto a polyester (Autostat, 250 micron thickness) flexible film. The carbon ink layer was cured in a fan oven at 60 degrees for 30 minutes. Next a silver/silver chloride reference electrode was included by screen-printing Ag/AgCl paste (product code: C2040308P2; Gwent Electronic Materials Ltd, UK) onto the polyester substrates which was subsequently cured once more in a fan oven at 60 degrees for 30 minutes. Finally, a dielectric paste (product code: D2070423P5; Gwent Electronic Materials Ltd, UK) was then printed onto the polyester substrate to cover the connections and define the active electrode areas including that of the working electrode (3 mm diameter). After curing at 60 degrees for 30 minutes the SPGEs are ready to be used. These electrodes have been characterised electrochemically in a prior paper and have heterogeneous rate constants of 1.08 × 10−3 cm s−1.17 A glassy carbon electrode (GCE) (3 mm diameter, BAS, USA) a boron-doped diamond electrode (BDDE) (3 mm diameter, BAS, USA) and a gold electrode (AuE) (1 mm diameter, BAS, USA) were also utilised with a platinum wire counter and a Saturated Calomel Electrode (SCE) as the reference electrode completing the circuit. The BDDE, GCE and AuE were all thoroughly cleaned and polished with 1 micron and 0.25 micron-sized diamond sprays prior to use.
3 Results and discussion
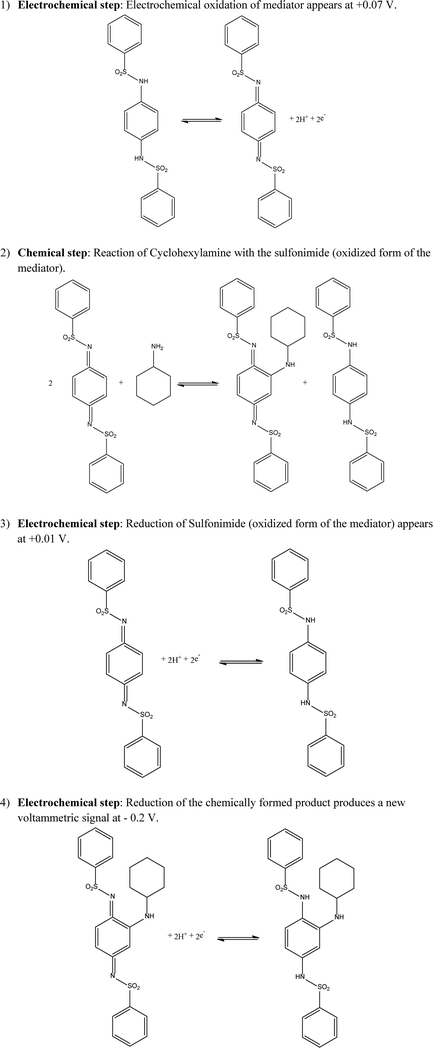
First, the direct electrochemical detection of cyclohexylamine and morpholine was explored. In order to achieve this, cyclic voltammetric responses of 10 mg L−1 cyclohexylamine and morpholine using SPGE, BDDE, GCE and AuE were explored in a pH 10 buffer. The observed voltammetric profiles are presented in ESI† Fig. S1–S8 where, for morpholine and cyclohexylamine, the similarity of the voltammograms with and without the analyte demonstrate that the direct electrochemical detection of morpholine and cyclohexylamine at pH 10 is not possible in the accessible aqueous potential window.Inspired by prior work by Adams and Schowalter18 who reported that morpholine and other amines such as dimethylamine, aniline, methylamiline and aliphatic primary amines chemically react with N,N′-(1,4-phenylene)dibenzenesulfonimide in organic solvents giving a variety of products which are dependent on the experimental conditions, we electrochemically adapt this novel system. In our approach within aqueous solutions, we electrochemically oxidise the compound N,N′-(1,4-phenylene)dibenzenesulfonamide (see Scheme 1, step 1) to the corresponding N,N′-(1,4-phenylene)dibenzenesulfonimide (producing a large oxidation wave) which then chemically reacts (Scheme 1, step 2) with the target amine at an appreciate rate. The product of this reaction is then electrochemically interrogated (Scheme 1, step 4) providing the analytical signal (see Scheme 1). A pH study of the mediators was untaken (see ESI†) in order to reveal the likely electrochemical mechanism in operation for the mediators which involves an equal ratio of m-protons and m-electrons, where m and n equal 2; this process is summarised in Scheme 1, step 1.
Next, N,N′-(1,4-phenylene)dibenzenesulfonamide and N-(4-amino-2-methyl-phenyl)-benzenesulfonamide for the indirect sensing of cyclohexylamine and morpholine respectively were investigated as potential mediators for their electrochemical detection. In order to select the optimum pH for the detection of the two neutralizing amines with the mediators, three parameters were taken into account. The pKa values for cyclohexylamine and morpholine, the voltammetric responses of the SPGE upon the two mediators at different pH and the optimum pH range for a mixed metallurgy system, which is 8–10.1 The pKa values for cyclohexylamine and morpholine are 10.6 (ref. 19) and 8.36 (ref. 20) respectively. The amine groups of both analytes are desirable to be as bases (pH above the pKa) and not as their conjugative acids, which do not react with the mediators.
The voltammetric responses of the mediators N-(4-amino-2-methyl-phenyl)-benzenesulfonamide and N,N′-(1,4-phenylene)dibenzenesulfonamide were investigated at different pH values using the SPGE. Plots of their electrochemical oxidation peak currents versus the pH value are shown in Fig. 1 and 2 respectively. The highest peak current for N-(4-amino-2-methyl-phenyl)-benzenesulfonamide is observed in a pH range between 9 and 10. N,N′-(1,4-phenylene)dibenzenesulfonamide presents the highest peak current in the pH range between 9 and 12. Considering the above factors, pH 10 was selected as the optimum pH for the detection of the two neutralizing amines with the two mentioned mediators.
 | ||
| Fig. 1 Analysis of the electrochemical oxidation peak current of 0.1 mg mL−1N-(4-amino-2-methyl-phenyl)-benzenesulfonamide (Scheme 1, Step 1) at different pH values using SPGEs. Scan rate: 50 mV s−1. | ||
 | ||
| Fig. 2 Analysis of the electrochemical oxidation peak current of 0.1 mg mL−1N,N′-(1,4-phenylene)dibenzenesulfonamide at different pH values using SPGEs. Scan rate: 50 mV s−1. | ||
Fig. 3 depicts typical cyclic voltammetric responses of SPGEs following additions of morpholine (1–10 mg L−1) into a pH 10 carbonate buffer solution containing 0.1 mg mL−1N-(4-amino-2-methyl-phenyl)-benzenesulfonamide. The electrochemical oxidation of the mediator is observed at +0.05 V (vs. Ag/AgCl) while its corresponding electrochemical reduction peak is observed to occur at −0.04 V (vs. Ag/AgCl). This electrochemically oxidised mediator reacts chemically with the target amine, morpholine over appreciable and useful timescale (no waiting time is required). The reduction of this product appears as a new voltammetric signal, observed at −0.15 V (vs. Ag/AgCl) which increases in proportion to the concentration of morpholine. The calibration plot corresponding to increasing concentrations of morpholine versus the voltammetric peak height (μA) are depicted in Fig. 4. The error bars for three different measurements are also shown. A linear response for the sensing of morpholine was achieved (IP/μA = −6.32 × 10−2 μA mg−1 L−1 + 3.31 × 10−2 μA; R2 = 0.9882 N = 3). Furthermore, the limit of detection (3σ) for morpholine was determined to be 1.0 mg L−1; the linear range and limit of detection are for FDA monitoring of these neutralizing amines.
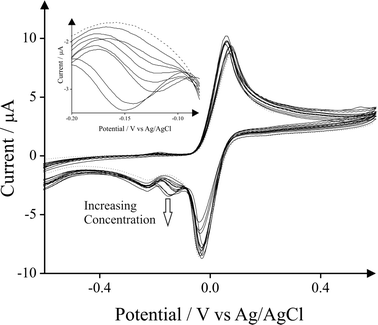
Fig. 5 depicts the cyclic voltammetric responses of SPGEs upon additions of cyclohexylamine (1–10 mg L−1) in pH 10 Carbonate buffer solution containing 0.1 mg ml−1N,N′-(1,4-phenylene)dibenzenesulfonamide; note that each measurement is conducted with a new SPGE. The electrochemical oxidation of the mediator to the corresponding sulfonimide and its electrochemical reduction are observed at +0.07 V (vs. Ag/AgCl) and at −0.01 V (vs. Ag/AgCl) respectively. The electrochemically oxidised mediator chemically reacts with cyclohexylamine. The reduction of this product then appears as a new peak observed at −0.20 V (vs. Ag/AgCl) which increases relative to the concentration of cyclohexylamine. The resulting calibration plot corresponding to increasing concentrations of cyclohexylamine versus the voltammetric peak height (μA) are presented in Fig. 6. A linear response for the sensing of cyclohexylamine is readily achieved (IP/μA = −19.29 × 10−2 μA mg−1 L−1 + 24.85 × 10−2 μA; R2 = 0.9899 N = 3). Furthermore, the limit of detection (3σ) for cyclohexylamine was determined to correspond to 0.9 mg L−1. The proposed mechanism for this novel indirect electroanalytical protocol is summarized in Scheme 1.
4 Conclusions
The first example has been reported of the sensing of morpholine and cyclohexylamine, two neutralizing amines widely used in water treatment in pH 10 carbonate buffer solutions using screen-printed graphite electrodes. These economical screen-printed sensors provide an appealing alternative to the laboratory based methods for the detection of these neutralizing amines with potential to be applied easily into the field. Screen-printed graphite electrodes were demonstrated to allow lowest limits of detection 0.9 mg L−1 for cyclohexylamine and 1.0 mg L−1 for morpholine in ideal conditions using the mediators N,N′-(1,4-phenylene)dibenzenesulfonamide and N-(4-amino-2-methyl-phenyl)-benzenesulfonamide respectively. The two mediators are required for the two analytes as one mediator cannot measure both. The limits of detection for these amines are 10 times lower than the level of the FDA for food industry. Such sensors provide a potential solution to the common problem of the transition of laboratory-based analytical procedures to real world applications in the ‘field’ combining the low-cost benefits of carbon based materials with ease of mass production and facile use of screen-printed sensors.This electroanalytical protocol has the potential to be applied into sensing the target corrosion inhibitors in steam condensate. This real sample/matrix consists of pure water and in order for our approach to work, the steam would be expected to be condensated with the sample then spiked with our identified mediators and altering the pH to the optimum (pH 10) value. Note that the steam condensate will be either acidic or in the range 8–10 depending if the neutralizing amines are present or not. Consequently, it is hard to replicate a “real sample”, which in this case, would be simply spiking deionised water (which has been done to prove our protocol). Future work is being coordinated with industries in order to explore the proposed analytical protocol for the implementation of sensing of neutralizing amines in steam condensate and has the potential to be used in an industrial or power generation cycle but note that cyclohexylamine concentrations would be much lower than 10 ppm for adequate pH control; such future work is underway.
References
- T. Suzuki, T. Hosokawa, M. Iwasaki, H. Komatsubara, Y. Makino, K. Matsubara, H. Morinaga, H. Suzuki, S. Takeda, M. Takemura and H. Takenaka, Kurita Handbook of Water Treatment, Kurita Water Industries Ltd., Tokyo, 2nd edn, 1999 Search PubMed.
- (a) C. De Waard and D. E. Milliams, Corrosion, 1975, 31, 177–181 CrossRef CAS; (b) H. Yung, U. Kim, G. Seo, H. Lee and C. Lee, Environ Eng Res, 2009, 14, 195–199 CrossRef.
- (a) H. A. Carlson, Ind. Eng. Chem., 1949, 41, 644–645 CrossRef CAS; (b) R. R. Villa Jr, J. R. V. Paraon and K. A. Lichti, GRC Trans., 2002, 26, 679–686 Search PubMed.
- J. Luong, R. A. Shellie, H. Cortes, R. Gras and T. Hayward, J. Chromatogr. A, 2012, 1229, 223–229 CrossRef CAS PubMed.
- I. S. Cole, P. Corrigan, S. Sim and N. Birbilis, Int. J. Greenhouse Gas Control, 2011, 5, 749–756 CrossRef CAS PubMed.
- Y. S. Choi, S. Nesic and D. Young, Environ. Sci. Technol., 2010, 44, 9233–9238 CrossRef CAS PubMed.
- V.-A. Glezakou, L. X. Dang and B. P. McGrail, J. Phys. Chem. C, 2009, 113, 3691–3696 CAS.
- S. Linnenberg, V. Darde, J. Oexmann, A. Kather, W. J. M. van Well and K. Thomsen, Int. J. Greenhouse Gas Control, 2012, 10, 1–14 CrossRef CAS PubMed.
- (a) F. Valentini, V. Biagiotti, C. Lete, G. Palleschi and J. Wang, Sens. Actuators, B, 2007, 128, 326–333 CrossRef CAS PubMed; (b) K. Murugappan, J. Lee and D. S. Silvester, Electrochem. Commun., 2011, 13, 1435–1438 CrossRef CAS PubMed.
- Considering Water Quality for Use in the Food Industry 2008, Report commissioned by ILSI (International Life Science Institute).
- U. S. F. a. D. Administration, Electronic Code of Federal Regulations 2014.
- (a) M. Joseph, V. Kagdiyal, D. K. Tuli, M. M. Rai, S. K. Jain, S. P. Srivastava and A. K. Bhatnagar, Chromatographia, 1993, 35, 173–176 CrossRef CAS; (b) R. Lindahl, A. Wasterby and J.-O. Levin, Analyst, 2001, 126, 152–154 RSC.
- R. Gilbert, R. Rioux and S. E. Saheb, Anal. Chem., 1984, 56, 106–109 CrossRef CAS.
- A. G. Kumbhar, S. V. Narasimhan and P. K. Mathur, Talanta, 1998, 47, 421–437 CrossRef CAS.
- J. P. Metters, R. O. Kadara and C. E. Banks, Analyst, 2011, 136, 1067–1076 RSC.
- (a) P. M. Hallam, D. K. Kampouris, R. O. Kadara and C. E. Banks, Analyst, 2010, 135, 1947–1952 RSC; (b) N. A. Choudry, D. K. Kampouris, R. O. Kadara and C. E. Banks, Electrochem. Commun., 2010, 12, 6–9 CrossRef CAS PubMed; (c) M. Khairy, D. K. Kampouris, R. O. Kadara and C. E. Banks, Electroanalysis, 2010, 22, 2496–2501 CrossRef CAS; (d) A. V. Kolliopoulos, J. P. Metters and C. E. Banks, Anal. Methods, 2013, 5, 3490–3496 RSC; (e) A. V. Kolliopoulos, J. P. Metters and C. E. Banks, Anal. Methods, 2013, 5, 851–856 RSC.
- J. P. Smith, J. P. Metters, D. K. Kampouris, C. Lledo-Fernandez, O. B. Sutcliffe and C. E. Banks, Analyst, 2013, 138, 6185–6191 RSC.
- R. Adams and K. A. Schowalter, J. Am. Chem. Soc., 1952, 74, 2597–2602 CrossRef CAS.
- Acute Exposure Guideline Levels for Selected Airborne Chemicals, National, Research and Council, Washington, D.C., 2007, vol. 5 Search PubMed.
- A. Asokan and M. J. Cho, Biochim. Biophys. Acta, Biomembr., 2003, 1611, 151–160 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ew00033a |
| This journal is © The Royal Society of Chemistry 2015 |