Potential exposure and treatment efficiency of nanoparticles in water supplies based on wastewater reclamation
Peter
Kirkegaard
,
Steffen Foss
Hansen
* and
Martin
Rygaard
Department of Environmental Engineering, Technical University of Denmark, Denmark. E-mail: sfh@env.dtu.dk
First published on 27th January 2015
Abstract
Water scarcity brings an increased focus on wastewater reclamation for drinking water supply. Meanwhile, the production volume of nanoparticles (NPs) is rapidly increasing, but to date there has been little attention given to the fate of NPs in water systems based on wastewater reclamation. We have investigated the possible concentrations of silver (Ag), titanium dioxide (TiO2), and zinc oxide (ZnO) nanoparticles in tap water for water supplies based on reclaimed wastewater. Tap water concentrations of the NPs were assessed by mass flow analyses of two typical wastewater reclamation concepts: 1) advanced membrane treatment and 2) bank infiltration, similar to systems established in Orange County, CA, USA and Berlin, Germany. The mass flow analyses are based on a literature review of known wastewater concentrations of NPs and removal efficiencies for the implemented treatment stages in two case systems. Few studies are available on the removal efficiencies of NPs by advanced water treatment processes with a majority of the identified studies focusing on removal efficiencies in wastewater treatment plants and fate in surface waters. The NP removal efficiency of several treatment processes is unknown at this stage. We found the worst case removal efficiencies for the two cases to be 97–99.97% for Ag-NPs, 91–99.2% for TiO2-NPs, and 92–93% for ZnO-NPs. The corresponding worst case concentrations in tap water for the advanced membrane treatment were 0.04 μg L−1 (Ag), 147 μg L−1 (TiO2), and 0.28 μg L−1 (ZnO). The concentration of ZnO-NPs also includes zinc ions, thus the concentration of ZnO-NPs is likely to be lower than that indicated here. The worst case removal by the wastewater reclamation bank infiltration system was predicted to lead to tap water concentrations of up to 3.3 μg L−1 (Ag), 13 μg L−1 (TiO2), and 0.25 μg L−1 (ZnO). Overall, it is found that the primary removal mechanisms of NPs are aggregation, sedimentation, coagulation, and biosorption; this supports observations that conventional biological treatment processes are likely to be effective barriers against NPs. Advanced treatment methods such as microfiltration and ultrafiltration can exhibit very low removal of ZnO-NPs or zinc ions due to dissolution of ZnO-NPs. There are marked knowledge gaps, and further research on NP fate in water treatment is encouraged.
Nano impactIn this article we present the first ever evaluation of the removal of nanoparticles in wastewater by different water treatment processes in order to estimate the concentrations of NPs in reclaimed wastewater for potable reuse. Based on the mass flow analysis of two specific water reclamation cases (i.e. Orange County and Berlin) and a literature review, we found that silver (Ag), titanium dioxide (TiO2), and zinc oxide (ZnO) nanoparticles may occur in concentrations up to 3 μg L−1 (Ag), 147 μg L−1 (TiO2), and 0.3 μg L−1 (ZnO). Critical research needs evolve around understanding the fate of nanoparticles treated by reverse osmosis, UV and disinfection processes and understanding which kinds of nanoparticles in various types of products end up in our water supply. |
1 Introduction
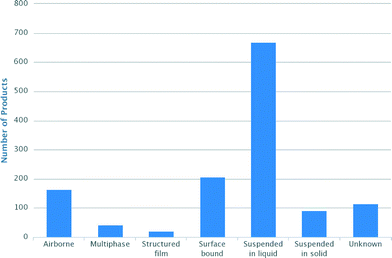
Engineered nanoparticles (NPs) are used to an ever increasing extent, e.g. in consumer products, but we know very little about how they are used and where they end up. According to The Nanodatabase (http://nanodb.dk/), which is a database maintained by the Danish Ecological Council, the Danish Consumer Council and the Department of Environmental Engineering at the Technical University of Denmark, more than 1200 products claimed to be based on nanotechnology or containing nanomaterials are now available to European consumers on-line. 52% of these products entail nanoparticles suspended in liquids, e.g. personal care products such as cosmetics, and only about 16% and 7% have surface bound NPs or NPs suspended in solids; hence it is reasonable to assume that most of these compounds will ultimately end up in our wastewater treatment systems (Fig. 1).Treated wastewater ends up in recipients such as rivers, lakes, and oceans, where it may, planned or unplanned (de facto), become an indirect source of drinking water supplies.1,2 Although few large scale reclamation plants for potable reuse are operational, it has been suggested that direct potable reuse can play a much larger role in future solutions to water scarcity.3 With the occurrence of nanoparticles in wastewater it is therefore relevant to investigate their potential presence and effective treatment in drinking water.
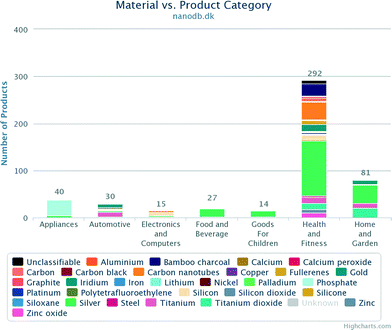
In this paper, we estimate the concentrations of silver (Ag), titanium dioxide (TiO2), and zinc oxide (ZnO) NPs that can be expected to end up in the water supply as a consequence of wastewater reclamation. NanoAg, nanoTiO2 and nanoZnO were chosen as they are the most commonly used NPs in consumer products (Fig. 2).4,5 These three NPs are often suspended in liquids when used in consumer products and hence can be expected to end up in wastewater. Other nanoparticles which are produced in large quantities annually are carbon black and silicon dioxide.6 However, carbon black nanoparticles are predominantly used in products (e.g. tires) which are disposed at landfills,7,8 while silicon dioxide is predominately used in food products and is not viewed as a health hazard even in a concentration of 1500 mg L−1.6 Therefore, these nanoparticles are not assessed in this study.
2 Method
The exposure and fate of nanoparticles in water treatment were investigated by mass flow analysis of two typical wastewater reclamation concepts based on a review of current knowledge on nanoparticle fate in water treatment systems. A literature review was conducted to find current knowledge on typical NP concentrations in wastewater, and their fate and transformation in wastewater treatment processes, advanced wastewater treatment, surface water, drinking water treatment, and natural filtration through a soil column.2.1 Two typical concepts for wastewater reclamation
We estimated the potential NP concentration in drinking water for two existing water reuse systems in Orange County, California, USA3 and Berlin.1 Orange County is situated in a water-scarce region and relies partly on the importation of water from outside the area. The Municipal Water District of Orange County (MWDOC) base its water supply on 62% groundwater, 34% imported water, and 4% surface water.9 Since the 1970's, groundwater has been replenished by reclaimed wastewater. The wastewater reclamation system of Orange County (Fig. 3A) consists of a conventional wastewater treatment plant (plant no. 1) which discharges its effluent to the Advanced Water Treatment Facility (AWTF) that is part of the Groundwater Replenishment System (GWRS) for the Orange County area. The Advanced Water Treatment Facility employs treatment methods such as disinfection, UV, microfiltration, and reverse osmosis. The effluent from the Advanced Water Treatment Facility is suitable for drinking and is subsequently delivered to the Santa Ana groundwater basin. After abstraction, the groundwater is disinfected before distribution to public water supply.10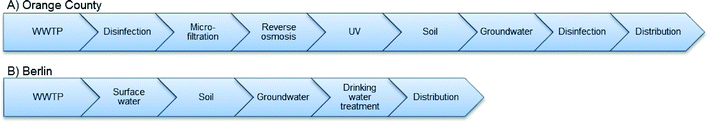 | ||
| Fig. 3 Conceptual diagram of the wastewater reclamation systems in (A) Orange County and (B) Berlin.1,2,54 | ||
Berlin's water supply is less technology-intensive than Orange County's. In Berlin, local groundwater is abstracted from local aquifers and then treated by aeration and sand filtration before distribution to the city (Fig. 3B). The aquifers are recharged with water from the local rivers and lakes.11 It is a “de facto” reclamation scheme because the same rivers and lakes also receive the effluent from local conventional wastewater treatment plants.12 Because of the recognized link between wastewater effluents and drinking water,13,14 Berlin has high awareness of keeping the state of the lakes and rivers healthy. Groundwater abstraction mainly occurs in soil layers dominated by sand and gravel in a depth of 30 to 50 m below the surface.12 In the central area of Berlin, the sewage system is combined, e.g. rain water and wastewater are collected by the same pipes. In the event of an overflow in this system, some of the sewage water is discharged to the rivers untreated because of WWTP capacity overload. In the aftermath of such an event, increased concentrations of usually well removed contaminants have been detected in the rivers, while contaminants which are usually difficult to remove are found in lower concentrations in the rivers due to dilution.15
2.2 Mass flow analysis
The removal efficiency by the two systems in Berlin and Orange County was assessed using mass flow analysis following the basic principles presented by Brunner.16 The considered mass flow analysis is a simple model which is based on the removal efficiencies identified for each of the treatment processes used in the investigated wastewater reclamation systems. The setup of the model shows that the nanoparticles that escaped from the previous treatment step are removed by the identified removal efficiency of the proceeding treatment process. Therefore, the model does not take the concentration dependency of the identified removal processes into account, e.g. it is well-known that removal by aggregation is concentration dependent.In order to properly evaluate the NP fate in the reclamation concepts, the removal efficiencies of the three NPs by the treatment stages are assessed in two scenarios representing the worst and best case evaluations of the assumed removal efficiencies. The lowest removal efficiency in each range is assumed to represent the worst case removal scenario, while the corresponding highest removal efficiency is used for the best case removal scenario. When only a single value for the NP removal efficiency by a given treatment stage has been identified, this single value is assumed to be the removal efficiency in both the minimum and maximum removal scenarios. When there is no documentation for the removal efficiency of a given NP by a given treatment stage, the minimum removal is assumed to be 0% and the maximum removal to be 100%.
3 Results
3.1 NP concentrations in wastewater
The estimation of the potential end concentration of NPs in drinking water starts with the estimation of the concentration in the wastewater influent. Gottschalk et al.17 modelled the concentrations of Ag-NPs, TiO2-NPs, and ZnO-NPs in the WWTP effluent for US, EU, and Switzerland. Tiede et al.8 used different forms of modeling to calculate the concentrations of TiO2-NPs and Ag-NPs in the WWTP effluent which again are based on the use, product concentration, and fate estimations reported by Boxall et al.,18 Mueller & Nowack,19 and Gottschalk et al.17 From their results we assumed WWTP influent concentrations of 107.2 μg L−1 for Ag-NPs, 1636.4 μg L−1 for TiO2-NPs, and 3.6 μg L−1 for ZnO-NPs.3.2 Fate of NPs in treatment processes
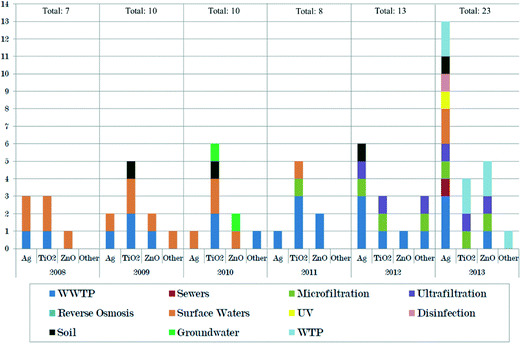
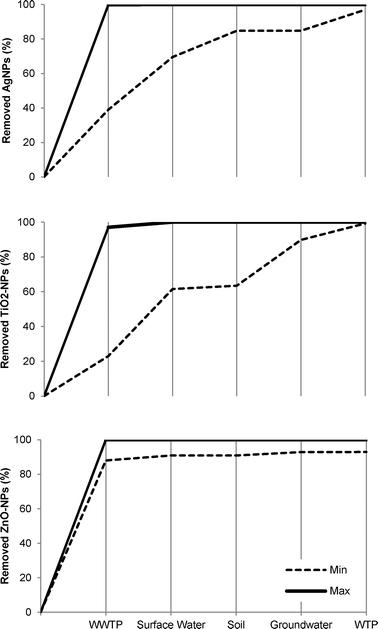
When it comes to understanding and mapping what happens to the NPs in the treatment processes, the specific fate and transformation process in the sewer, the WWTP, microfiltration, etc. is of vital importance. Out of the 71 studies identified for the period 2008–2013 (Fig. 4), wastewater treatment plants (27) and surface water (17) have received the most attention. Then come microfiltration (7), drinking water treatment plants (7), ultrafiltration (6), and soil and groundwater (6). Sewers, reverse osmosis, UV and disinfection have been covered by 0–1 study each.The estimated removal efficiencies of Ag-NPs, TiO2-NPs, and ZnO-NPs by each treatment stage identified in the literature are presented in Table 1 and will be discussed in the following paragraphs in the light of Orange County and Berlin.
| Stage | Reference | Comments | Ag | TiO2 | ZnO | Type of study | Considered in the mass flow analysis |
|---|---|---|---|---|---|---|---|
| a The removal efficiencies are read from graphs, tables or data. b Not considered since the microfiltration influent and effluent in the ATWF of Orange County have pH values of 7.3 and 7.5 (GWRS, 2013). c The conventional water treatment simulated by Abbott Chalew et al. (Abbott Chalew et al., 2013) is based upon coagulation and the removal efficiencies are therefore not necessarily the correct removal efficiencies for the drinking water treatment plants in Berlin. | |||||||
| Sewers | (Kaegi et al., 2013) | The Ag-NPs are observed to be stabilized by adsorption to sulfides and suspended solids. | 0 | — | — | Lab.+field | Yes |
| Wastewater treatment plants | (Kaegi et al., 2013) | Overall, sedimentation, aggregation and adsorption are identified as the primary mechanisms for the removal of the NPs. The increased concentration of Total Suspended Solids (TSS) is observed to increase the removal of the NPs (M. A. Kiser et al., 2010; Wang et al., 2012). In addition, Li et al. (L. Li et al., 2013) found the removal efficiencies of mechanical treatment and biological treatment to be 35% and 72%, respectively. | 98.9–99.9 | — | — | Lab.+field | Yes |
| (Jeong et al., 2012) | 70–90 | — | — | Lab. | Yes | ||
| (L. Li et al., 2013) | 95 | — | — | Lab. | Yes | ||
| (Wang et al., 2012) | 39–59 | 65–98 | — | Lab. | Yes | ||
| 84–92 | |||||||
| (M. A. Kiser et al., 2010) | — | 23–88 | — | Lab. | Yes | ||
| (Mueller and Nowack, 2008) | 81–92a | 81–87.6a | — | Sim. | Yes | ||
| (Gottschalk et al., 2009) | ~76a | ~76a | ~88a | Sim. | Yes | ||
| (Gottschalk et al., 2010) | — | ~86.6a | — | Sim. | Yes | ||
| (Johnson et al., 2011) | — | ~89.5 | — | Field | Yes | ||
| (Hou et al., 2013) | — | — | ~100 | Lab. | Yes | ||
| Microfiltration | (Abbott Chalew et al., 2013) | Abbott Chalew et al. report for pH 7–8 and Ladner et al. report for pH 8.2–9.6. | 55–99 | 56–100 | 17–64 | Lab. | Yes |
| (Ladner et al., 2012) | 0–10 | 0–10 | — | Lab. | Nob | ||
| ~100 | |||||||
| Ultrafiltration | (Abbott Chalew et al., 2013) | Abbott Chalew et al. report for pH 7–8 and Ladner et al. report for pH 8.2–9.6. | 98–100 | 96–100 | 4–98 | Lab. | Yes |
| (Ladner et al., 2012) | 60–90 | 95–100 | — | Lab. | Nob | ||
| Reverse osmosis | N/A | It is expected that reverse osmosis should have a removal efficiency better than ultrafiltration (Abbott Chalew et al., 2013; Ganzleben et al., 2011); the actual removal efficiency has not been investigated. | N/A | N/A | N/A | N/A | N/A |
| UV | (Yuan et al., 2013) | Oxidative dissolution was found to be the dominant reaction when Ag-NPs were exposed to disinfectants. In addition, the level of pH, sodium nitrate, humic acid and the type of disinfectant are important for predicting the removal of NPs by disinfection. | 17–67a | — | — | Lab. | Yes |
| Disinfection | (Yuan et al., 2013) | 95a | — | — | Lab. | Yes | |
| Surface waters | (Gottschalk et al., 2009) | The dominant removal mechanisms in surface waters seem to be the low pH and flow rate as well as increased flocculation, sedimentation, aggregation, and electrostatic attraction. | ~50 | ~99 | ~70 | Sim. | Yes |
| (Gottschalk et al., 2010) | — | ~50 | — | Sim. | Yes | ||
| (Ticiana Boncagni et al., 2009) | — | ~100 | — | Lab. | Yes | ||
| (Zhang et al., 2008) | — | 53–75a | ~75a | Lab. | Yes | ||
| (Keller et al., 2010) | — | ~77a | ~24a | Lab. | Yes | ||
| Soil | (Sagee et al., 2012) | In general, the studies observed that the low grain sizes, low concentration of humic acid, low flow conditions, low pH value, and high ionic strength of the soil promote electrostatic attraction and mechanical straining of the NPs. | 22a | — | — | Lab. | Yes |
| (Fang et al., 2009) | — | 17–99.8 | — | Lab. | Yes | ||
| (Solovitch et al., 2010) | — | 5–99 | — | Lab. | Yes | ||
| Groundwater | (Keller et al., 2010) | The increased ionic strength and concentration of Total Organic Carbon (TOC) promote retention of NPs. | — | 72a | 21.5a | Lab. | Yes |
| Water treatment plants | (Abbott Chalew et al., 2013) | Sand filtration is observed to remove 100% of the uncoated NPs while below 40% of the coated NPs (depending on the type and surface coating) can be removed by sand filters. Moreover, sand filtration seems to be susceptible towards a continuous influent resulting in a larger break through of the NPs than in a peak flow (Z. Li et al., 2013). | 80–98 | 92–97 | 1–52 | Lab. | Yesc |
Similar to the removal efficiency of Ag-NPs, the increased concentration of TSS is likely to result in the increased removal of the influent TiO2-NPs.24,27 The removal efficiencies of TiO2-NPs at 23–97% depending on the type of surface coating and concentration of TSS have been observed.24,27 Due to the very low solubility of TiO2-NPs, the presence of ionic Ti is not expected.24 The results presented by Johnson et al.26 indicate that the primary treatment can remove about 13% and the secondary treatment (activated sludge) removes more than 88%. Overall, Johnson et al. found the removal efficiency of TiO2-NPs by WWTPs to be 90%.26
Most of the ZnO-NPs are estimated to agglomerate and aggregate in WWTPs.28,29 Overall, the removal efficiency of ZnO-NPs by WWTPs is likely to be 88–100%.17,28
It should be noted that all three NPs are found to have a potential inhibitive effect on the microbial community in the WWTPs, at least in a short term until the microbes have adapted to the new compounds.21,24,25,28,30–34
In Orange County, the water is treated by microfiltration after the disinfection stage in the Advanced Water Treatment Facility. The pH values of the microfiltration influent and effluent are 7.3 and 7.5, respectively.10 Therefore, we assume that the removal efficiencies obtained by Abbott Chalew et al.35 should be applicable to the microfiltration units in the AWTF. It is noted that the removal efficiency by microfiltration/ultrafiltration membranes possibly can increase for ZnO-NPs by adjusting the pH to a higher level as some results indicate.35
In Orange County, the groundwater is typically disinfected prior to distribution to the consumer. 14% of the extracted groundwater is treated by ultrafiltration and reverse osmosis among others before distribution.39 However, for this mass flow analysis, these water treatment processes are disregarded at this stage due to their insignificant effect. In Anaheim, Orange County, sodium hypochlorite (12.5%) is used as the only treatment of groundwater prior to distribution (personal communication), while the water utility in Irvine Ranch adds chloramine at about 3 ppm in order to keep a ratio of 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloramine to ammonia (personal communication). The pH levels of the groundwater in Anaheim and Irvine Ranch are 7.9 and 8.2, respectively (personal communication). However, Yuan et al. found that changes in the pH level would have little effect on the removal efficiency of Ag-NPs.38 Therefore, the removal efficiency of the remaining Ag-NPs by the treatment of groundwater is estimated to be approximately 95%. The literature does not provide the corresponding values for TiO2-NPs and ZnO-NPs, therefore, their removal efficiencies are assumed to be 0% and 100%, respectively, in the mass flow analysis.
1 chloramine to ammonia (personal communication). The pH levels of the groundwater in Anaheim and Irvine Ranch are 7.9 and 8.2, respectively (personal communication). However, Yuan et al. found that changes in the pH level would have little effect on the removal efficiency of Ag-NPs.38 Therefore, the removal efficiency of the remaining Ag-NPs by the treatment of groundwater is estimated to be approximately 95%. The literature does not provide the corresponding values for TiO2-NPs and ZnO-NPs, therefore, their removal efficiencies are assumed to be 0% and 100%, respectively, in the mass flow analysis.
The effluent from the Advanced Water Treatment Facility in Orange County is pumped to the Kraemer, Miller, and Miraloma Basins or to the Talbert Seawater Intrusion Barrier at the coast. At the Kraemer, Miller, and Miraloma Basins, the treated water percolates into the groundwater while the treated water directed to the intrusion barrier is pumped into the groundwater reservoir. In both cases, the water is mixed with the existing groundwater. Due to the assumed short residence time in the basins, no interactions or transformation of NPs are expected.
Several studies have been made on the fate and transformation of Ag-NPs in surface waters. The potential retention of Ag-NPs in surface water has been predicted by Monte Carlo simulations to be around 50%.17,19 The possible retention of TiO2-NPs and ZnO-NPs in surface water has been assessed by experimental results and Monte Carlo simulations which estimate that 53–100% of TiO2-NPs are likely to be retained while 24–75% of ZnO-NPs will be removed.17,19,42,43 The retention of Ag-NPs, TiO2-NPs, and ZnO-NPs in surface water is likely to be determined by several factors such as the surface coating of the NPs, flow rate, and pH.17,19,42,43 The found percentage ranges for retention are estimated to be the best guess for Berlin while no retention is assumed for the basins in Orange County.
Due to the sandy soil in the two areas,12,49 the removal efficiencies for both Berlin and Orange County are estimated to be 50–71% for Ag-NPs in the unsaturated zone, 5–99% for TiO2-NPs in unsaturated soil and 72% in saturated soil layers, and about 21.5% for ZnO-NPs in saturated soil layers.43,45–47
Abbott Chalew et al. found the average removal efficiency of Ag-NPs by traditional water treatment plants to be around 80–98%.35 For TiO2-NPs, the average removal efficiency was found to be in the range of 92–97% for the simulated traditional water treatment, while for ZnO-NPs it was found to be 1–52%. The removal efficiency of ZnO-NPs includes zinc ions, indicating that the removal of ZnO-NPs is likely to be greater than that presented. By comparing the observed removal efficiencies by Z. Li et al.50 and Abbott Chalew et al.,35 aeration should remove 50% or more of the NPs in the influent to the water treatment plant.
The six drinking water treatment plants in Berlin treat the extracted groundwater by conventional processes such as sand filtration, primary treatment, and secondary treatment. Li et al. found that less than 50% of the surface coated NPs will be retained by sand filters.50 As many engineered NPs are surface coated, this is likely to be the removal efficiency by sand filters in most cases. Abbott Chalew et al. found the removal efficiencies for conventional drinking water treatment processes of Ag-NPs, TiO2-NPs, and ZnO-NPs to be 80–98%, 92–97%, and 1–52%, respectively.35 In Orange County, the extracted groundwater is treated by disinfection which is covered in section 3.2.6.
3.3 Mass flow analysis
Fig. 6 depicts the concentrations after treatment by each treatment stage in Orange County. The estimated worst case concentration of TiO2-NPs in the influent to the WWTP is predicted to be 147 μg L−1 while ZnO-NPs and Ag-NPs were predicted to be in concentrations of 280 ng L−1 and 37 ng L−1, respectively.
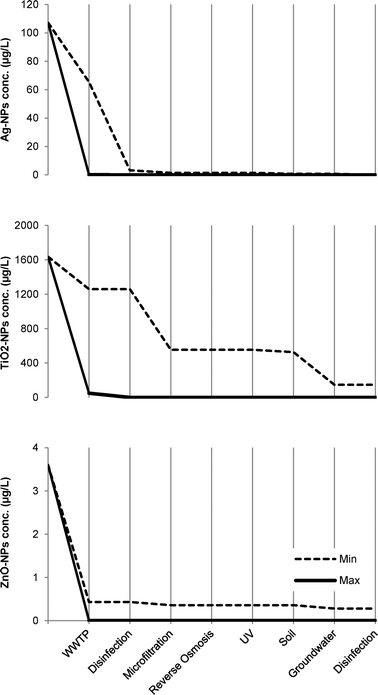 | ||
| Fig. 6 Concentration of NPs (μg L−1) after each treatment stage in the Orange County wastewater reclamation system for the removal scenarios: minimum and maximum. The estimated concentrations of Ag-NPs and TiO2-NPs in the influent to the WWTP are based on the study by Tiede et al.8 while the corresponding concentration of ZnO-NPs is based on the study by Gottschalk et al.17 | ||
The estimated concentrations of the three NPs in the tap water distributed to the consumers in Berlin indicate that TiO2-NPs can be found in the largest concentrations followed by Ag-NPs. The worst case scenario predicts that all three NPs may end up in the tap water in concentrations between 0.25–13 μg L−1 (Table 2). In addition, the system in Berlin utilizes surface water treatment plants (SWTPs) which treat the river water in order to minimize the concentration of phosphorus among others in the local surface water. In these plants flocculation, sedimentation, and filtration occur.12 These processes are likely to contribute to the overall NP removal efficiency by the system in Berlin. However, no information regarding the treated volume could be found. The SWTPs are therefore not included in the mass flow analyses (Fig. 8).
| Ag | TiO2 | ZnO | ||
|---|---|---|---|---|
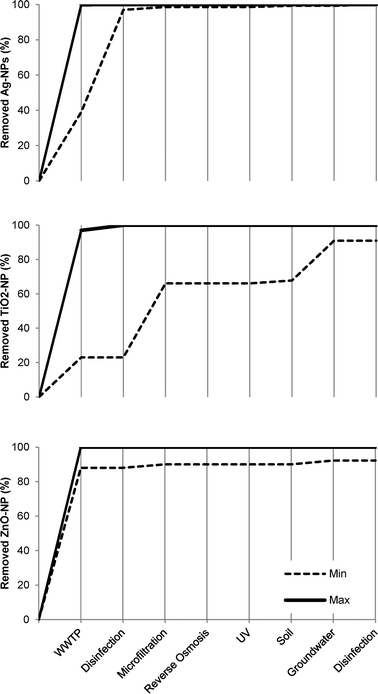
| Removal efficiency range (%) | Orange County | 99.97–100 | 91–100 | 92–100 |
| Berlin | 97–100 | 99.2–100 | 93–100 | |
| Worst case concentrations in tap water (μg L−1) | Orange County | 0.04 | 147 | 0.28 |
| Berlin | 3.3 | 13 | 0.25 |
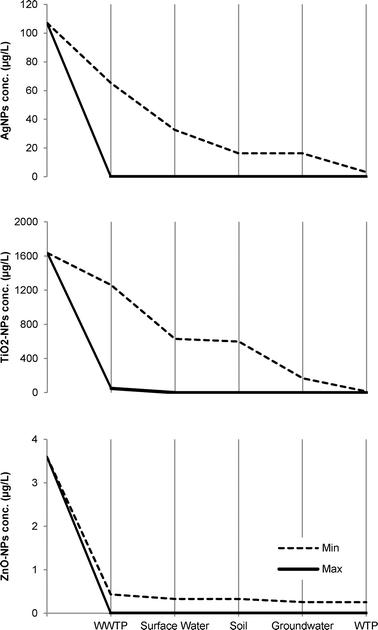 | ||
| Fig. 8 Concentration of NPs (μg L−1) after each treatment stage in the Berlin wastewater reclamation system for the removal scenarios: minimum and maximum. The estimated concentrations of Ag-NPs and TiO2-NPs in the influent to the WWTP are based on the study by Tiede et al.8 while the corresponding concentration of ZnO-NPs is based on the study by Gottschalk et al.17 WTP: conventional drinking water treatment. | ||
4 Discussion
4.1 Evaluation of the wastewater reclamation systems in Orange County and Berlin
The mass flow analyses in the previous sections of the wastewater reclamation systems in Orange County and Berlin indicate that considering a worst case scenario, the advanced treatment currently in operation in Orange County is likely to be more efficient towards Ag-NPs than the corresponding system in Berlin while the opposite is the case for TiO2-NPs and ZnO-NPs (Table 2).The concentration of TiO2-NPs in the tap water for the worst case scenario in Berlin is less than 10% of the corresponding concentration in Orange County. On the other hand, the system in Orange County has a worst case overall treatment efficiency of 99.97% for Ag-NPs, whereas in Berlin, the system is predicted to remove just 97% of the Ag-NPs. For ZnO-NPs, the found concentrations in the worst case scenario are almost equivalent for both systems.
The two disinfection stages (sodium hypochlorite) in the wastewater reclamation system in Orange County are the main cause of the higher worst case removal efficiency of Ag-NPs compared to the system in Berlin. For Ag-NPs, the difference in the worst case removal efficiencies for the two systems is mainly a 95% removal efficiency by the two disinfection stages and 55% removal efficiency by the microfiltration treatment in Orange County versus a 50% and 80% removal by the surface water and WTP, respectively, in the Berlin system. Therefore, the combination of size exclusion by microfiltration35 and especially oxidative dissolution and aggregation of Ag-NPs by disinfectants38 seem to be a more effective barrier than the combination of surface water (aggregation) and drinking water treatment plants (coagulation and bioadsorption) in Berlin.35,42,43,50,51
The primary reason for the difference in the worst case removal efficiency of TiO2-NPs by the two wastewater reclamation systems is the process in surface waters and water treatment plants in Berlin. The found worst case removal efficiencies of TiO2-NPs by the advanced treatment in Orange County are down to the lower minimum removal efficiency by microfiltration. The different removal mechanisms provide the system in Berlin with an advantage as no removal efficiency of TiO2-NPs by disinfection, reverse osmosis, or UV could be identified. The differences between the microfiltration treatment used in Orange County and the processes occurring in water treatment plants and rivers are predominantly due to the removal mechanism in microfiltration being reliant on size exclusion,35 while the natural attraction of TiO2-NPs to the particles and matter in surface water (including mutual attraction between the TiO2-NPs)42,43,51 as well as the coagulation35 and bioadsorption50 in conventional water treatment are observed to be more effective.
The difference between the found worst case removal efficiencies of ZnO-NPs for Berlin and Orange County is the smallest compared to those corresponding for Ag-NPs and TiO2-NPs. However, the WWRS in Berlin is still predicted to be slightly more effective than the corresponding system in Orange County due to the slightly higher overall estimated worst case removal efficiency of ZnO-NPs by surface waters and WTPs than the corresponding efficiency by microfiltration.
4.2 The known barriers against NPs
The literature search results indicate that ultrafiltration provides the best removal efficiency of Ag-NPs (98–100% removed) due to size exclusion.35 Moreover, the heteroaggregation occurring in treatment processes in WWTPs and drinking water treatment plants is likely to provide a significant retention of Ag-NPs. The dominant removal mechanism in WWTPs suggested by the identified studies is the natural attraction of Ag-NPs to the total suspended solids in the medium and the bacteria in the activated sludge.20,30 Disinfection and microfiltration are also likely to retain a large ratio of Ag-NPs. UV disinfection was effective predominantly due to the dissolution and aggregation of Ag-NPs.38By similar comparison, ultrafiltration is likely to be the most efficient barrier against TiO2-NPs (96–100% removed) due to size exclusion.35 However, the coagulation, sedimentation, and flocculation in the water treatment result in a high removal efficiency of TiO2-NPs (92–97%). Furthermore, high single removal efficiencies of TiO2-NPs were documented for WWTPs, surface waters, soil, and microfiltration, in which the highest estimated removal efficiencies of TiO2-NPs were found to be 97%, 99.9%, 99%, and 100%, respectively. The aggregation and biosorption of TiO2-NPs in WWTPs were observed to be the primary removal mechanisms.24,27 The removal efficiency of TiO2-NPs by (sandy) soil was very much dependent on the pH value of the soil which could lead to an almost negligible removal efficiency (5%). Moreover, if the soil has a high dissolved organic carbon content, a high removal efficiency (>95%) can be expected due to the composition of the clay. The size exclusion of the aggregated TiO2-NPs due to the pore size of the microfiltration membrane was the main reason for the high removal efficiency.35 In surface waters with acidic conditions and low flow rate (3.2 L s−1), the sedimentation of TiO2-NPs is found to be almost 100% regardless of surface coating. However, by increasing the pH and flow rate, the TiO2-NPs are more difficult to settle.42
The processes in the WWTP are likely to be the most efficient barrier against the break through of ZnO-NPs. Hou et al. found that about 70% of ZnO-NPs (no surface coating) are likely to rapidly settle in the primary clarification tank and the remaining ZnO-NPs are suggested to be completely removed by processes in the aeration and activated sludge treatment stages.28 The primary removal mechanism was found to be biosorption. Rapid settling is also observed in surface water media in which 8 hours of flocculation and 1 hour of sedimentation of ZnO-NPs are likely to result in 75% removal of ZnO-NPs.51 However, a removal efficiency of ZnO-NPs of 24% in river water media indicates that the flow rate is the primary parameter for the removal of ZnO-NPs in surface water.43 Ultrafiltration and microfiltration can also exhibit high removal efficiencies of ZnO-NPs (98% and 64%, respectively) but due to the dissolution of ZnO-NPs into zinc ions, these barriers are also observed to exhibit very low removal efficiencies (17% and 4%, respectively).35
In general, the identified studies highlight aggregation – especially heteroaggregation – and size exclusion as essential removal mechanisms in wastewater and water treatments.20,52
4.3 Knowledge gaps
In our attempt to complete a mass flow analysis for potable water reclamation in Orange County and Berlin, a marked lack of observations from several treatment stages and compartments is evident. This prevents an accurate estimation of the fate of the investigated NPs. Of the three NPs in question, ZnO-NPs have received the least attention. Only single observations were found for several combinations of NP types and treatment stages, for example ZnO-NPs in WWTP and groundwater (Table 1). The ranges of the removal efficiencies of Ag-NPs and TiO2-NPs were found for wastewater treatment, microfiltration, and soil layers whereas only single observations for the removal efficiencies of Ag-NPs and TiO2-NPs could be found for disinfection and groundwater, respectively.No studies which examined the removal efficiency of the NPs by reverse osmosis were identified. The pore size of the reverse osmosis membranes is below 0.1 nm and it is justified to assume that the reverse osmosis membranes will have a removal efficiency at least equal to the ultrafiltration membranes or better. But as Bellona et al.40 have found, rejection at the membrane might not be straightforward, because the size exclusion of the compounds may not be the only parameter controlling rejection. In addition, primarily due to their tendency to dissolve into zinc ions, ZnO-NPs have been observed to be difficult to remove by size exclusion35 and further studies are needed in order to determine the exact concentrations of ZnO-NPs which are able to break through the barriers in a given system. In the review of the identified studies, the WWTPs were found to exhibit the highest removal efficiency of ZnO-NPs. Moreover, WWTPs are also likely to exhibit relatively high removal efficiencies of Ag-NPs and TiO2-NPs which indicate that the biological processes in the WWTPs at the current knowledge level appear as the most efficient NP barriers in wastewater reclamation systems.
In general, further studies are needed in order to attain more knowledge on the fate of NPs in the various treatment stages. Only the studies on the removal efficiencies of the three NPs by WWTPs and surface waters can be regarded to provide a minimum level of nuanced understanding on the fate and behavior of the NPs. This indicates that the primary focus of the research community has been on the release to and fate in the environment and to a lesser extent the risk of exposure to humans through drinking water. Therefore, there is a lack of knowledge on the removal efficiency of more advanced treatment processes, with no observations reported for reverse osmosis. Furthermore, the studies evaluated in this study have primarily been conducted in laboratory settings or by modelling and simulation, which might provide a distorted perception of the transformation of NPs in real environments which is also highlighted in the work of Garner and Keller.52
Finally, an important knowledge gap lies in the estimation of the influent concentration. The influent concentrations of each NP in the mass flow analyses are based on the results found by Tiede et al.8 and Gottschalk et al.17 and are based primarily on the observed behavior of the NPs in the various treatment processes and previous results from similar studies as well as assumptions on the production volume and WWTP influent concentration of the NPs. Although, we do believe that these concentrations represent the best known emission concentrations of the NPs, it should be acknowledged that different estimations are available and that influent and effluent estimations will of course depend on fundamental assumptions about, for instance, production volumes.53
5 Conclusions
Based on a mass flow analysis of possible nanoparticle fate and treatment in two typical potable reuse systems, we have found that:•Considering a worst case scenario, nanoparticles may reach the drinking water supply in ng L−1 to μg L−1 concentrations after both advanced membrane-based reclamation and simpler conventional water treatment have been employed.
•There are marked knowledge gaps and actual removal efficiencies by several combinations of nanoparticle and treatment stages are largely unknown.
•Observations reported so far support biological treatment processes as the most efficient engineered barriers against nanoparticles in wastewater reclamation systems for potable reuse.
Whether the estimated concentrations pose a risk to humans is yet to be determined.
References
- M. Rygaard, P. J. Binning and H.-J. Albrechtsen, J. Environ. Manage., 2011, 92, 185–194 CrossRef PubMed.
- D. Gerrity, B. Pecson, R. S. Trussell and R. R. Trussell, J. Water Supply: Res. Technol.--AQUA, 2013, 62, 321 CrossRef CAS.
- H. L. Leverenz, G. Tchobanoglous and T. Asano, J. Water Reuse Desalin., 2011, 1, 2 CrossRef CAS.
- Nanodb.dk, 2014.
- Nanotechproject.org, 2014.
- European Commission, Types and Uses of Nanomaterials, including Safety Aspects, 2012 Search PubMed.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Model. Softw., 2010, 25, 320–332 CrossRef PubMed.
- K. Tiede, P. K. Westerhoff, S. Foss Hansen, G. Fern, R. J. Aitken, Q. Chaudhry and A. B. A. Boxall, Review of the risks posed to drinking water by man-made nanoparticles, 2011 Search PubMed.
- MWDOC, 2010.
- GWRS, Technical Brochure, 2013 Search PubMed.
- Veolia Water, Information Sheets Regarding Water Recycling, 2005 Search PubMed.
- Berliner Wasserbetriebe, Water for Berlin, 2013 Search PubMed.
- G. Massmann, J. Sultenfuss, U. Dunnbier, A. Knappe, T. Taute and A. Pekdeger, Hydrol. Processes, 2008, 22, 788–801 CrossRef CAS.
- T. Heberer, J. Hydrol., 2002, 266, 175–189 CrossRef CAS.
- P. Weyrauch, A. Matzinger, E. Pawlowsky-Reusing, S. Plume, D. von Seggern, B. Heinzmann, K. Schroeder and P. Rouault, Water Res., 2010, 44, 4451–4462 CrossRef CAS PubMed.
- P. H. Brunner and H. Rechberger, Practical handbook of material flow analysis : Advanced methods in resource and waste management, Lewis Publishers, 2004 Search PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- A. B. A. Boxall, Q. Chaudhry, C. Sinclair, A. Jones, R. J. Aitken, B. Jefferson and C. Watts, Current and Future Predicted Environmental Exposure to Engineered Nanoparticles, 2007 Search PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- E. Jeong, S. R. Chae, S. T. Kang and H. S. Shin, Water Sci. Technol., 2012, 65, 1298–1303 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS PubMed.
- L. Li, G. Hartmann, M. Döblinger and M. Schuster, Quantification of Nanoscale Silver Particles Removal and Release from Municipal Wastewater Treatment Plants in Germany, Environ. Sci. Technol., 2013, 13, 7317–7323 Search PubMed.
- Y. Wang, P. Westerhoff and K. D. Hristovski, J. Hazard. Mater., 2012, 201–202, 16–22 CrossRef CAS PubMed.
- N. Musee, J. N. Zvimba, L. M. Schaefer, N. Nota, L. M. Sikhwivhilu and M. Thwala, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, 49, 59–66 CrossRef CAS PubMed.
- A. C. Johnson, M. J. Bowes, A. Crossley, H. P. Jarvie, K. Jurkschat, M. D. Jürgens, A. J. Lawlor, B. Park, P. Rowland, D. Spurgeon, C. Svendsen, I. P. Thompson, R. J. Barnes, R. J. Williams and N. Xu, Sci. Total Environ., 2011, 409, 2503–2510 CrossRef CAS PubMed.
- M. A. Kiser, H. Ryu, H. Jang, K. Hristovski and P. Westerhoff, Water Res., 2010, 44, 4105–4114 CrossRef CAS PubMed.
- L. Hou, J. Xia, K. Li, J. Chen, X. Wu and X. Li, Water Sci. Technol., 2013, 67, 254–260 CrossRef CAS PubMed.
- L. K. Limbach, R. Bereiter, E. Müller, R. Krebs, R. Galli and W. J. Stark, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS.
- S. P. Dhas, P. J. Shiny, S. Khan, A. Mukherjee and N. Chandrasekaran, J. Basic Microbiol., 2013, 1–12 Search PubMed.
- S. Eduok, B. Martin, R. Villa, A. Nocker, B. Jefferson and F. Coulon, Ecotoxicol. Environ. Saf., 2013, 95, 1–9 CrossRef CAS PubMed.
- G. Liu, D. Wang, J. Wang and C. Mendoza, Sci. Total Environ., 2011, 409, 2852–2857 CrossRef CAS PubMed.
- X. Sun, Z. Sheng and Y. Liu, Sci. Total Environ., 2013, 443, 828–835 CrossRef CAS PubMed.
- X. Zheng, R. Wu and Y. Chen, Environ. Sci. Technol., 2011, 45, 2826–2832 CrossRef CAS PubMed.
- T. E. Abbott Chalew, G. S. Ajmani, H. Huang and K. J. Schwab, Environ. Health Perspect., 2013, 121, 1161–1166 Search PubMed.
- D. A. Ladner, M. Steele, A. Weir, K. Hristovski and P. Westerhoff, J. Hazard. Mater., 2012, 211–212, 288–295 CrossRef CAS PubMed.
- R. W. Baker, Membrane Technology and Applications, John Wiley & Sons, Ltd, Chichester, UK, 2012 Search PubMed.
- Z. Yuan, Y. Chen, T. Li and C.-P. Yu, Chemosphere, 2013, 93, 619–625 CrossRef CAS PubMed.
- City of Huntington Beach, 2010Urban Water Management Plan, 2011 Search PubMed.
- C. Bellona, J. E. Drewes, P. Xu and G. Amy, Water Res., 2004, 38, 2795–2809 CrossRef CAS PubMed.
- P. Xu, J. E. Drewes, T.-U. Kim, C. Bellona and G. Amy, J. Membr. Sci., 2006, 279, 165–175 CrossRef CAS PubMed.
- N. Ticiana Boncagni, J. M. Otaegui, E. Warner, T. Curran, J. Ren and M. Marta Fidalgo de Cortalezzi, Exchange of TiO2 Nanoparticles between Streams and Streambeds, Environ. Sci. Technol., 2009, 7699–7705 CrossRef PubMed.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- MWDSC, Final Groundwater Assessment Study, 2007 Search PubMed.
- O. Sagee, I. Dror and B. Berkowitz, Chemosphere, 2012, 88, 670–675 CrossRef CAS PubMed.
- N. Solovitch, J. Labille, J. Rose, P. Chaurand, D. Borschneck, M. R. Wiesner and J.-Y. Bottero, Environ. Sci. Technol., 2010, 44, 4897–4902 CrossRef CAS PubMed.
- J. Fang, X. Shan, B. Wen, J. Lin and G. Owens, Environ. Pollut., 2009, 157, 1101–1109 CrossRef CAS PubMed.
- S. Bae, Y. S. Hwang, Y.-J. Lee and S.-K. Lee, Environ. Health Toxicol., 2013, 28, e2013006 CrossRef PubMed.
- OCWD, 2010.
- Z. Li, A. Aly Hassan, E. Sahle-Demessie and G. A. Sorial, Water Res., 2013, 47, 6457–6466 CrossRef CAS PubMed.
- Y. Zhang, Y. Chen, P. Westerhoff, K. Hristovski and J. C. Crittenden, Water Res., 2008, 42, 2204–2212 CrossRef CAS PubMed.
- K. L. Garner and A. A. Keller, J. Nanopart. Res., 2014, 16, 2503 CrossRef.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanoparticle, 2013, 15, 1692 CrossRef.
- G. Tchobanoglous, H. Leverenz, M. H. Nellor and J. Crook, Direct potable reuse. A path forward, 2011 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |