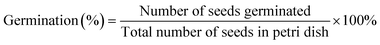Effect of N-acetyl cysteine coated CdS:Mn/ZnS quantum dots on seed germination and seedling growth of snow pea (Pisum sativum L.): imaging and spectroscopic studies†
Smruti
Das
a,
Brandon P.
Wolfson
a,
Laurene
Tetard
abc,
Jeremy
Tharkur
ad,
Joshua
Bazata
ad and
Swadeshmukul
Santra
*acde
aNanoScience Technology Center, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA
bDepartment of Physics, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA
cDepartment of Materials Science and Engineering, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA
dBurnett School of Biomedical Sciences, 12424 Research Parkway, Suite 400, Orlando, FL 32826, USA
eNanoScience Technology Center, Department of Chemistry, Department of Materials Science and Engineering, and Burnett School of Biomedical Sciences, University of Central Florida, 12424 Research Parkway, Suite 400, Research Pavilion, Orlando, FL 32826, USA. E-mail: ssantra@mail.ucf.edu; Fax: +1 407 882 2819; Tel: +1 407 882 2848
First published on 5th February 2015
Abstract
Anthropogenic nanomaterials (ANMs), once produced, will inevitably be present in the environment. Depending on their environmental stability and level of toxicity, ANMs raise some concern regarding their potential impact on the surrounding animal, aquatic and plant life. In this study, we demonstrate for the first time the effect of ultra-small size (<5 nm) semiconductor ANMs on the germination and growth of seeds of a snow pea model plant system (Pisum sativum) using a N-acetyl cysteine (NAC) coated core–shell CdS:Mn/ZnS Qdots as a heavy metal ion containing model ANM. We present combined results of fluorescence confocal, atomic force microscopy (AFM) and Raman imaging of quantum dot (Qdot) to track the uptake and localization (translocation) in plant tissue. It was found that Qdots were localized on the surface seed coat, epidermis and intercellular regions. The germination, growth and chlorophyll content of the seedlings were found to be strongly dependent on Qdot dosage and time of seed incubation with Qdots. Interestingly, no acute Cd metal toxicity was observed at Qdot concentration below 40 μg mL−1, and seed germination and growth processes were promoted.
Nano impactThe ultra small size water soluble NAC-Qdot used in this study can be a taggant and delivery cargo inside the plant system. Its fluorescence properties can help to track its localization inside the plant system. Fluorescence imaging has been used as an efficient tool. Along with this we have observed that it can be used as a nutrient medium to boost the germination and growth of snow pea plants (Pisum sativum). We believe that our study can help and bring a new direction to research going on in the new emerging nano-agricultural studies. |
1. Introduction
Anthropogenic nanomaterials (ANMs) are a class of material specifically engineered to exhibit unique physical and chemical properties on the nanoscale (size below 100 nm). After their useful life, ANMs are bound to interact with their environment, but their potential impact on animal, plant and aquatic life is not yet fully understood, thus potentially raising serious environmental concerns.1 Studying the interaction of ANMs with the plant vascular system to assess their potential ecotoxicity in physiological conditions (in vivo) and understand ANM uptake by plant cells and tissues is therefore necessary, and calls for suitable imaging and spectroscopy techniques. In addition to nano- and microscopic interactions of ANMs with plants, their immediate phenotypic effects on seed germination and plant growth should be considered. Hence due to the intrinsic complexity of plants, several routes of nanoparticle exposure must be taken into account.Some of these parameters have been examined in previous related studies.2–11 As seed germination and vegetative phase are the two most important stages of plant growth, during which the plant is most sensitive to its different environmental conditions, various studies have considered tolerance, uptake and possible accumulation of environmental contaminants in roots and shoots, and the effect of nanoparticles (NPs) on seed germination and seedling growth.2–12 Furthermore, a number of other parameters including the particle size, surface charge, surface coating chemistry, aspect ratio, and composition of ANMs are expected to greatly affect the overall plant physiology.3,4 An inhibitory effect of zinc oxide (ZnO) NPs (20 ± 5 nm) on corn seed germination at a concentration of 2000 ppm, root growth inhibition of 50% at 50 ppm in radish, and at 20 ppm in rape and ryegrass plant systems has also been shown.6 However another study reported beneficial effects on seed germination and seedling growth at 1000 ppm with 25 nm mean particle size, while an inhibitory effect at higher concentration.5 Priester et al. also considered the translocation of ZnO NPs in plant tissues after soil application.7 The overall metal concentrations increased in plant tissues as a result of the treatment, somewhat compromising food quality, but showing no toxicity.7 Similar treatments with CeO2 NPs led to the plant nitrogen fixation process being shut down, and created a negative ecotoxicological impact.13 The impact of NP containing non-nutrient based metal ions such as silver (Ag) and gold (Au) on seed germination and plant growth has also been considered. Yin et al. reported an inhibitory effect of gum arabic coated Ag NPs on seed growth at 40 ppm, whereas no inhibitory effect was observed for polyvinylpyrrolidone coated Ag NPs and AgNO3 salts, suggesting that phytotoxicity of Ag NPs is dependent on surface coating chemistry.8 Similar inhibitory effects of Ag NPs were also observed on rice seed germination and seedling growth with increasing NP sizes and concentrations.11 However, Prasad et al. showed that Au NPs of 24 nm size induced vegetative growth and enhanced seed yield of an Arabidopsis thaliana model plant system.5 Finally, carbon nanotubes did not exhibit any significant adverse impact on the plant growth.6 In fact, carbon nanotubes enhanced growth in tobacco plant cells.9
One critical aspect to understand ANMs' translocation and their ultimate fate in plant vascular systems and their effect on the overall behavior of the plant is the necessity to develop suitable imaging techniques capable of resolving nanoparticle signatures. Photoacoustic and photothermal based detection of carbonaceous nanomaterials were used to monitor the interaction of activated carbon, carbon nanotubes and graphene with tomato plant.10 However, the metrology available for such studies remains underexplored.
Herein we investigated the effect of ultra-small size (~5.0 nm) NAC-coated CdS:Mn/ZnS Qdots on the germination of seeds and seedling growth of snow peas (P. sativum). NAC is an antioxidant biomolecule as well as a water-soluble carboxylated (negatively charged) capping agent for Qdots. We report fluorescence, atomic force microscopy (AFM) and Raman spectroscopy based surface and chemical imaging investigation of the structural and content analysis of seeds to monitor possible nanoparticle–cell interaction inside plant cells resulting from Qdot treatments. The study was complemented by a physiological study of germination and growth parameters.
2. Materials and methods
2.1. Materials
All reagents were purchased from commercial vendors and used without any further purification unless stated specifically. Cadmium acetate dihydrate, manganese acetate tetrahydrate, N-acetyl-L-cysteine (NAC) and dioctyl sulfosuccinate sodium salt (Aerosol OT, AOT) were purchased from Acros Organics (New Jersey, US). Zinc acetate dihydrate and sodium sulfide were purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade heptane and ethanol (95%; V/V) were obtained from Fisher Scientific (Pittsburgh, PA) and acetone was obtained from VWR (Radnor, PA). Purified deionized water (DI) was obtained from Nanopure (Barnstead Nanopure Diamond purifier; model # D11931).2.2. Synthesis of NAC coated CdS:Mn/ZnS Qdots
CdS:Mn/ZnS Qdots were synthesized via a reverse micelle method following a published protocol21,22 with some modifications as stated below. Three stock solutions (A, B and C) of salts (cadmium acetate dihydrate, manganese acetate, sodium sulfide, zinc acetate) were separately prepared. Stock solution “A” contains a mixture of 266 mg (0.1 mol) of cadmium acetate and 4.9 mg of manganese acetate (1.8 mol% with respect to metallic Cd) in 10 mL of DI water. Stock solutions (5 mL; DI water) of “B” and “C” contain 257.5 mg of sodium sulfide and 285.3 mg of zinc acetate dehydrate (0.26 mol), respectively. Then three separate AOT/heptane/water water-in-oil (W/O) microemulsion solutions 1, 2 and 3 were prepared containing 0.9 mL of stock solution “A”, 2.7 mL of stock solution “B” and 2.7 mL of stock solution “C”, respectively. Microemulsion 1 contains 2.23 g of AOT and 25 mL of heptane, whereas microemulsions 2 and 3 contain 6.69 g of AOT and 75 mL of heptane. Microemulsion 1 was added to 2 followed by magnetic stirring for 15 min. Thereafter microemulsion 3 was added to the combined mixture of 1 and 2 dropwise. After 24 h of stirring, bright yellow-orange color emission was seen when the microemulsion solution was exposed to a handheld UV lamp (Mineralight®, multiband UV 254/365 nm lamp, model UVGL-58) confirming formation of CdS:Mn/ZnS Qdots. Post coating with NAC was done while Qdots were present in W/O microemulsion. The NAC coating procedure involved the following steps. A W/O microemulsion containing NAC was prepared by combining 175 mL of heptane, 15.61 g of AOT and 0.55 mg of NAC solution (prepared in 6.3 mL of DI water). Next, NAC containing microemulsion was added dropwise to Qdot microemulsion followed by stirring for 24 h. NAC coated Qdots (NAC-Qdots) were then recovered by first destabilizing the microemulsion using a solvent mixture of acetone and ethanol (95% V/V) followed by centrifugation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min). The NAC-Qdot pellet was then re-dispersed in 10 mL of acetone followed by centrifugation (11
000 rpm for 10 min). The NAC-Qdot pellet was then re-dispersed in 10 mL of acetone followed by centrifugation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min), sonication (5 min) and vortexing (2–3 min). This washing procedure was repeated using acetone (2×), heptane (2×) and acetone (1×). Finally, the NAC-Qdot pellet was dried at 60 °C for 4 min to obtain the product in powder form.
000 rpm, 5 min), sonication (5 min) and vortexing (2–3 min). This washing procedure was repeated using acetone (2×), heptane (2×) and acetone (1×). Finally, the NAC-Qdot pellet was dried at 60 °C for 4 min to obtain the product in powder form.
2.3. UV-Visible and fluorescence spectroscopy
All UV-Visible absorption and fluorescence emission spectra were recorded at room temperature using a Cary 300 UV-Vis spectrophotometer and a NanoLog spectrofluorometer (SPEX, Jobin Yvon Horiba), respectively. A quartz cuvette (1 cm path length) was used for all UV-Vis and fluorescence measurements. For UV-Vis measurements, the instrument was calibrated against DI water in the wavelength range of 200–800 nm. 450 μl of a stock solution of 1 mg ml−1 NAC-Qdot diluted with 2 ml of DI water was used to take the OD. The fluorescence emission spectrum was recorded at 375 nm excitation keeping the slit width (both excitation and emission) fixed at 5 nm and the scan rate at 1 nm s−1.2.4. High resolution transmission electron microscopy (HRTEM)
Characterization of Qdot crystal size and crystallinity was performed using a FEI Tecnai F30 High-Resolution Transmission Electron Microscope (HRTEM). Carbon coated copper grids with 400 mesh (Electron Microscopy Sciences) were submerged in NAC-Qdot solution (in DI water) for 20–30 min and then placed on absorbing task wipers (Kimwipes, Kimtech Science brand; Kimberly-Clark) in a plastic Petri dish to dry overnight. Grids were transported for HRTEM analysis for measuring crystal size and visualizing the crystallinity.2.5. Preparation of seeds and plant growth conditions
Snow pea (P. sativum) seeds were purchased from Lucas Nursery, Orlando, FL and stored in a dry opaque packet at room temperature. Prior to use, seeds were soaked in DI water for 30 min to soften the seed coat. Thereafter surface sterilization was performed by washing the seeds with separate solutions of 70% ethanol and 1% sodium hypochlorite for 1 minute. Seeds were then washed five times with DI water to ensure the removal of additional sterilizing solutions remaining on the surface. The sets of Qdot treated and untreated seeds were sown in cups containing a potting mixture at a constant day/night temperature (70/65 °F) to see the effect of Qdot on seedling growth (root and shoot length).2.6. Plant germination tests
Exposure of plant cells to an excess amount of Qdots could raise ROS levels (i.e. beyond a maximum tolerable dose) which could negatively impact plant genotype. To test this hypothesis, we investigated the effect of different concentrations of NAC-Qdot on the seed germination, root length, shoot length and chlorophyll content of snow pea genotype. Different concentrations of NAC-Qdots (0, 2, 5, 10, 20, 40, 60, 80, 100 μg mL−1) were prepared in DI water to study their effect on seed germination and growth of seedling of snow pea (P. sativum). Seeds were soaked under different concentrations of freshly prepared NAC-Qdot for 24 h. Seed germination was tested on moist filter paper (Whatman™ filter paper size 41; GE Healthcare Life Sciences). In each Petri dish (85 mm diameter), 20 seeds were randomly placed on the filter paper, then 5 mL of NAC-Qdot solution was added (i.e. treated seed samples). The controls were maintained by similarly treating seeds with 5 mL of DI water. Seeds were germinated for five days in the dark in controlled environmental conditions (25 °C and 50% Relative Humidity, RH). Germination was considered successful when the coleoptiles were longer than 2 mm. Three replicates were carried out for each treatment. The germination rate is the average number of seeds that germinate over the five-day time period.2.7. Chlorophyll estimation
Leaves were collected from a 15 day old seedling to estimate the chlorophyll content. Freshly cut leaves (40 mg) were weighed and put in 80% acetone for chlorophyll estimation. Chlorophyll a, chlorophyll b and total chlorophyll were estimated following Arnon's method using the specific absorption coefficient given by Mackinney in 1941.23,24 The Optical Density (OD values) of the solutions was read at 645 and 663 nm using a SpectraMax 190 absorbance plate reader equipped with SoftMax® ProData acquisition & analysis software.| Total Chlorophyll(mg g−1) = (20.2(OD645) + 8.02(OD663)) |
V = Final volume of 80% acetone (i.e. 25 ml)
w = Dry weight of sample taken (i.e. 40 mg)
2.8. Sample preparation for microscopy
For the microscopic studies, cross sections (~300 μm thick slice) of treated and untreated seeds without seed coats (cotyledons) were prepared manually using a single edge blade (~230 μm thickness; American Safety Razor Company; catalogue # 66-0392; Verona, VA) and mounted on microscope slides (Fisherbrand®; size 12 × 75 × 1 mm; catalogue # 12-550-003).2.9. Raman spectroscopy
Raman spectroscopy measurements were performed using a Witec alpha300 RA under ambient conditions with an excitation wavelength of 532 nm and using a Zeiss 20× objective. A full Raman spectrum was collected at each point of the image with a 600 g mm−1 grating and an integration time of 0.05 s. The Raman intensity and peak position maps were reconstructed using the data analysis toolbox available in Witec Project Plus software.2.10. Atomic force microscopy
Atomic force microscopy (AFM) images were obtained in tapping (AC) mode using a Witec alpha300 RA under ambient conditions with a standard cantilever (Al coating, fres = 247 kHz).2.11. Statistical analysis
Each treatment was conducted with three replicates, and the results were presented as mean ± SD (standard deviation). The statistical analysis of experimental data utilized the PASW Statistics 18 (IBM SPSS). Tukey's least significant difference (LSD) was used to compare each of the experimental value to its corresponding control at a significance level of P < 0.05.3. Results and discussion
3.1. Characterization of NAC-Qdot
HRTEM images of NAC-Qdots are shown in Fig. 1a. Despite a few clusters, Qdots (dark contrast) were fairly monodispersed as seen in the low-magnification image (Fig. 1a (i)). Average particle size was estimated to be around 5 nm based on individual measurements of 21 particles using ImageJ, as shown in the histogram (Fig. 1a (ii)). High magnification HRTEM imaging revealed crystal lattice planes 101 and 110 for CdS and ZnS, respectively (Fig. 1a (iii)), with the corresponding electron diffraction pattern indicating Qdot crystallinity. HRTEM, therefore, confirmed that the Qdot surface coating with NAC did not compromise the particle crystallinity (Fig. 1a (iv)). Next, we performed comprehensive characterization of NAC and Qdots with UV-Vis, fluorescence, and FTIR spectroscopy. The results are of great importance in order to track the NPs in the plant and understand how their potential interactions with plant tissues can affect their intrinsic properties. UV-visible spectra show an absorption peak of NAC around 225 nm, which was not present in the NAC-Qdot spectrum. However, the NAC-Qdot compound exhibits an absorption peak of 320 nm (Fig. 1b). A bare Qdot shows typical absorption characteristics of a semiconductor material with no clear band maximum, rather a shoulder appeared around 250 nm which is considered as the Qdot absorption peak. This absorption peak red-shifts by about 70 nm once the Qdot surface is conjugated to NAC. The shift in Qdot absorption is attributed to the formation of surface-related defects during chemical conjugation of NAC to the Qdot surface. The absence of the 225 nm NAC absorption peak could not be resolved in the NAC-Qdot absorption spectrum as it was suppressed by the strong Qdot absorption in the 225 nm region. The fluorescence intensity of the Qdot and NAC-Qdot was observed at 590 nm, the peak emission wavelength, whereas NAC presented no fluorescence. The fluorescence of the Qdots is attributed to the Mn dopant (4T1 → 6A1 transition). The results show that the presence of NAC on the surface did not alter the Qdot emission properties. The bright yellow-orange fluorescence of the NAC-Qdot solution can be seen in the inset (Fig. 1c). The strong Qdot fluorescence can be used as a powerful biomarker to uncover its uptake by plant cells.14 These results were used to select appropriate filters in fluorescence imaging to detect NAC-Qdot in plant cells. Next, FTIR signatures of NAC, NAC-Qdot and bare Qdot were compiled (Fig. 1d). The characteristic NAC S–H peak at 2552 cm−1 was not observed in NAC-Qdot, indicating chemical conjugation of NAC to the Qdot surface through the formation of Zn–S bonds. NAC is highly water soluble in a wide pH range (2.4–12.0) due to the presence of hydrophilic carboxyl (–COOH) and acetamide (–NHCOCH3) groups. These hydrophilic groups are available in the NAC-Qdot composite as NAC is conjugated to the Qdot surface through the thiol (S–H) group. Therefore, NAC-Qdot exhibits excellent water solubility, as also reported by Zhao et al.153.2. Effect on seed germination
We then studied the effect of the ultra-small size (~5.0 nm) NAC-Qdots on the germination of seeds and seedling growth of snow peas (P. sativum). A schematic diagram represents different possibilities of the NAC-Qdot impact on snow pea seed germination and seedling growth (Fig. 2). When entering a plant, external agents can trigger reactive oxygen species (ROS) production, leading to oxidative stress resulting in antioxidant enzyme production.16 Qdot, being an external agent, is therefore expected to trigger antioxidant enzyme production, leading to enhanced nutrient uptake and metabolism. This might lead to a change in physiological activities in the plant system. | ||
| Fig. 2 Schematic representation of the possible effect of NAC-Qdot on seed germination and plant growth of snow pea (Pisum sativum). | ||
Physiological changes in seed germination against treatments of different concentrations for NAC-Qdots were observed (Table S1†). Seed germination reached 100% within 48 hours under NAC-Qdot exposure. The radicle and coleoptile exhibited signs of enhanced growth for treatments up to 40 μg mL−1 NAC-Qdot concentration, despite the presence of toxic Cd ions in NAC-Qdot (Fig. 3a). Enhanced nitrogen assimilation17 and reduction in free radical production18,19 are considered to be possible contributing factors towards enhancement of seed germination. Interestingly, inhibition of seed germination and seedling growth was clearly noticed for all treatments above 40 μg mL−1 NAC-Qdot concentration. To further understand the role of Qdot surface chemicals i.e. NAC and ZnS shell which protects the CdS core (Fig. 3), the effect of each individual component of NAC-Qdot on seed germination was tested. These chemical components include NAC, zinc acetate, zinc acetate–NAC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio), cadmium acetate, sodium sulfide, AOT and DI water (control). Only the soluble form of Qdot components was used as control. We could not involve other possible controls such as CdS and ZnS particles due to their aqueous insolubility. Seeds were allowed to germinate over 5 days and observed that NAC (vi), zinc acetate (iii), and zinc acetate–NAC (ii) accelerated germination rate, as seen by the increase in radical length over time (Fig. 3b). However, cadmium acetate (iv), sodium sulfide (v) and AOT (vii) completely suppressed the growth (Fig. 3b). NAC is considered as a free radical scavenger and is capable of reducing cellular toxicity induced by free radicals.2 Heavy metal ions such as cadmium (Cd) ions are known to be cytotoxic because of their ability to produce free radicals. Cd transport from plant roots to above ground tissues has been observed and suggests possible threats to human health.17 Cd accumulation in plants is regulated by several physiological processes, including uptake from soil (via roots) and atmosphere (via shoots), resulting in xylem transport from root to shoot and phloem movement into grain during maturation.19 Its high phytotoxicity associated with uptake and accumulation in plants can influence plant growth.18,20 Under most environmental conditions, it has been shown that Cd causes damage after its uptake by the roots, which in turn reduces the absorption and affects the transport of nitrate from roots to shoots, by inhibiting the nitrate reductase activity in the shoots.21 Cd-induced deficiencies in Fe(II) have also been reported to severely affect photosynthesis.22 In our study we have used core–shell CdS:Mn/ZnS where the CdS crystalline core is protected by a wide band-gap ZnS semiconductor material. The ZnS shell not only stabilizes the CdS core from oxidation but minimizes release of free Cd ions. Furthermore, the Qdot surface capping agent, NAC, is expected to passivate any Cd toxicity through scavenging free radicals generated by the Qdot core. Enhancement in seed germination and seedling growth for NAC-Qdot treated seeds below 40 μg mL−1 is therefore attributed to the combined effect of NAC and ZnS. Photographs of NAC-Qdot treated and untreated control seeds were illuminated using a handheld 366 nm multi-band UV excitation source (Fig. 3c, d). A bright glow was observed from radicals of NAC-Qdot treated seeds in comparison to the radicals of the control seeds, which we believe to be due to the combined emission of Qdot and plant tissue auto-fluorescence. This indicates the uptake of Qdots by the seed and their systemic distribution in plant cells. Some radicals however appeared brighter than others, which could be due to non-uniform distribution of Qdots.
1 molar ratio), cadmium acetate, sodium sulfide, AOT and DI water (control). Only the soluble form of Qdot components was used as control. We could not involve other possible controls such as CdS and ZnS particles due to their aqueous insolubility. Seeds were allowed to germinate over 5 days and observed that NAC (vi), zinc acetate (iii), and zinc acetate–NAC (ii) accelerated germination rate, as seen by the increase in radical length over time (Fig. 3b). However, cadmium acetate (iv), sodium sulfide (v) and AOT (vii) completely suppressed the growth (Fig. 3b). NAC is considered as a free radical scavenger and is capable of reducing cellular toxicity induced by free radicals.2 Heavy metal ions such as cadmium (Cd) ions are known to be cytotoxic because of their ability to produce free radicals. Cd transport from plant roots to above ground tissues has been observed and suggests possible threats to human health.17 Cd accumulation in plants is regulated by several physiological processes, including uptake from soil (via roots) and atmosphere (via shoots), resulting in xylem transport from root to shoot and phloem movement into grain during maturation.19 Its high phytotoxicity associated with uptake and accumulation in plants can influence plant growth.18,20 Under most environmental conditions, it has been shown that Cd causes damage after its uptake by the roots, which in turn reduces the absorption and affects the transport of nitrate from roots to shoots, by inhibiting the nitrate reductase activity in the shoots.21 Cd-induced deficiencies in Fe(II) have also been reported to severely affect photosynthesis.22 In our study we have used core–shell CdS:Mn/ZnS where the CdS crystalline core is protected by a wide band-gap ZnS semiconductor material. The ZnS shell not only stabilizes the CdS core from oxidation but minimizes release of free Cd ions. Furthermore, the Qdot surface capping agent, NAC, is expected to passivate any Cd toxicity through scavenging free radicals generated by the Qdot core. Enhancement in seed germination and seedling growth for NAC-Qdot treated seeds below 40 μg mL−1 is therefore attributed to the combined effect of NAC and ZnS. Photographs of NAC-Qdot treated and untreated control seeds were illuminated using a handheld 366 nm multi-band UV excitation source (Fig. 3c, d). A bright glow was observed from radicals of NAC-Qdot treated seeds in comparison to the radicals of the control seeds, which we believe to be due to the combined emission of Qdot and plant tissue auto-fluorescence. This indicates the uptake of Qdots by the seed and their systemic distribution in plant cells. Some radicals however appeared brighter than others, which could be due to non-uniform distribution of Qdots.
3.3. Raman spectroscopy, AFM and fluorescence microscopic studies
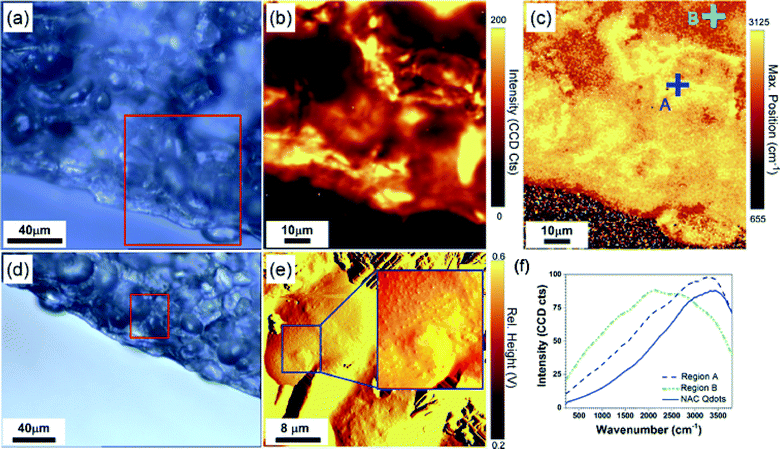
For these measurements, slices of the treated seed (about 5 mm diameter) were sectioned ~3–4 mm beneath the seed surface and then mounted on a microscope slide for imaging. To explore the NAC-Qdot content with higher spatial resolution, we performed Raman spectroscopy mapping and AFM. The Raman signatures in the epidermis and inner region of the seed suggest a non-homogeneous distribution of the nanoparticles in the tissue of the treated seeds (Fig. 4). We first acquired an optical image of the seed (Fig. 4a), and determined the region of interest (red box in Fig. 4a) for analysis with the confocal Raman system (Fig. 4b, c). Due to the fluorescence of the samples resulting from the excitation wavelength of 532 nm of the Raman system, we used the variations in the fluorescence background of the Raman spectra to probe the changes in nanoparticle distribution across the section of the seed. Raman spectra were collected every 500 nm to cover the area of the selected region (Fig. 4b, c). The resulting data set displayed the position of the fluorescence peak at each point in Fig. 4c. As can be seen in the scale bar, the position of the maximum peak varied from 655 cm−1 to 3125 cm−1. For comparison, we present the characteristic signature of the pure NAC-Qdot solution (solid blue curve) in Fig. 4f. For the NAC-Qdot solution, the fluorescence signature presented a characteristic peak around 3125 cm−1, which was not observed in the non-treated seed cross section. Hence the map presented in Fig. 4c is a good indicator of the presence of NAC-Qdot in the tissue. To illustrate this, we presented two representative Raman spectra in Fig. 4f. Locations labeled A and B in Fig. 4c correspond to maximum peaks at 3125 cm−1 and 200 cm−1, respectively. As shown in Fig. 4f, the spectrum acquired at point A clearly resembles the signature of the NAC-Qdots. Thus, a position of the peak maximum around ~3100 cm−1 is likely linked to the presence of NAC-Qdots, as the fluorescence background of untreated pea tissue is similar to the one obtained in point B (Fig. 4c), hence suggesting the presence of NAC-Qdots in the seed, with high concentration in the bright (yellow) regions of the map presented in Fig. 4c. Despite the important chemical information provided by Raman spectroscopy in Fig. 4b, c, and f, the need for nanoscale imaging tools to precisely determine the organization of the nanoparticles in the plant tissue clearly emerges. Imaging plant tissues with high resolution is quite challenging. Thus, we performed AFM imaging at a location where a peak around 3100 cm−1 was found in the Raman spectrum. The resulting AFM image (Fig. 4e) revealed nanoparticle-like structures in the tissue. Such nanoscale structures were not present in other areas of the section. However, AFM only provides topographical information, and the composition of the features can only be accessed by Raman spectroscopy with lower spatial resolution. This constitutes a known limitation of current nanometrology tools, and will be the subject of further studies. Nonetheless, given that the Raman spectra indicate the presence of nanoparticles and that the AFM images clearly show nanoscale particles at the same location, our data suggest that the particles indeed correspond to the NAC-Qdots. The image shows possible signs of aggregation (inset in Fig. 4e) surrounded with well dispersed nanoparticles inside the tissue. Beyond confirmation of the presence of the Qdot in the section, we used confocal and phase contrast microscopy to localize NAC-Qdot in the treated seeds. Confocal fluorescence images were acquired ~1 mm beneath the cotyledon surface. A stack of 20 images was collected in the Z-direction of the sectioned tissue to localize the presence of any NAC-Qdot in the surroundings of the cells. Phase contrast images (Fig. 5) in transmission (i) and fluorescence (ii) mode are shown. The presence of NAC-Qdots in the seeds is shown by overlay of the phase contrast images with false blue-color transmission background and red color fluorescence of the NAC-Qdots (Fig. 5iii). Interestingly, Qdots were mostly populated in the intracellular regions and the cell walls (red colored areas), and no sign of fluorescence could be found inside the cells. The same cotyledon section was then imaged using the confocal microscope to find the location of NAC-Qdots. An overlay of confocal images (DIC and fluorescence images) is also shown (Fig. 5iv). The DIC images (Fig. 5) represent a layer about 10 μm below the top surface of the sectioned tissue sample. The fluorescence signature of NAC-Qdot in the intracellular regions is evident as shown by false red color. Thus, this multi-prong microscopy study suggests that NAC-Qdots, with sizes around 5 nm, are small enough to penetrate the seed coat and reach the pith (i.e. about 2–3 mm beneath the cotyledon surface), while remaining in the intracellular region of the tissue. These findings suggest that NAC-Qdot migration to other plant parts including stems and leaves during the growth of the plant should be considered.3.4. Physiological impact of NAC-Qdot on plants
Finally, to evaluate the impact of NAC-Qdot dosage during seed germination on seedling growth, we have measured root and shoot lengths of pea (P. sativum) seedlings. Seeds were exposed to different treatments for 48 hours followed by sowing in cups for 15 days. An average length of root and shoot at day 15 versus NAC-Qdot concentrations ranging from 0 to 100 μg mL−1 has been plotted to represent the changes occurring at different treatments (Fig. 6a). Several interesting behaviors could be observed from these plots. First, NAC-Qdot dosage affects the growth of shoots and roots non-linearly, where a solution of NAC-Qdot with concentration above 5 μg mL−1 can induce shoot and root growth. The roots exhibited a significant increase in length from about 19 cm long in control plants to an average of 22 cm for treatment concentration ranging from 5 to 40 μg mL−1. However no significant change in length was observed in root and shoot lengths for treatment concentrations between 10 μg mL−1 and 40 μg mL−1. Above the 40 μg mL−1 NAC-Qdot concentration, a close to linear decrease in shoot and root length was observed. Interestingly, root growth was sharply inhibited (about 5 cm decrease per additional 20 μg mL−1) in comparison to shoot growth (about 1 cm decrease per additional 20 μg mL−1) above the 40 μg mL−1 NAC-Qdot concentration threshold. The effect on root and shoot length was statistically significant at P < 0.05 (Table S1†). While the longest root (22.8 cm) was observed for the 20 μg mL−1 group, the shortest root (9.1 cm) was recorded at 100 μg mL−1 treatment. Shoot length of the three pea seedlings ranged from 27.9 to 34.1 cm and the variety, concentration and interactions of variety–concentration were found to be significant at 0.1% confidence level (Table S1†). Similarly the shortest shoot lengths were seen at a 100 μg mL−1 concentration (27.9 cm), on the other hand the highest shoot length was obtained at 10 μg ml−1 (34.1 cm) NAC-Qdot (Fig. 6a). Thus, the initial boost in seedling growth reflected in the increase in root and shoot length is attributed to NAC-Qdot treatment followed by antioxidant enzyme production to suppress the ROS activity. It can then be inferred that antioxidant enzymes along with plant growth regulators involved in cell division and cell elongation, such as cytokinins and gibberellins, facilitate plant growth.20 However, the difference in effect on the shoot and roots suggests a different distribution of the NAC-Qdot throughout the plant, and a higher impact of the treatment on the root activity. We surmise that the release of NAC and Zn from the shell of NAC-Qdot over time could be another contributing factor to the nutrient mediated root and shoot growth. Between 10 μg mL−1 and 40 μg mL−1 concentration, we believe that the NAC and Zn induced increase in the growth process is partially countered by the Cd ion induced phytotoxicity. Above 40 μg mL−1 NAC-Qdot concentration, Cd ion induced phytotoxicity becomes prominent causing overall decline in seedling growth. At the highest NAC-Qdot concentration, the possibility of particulate aggregation and deposition in the plant vascular system could also contribute to seedling growth inhibition. The chlorophyll content in plant leaves is one of the contributing factors to assess the level of phytotoxicity. Therefore, in addition to the shoot and root length, the effect of increasing NAC-Qdot concentration on the chlorophyll content of snow pea (P. sativum) seedlings was considered. The average chlorophyll content (chlorophyll a, chlorophyll b and total chlorophyll) with respect to NAC-Qdot is also plotted at concentrations ranging from 0 to 100 μg mL−1 (Fig. 6b). A significant increase (P < 0.05) in chlorophyll a and total chlorophyll (a + b) content was observed in seedlings treated with solution concentration ranging from 5 to 40 μg mL−1 (Table S1† & Fig. 6b). An increase in chlorophyll b content between 5 and 10 μg mL−1 was also measured. Chlorophyll a and total chlorophyll (a + b) contents were highest at 20 μg mL−1 NAC-Qdot concentration and the estimated values were 1.058 and 1.357 mg per gram fresh weight (mg per g FW), respectively. The lowest value of chlorophyll a content (0.551 mg per g FW) was found at 100 μg mL−1 while the lowest value of total chlorophyll (a + b) content (0.857 mg per g FW) was at 80 μg mL−1 NAC-Qdot concentration. Chlorophyll b content was highest (0.384 mg per g FW) at 10 μg mL−1 and lowest (0.290 mg per g FW) after treatment with 2 μg mL−1 NAC-Qdot concentration. As chlorophyll a and chlorophyll b are the pigments present inside chloroplast and help in absorbing light energy to synthesize glucose, an increase in chlorophyll content contributes to an enrichment of the photosynthetic rate, hence potentially leading to an increased amount of nutrient synthesis. In the present study, NAC-Qdot dosage above 40 μg mL−1 exhibited a negative impact on chlorophyll a and total chlorophyll (a + b) content, indicating a probable disruption of the normal photosynthetic process. This is again attributed to Cd ion related phytotoxicity as described previously. | ||
| Fig. 6 Comparison of (a) root length and shoot length; (b) chlorophyll a, chlorophyll b and total chlorophyll content at different levels of NAC-Qdot based on Duncan's test P ≤ 0.05. | ||
4. Conclusion
We investigated, for the first time, the impact of ultra-small size (~5 nm) NAC coated CdS:Mn/ZnS Qdot on seed germination and seedling growth on a snow pea (P. sativum) model system. The fluorescence of NAC-Qdot constituted an important marker to track the dispersion of the particulates in the seeds during germination, and for higher resolution microscopy. Upon 48 hours of exposure of seeds to NAC-Qdots, it was found that the Qdots had translocated a few cell layers beneath the seed surface as revealed from fluorescence and Raman imaging studies. The NAC-Qdots were found to accumulate in the inter-cellular region of the plant tissues. Interestingly, our results show great influence of the NAC-Qdot dosage on seed germination and seedling growth patterns. Surface coating agents, NAC and Zn ions (of the Qdot ZnS shell), appeared to passivate Cd ion related phytotoxicity (of the Qdot core) for NAC-Qdot concentration up to 40 μg mL−1, which was identified as the threshold above which toxicity mechanisms take place in the snow pea plants. Above this threshold, drastic reduction in seedling growth was observed as well as negative impact on total chlorophyll (a + b) content. The molecular mechanisms of the dose dependence of NAC-Qdot will be the focus of further studies.Acknowledgements
Authors acknowledge in kind support from Mona Doshi and Dr. Andre Gesquiere (UCF NanoScience Technology Center, Department of Chemistry and CREOL College of Optics and Photonics) for fluorescence and phase contrast microscopy. Authors acknowledge in kind support from Dr. Helge Heinrich (UCF Department of Physics) with HRTEM. Authors also acknowledge UCF-Advanced Materials Processing and Analysis Center (AMPAC)-Materials Characterization Facility (MCF) for the electron microscopy facility. This research is partially supported by the National Science Foundation (NSF-CBET grant number 1159500) and the Citrus Research and Development Foundation, Inc. (project # 907).References
- M. J. McCall, Nat. Nanotechnol., 2011, 6, 613–614 CrossRef CAS PubMed.
- Y. X. Chen, Y. F. He, Y. M. Luo, Y. L. Yu, Q. Lin and M. H. Wong, Chemosphere, 2003, 50, 789–793 CrossRef CAS.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Plant Sci., 2010, 179, 154–163 CrossRef CAS PubMed.
- T. Yadav, A. A. Mungray and A. K. Mungray, Rev. Environ. Contam. Toxicol., 2014, 230, 83–110 Search PubMed.
- T. N. V. K. V. Prasad, P. Sudhakar, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. R. Reddy, T. S. Sreeprasad, P. R. Sajanlal and T. Pradeep, J. Plant Nutr., 2012, 35, 905–927 CrossRef CAS.
- D. H. Lin and B. S. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS PubMed.
- J. H. Priester, Y. Ge, R. E. Mielke, A. M. Horst, S. C. Moritz, K. Espinosa, J. Gelb, S. L. Walker, R. M. Nisbet, Y.-J. An, J. P. Schimel, R. G. Palmer, J. A. Hernandez-Viezcas, L. Zhao, J. L. Gardea-Torresdey and P. A. Holden, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E2451–E2456 CrossRef CAS PubMed.
- L. Yin, B. P. Colman, B. M. McGill, J. P. Wright and E. S. Bernhardt, PLoS One, 2012, 7, e47674 CAS.
- M. V. Khodakovskaya, K. de Silva, A. S. Biris, E. Dervishi and H. Villagarcia, ACS Nano, 2012, 6, 2128–2135 CrossRef CAS PubMed.
- M. V. Khodakovskaya, K. de Silva, D. A. Nedosekin, E. Dervishi, A. S. Biris, E. V. Shashkov, E. I. Galanzha and V. P. Zharov, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1028–1033 CrossRef CAS PubMed.
- P. Thuesombat, S. Hannongbua, S. Akasit and S. Chadchawan, Ecotoxicol. Environ. Saf., 2014, 104, 302–309 CrossRef CAS PubMed.
- Z. W. Rang, S. V. K. Jagadish, Q. M. Zhou, P. Q. Craufurd and S. Heuer, Environ. Exp. Bot., 2011, 70, 58–65 CrossRef PubMed.
- S. Bandyopadhyay, J. R. Peralta-Videa, G. Plascencia-Villa, M. José-Yacamán and J. L. Gardea-Torresdey, J. Hazard. Mater., 2012, 241–242, 379–386 CrossRef CAS PubMed.
- S. Pathak, E. Cao, M. C. Davidson, S. Jin and G. A. Silva, J. Neurosci., 2006, 26, 1893–1895 CrossRef CAS PubMed.
- D. Zhao, Z. He, W. H. Chan and M. M. F. Choi, J. Phys. Chem. C, 2009, 113, 1293–1300 CAS.
- S. Das, P. Krishnan and M. Nayak, Exp. Agric., 2013, 49, 53–73 CrossRef.
- F. Yang, F. Hong, W. You, C. Liu, F. Gao, C. Wu and P. Yang, Biol. Trace Elem. Res., 2006, 110, 179–190 CrossRef CAS.
- G. Barba-Espin, P. Diaz-Vivancos and J. Clemente-Moreno, Plant, Cell Environ., 2010, 33, 981–994 CrossRef CAS PubMed.
- F. Hong, J. Zhou, C. Liu, F. Yang, C. Wu, L. Zheng and P. Yang, Biol. Trace Elem. Res., 2005, 105, 269–279 CrossRef CAS.
- D. Stampoulis, S. K. Sinha and J. C. White, Environ. Sci. Technol., 2009, 43, 9473–9479 CrossRef CAS PubMed.
- S. Santra, H. S. Yang, P. H. Holloway, J. T. Stanley and R. A. Mericle, J. Am. Chem. Soc., 2005, 127, 1656–1657 CrossRef CAS PubMed.
- S. Santra, H. Yang, J. T. Stanley, P. H. Holloway, B. M. Moudgil, G. Walter and R. A. Mericle, Chem. Commun., 2005, 3144–3146 RSC.
- D. I. Arnon, Plant Physiol., 1949, 24, 1–15 CrossRef CAS PubMed.
- G. Mackinney, J. Biol. Chem., 1941, 140, 315–322 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4en00198b |
| This journal is © The Royal Society of Chemistry 2015 |