Nitrate behaviors and source apportionment in an aquatic system from a watershed with intensive agricultural activities
Lu
Lu
a,
Hongguang
Cheng
*a,
Xiao
Pu
b,
Xuelian
Liu
a and
Qianding
Cheng
a
aSchool of Environment, Beijing Normal University, No. 19 Xinjiekouwai Street, Beijing, 100875, China. E-mail: chg@bnu.edu.cn; Fax: +86-10-59893086; Tel: +86-10-59893086
bState Key Laboratory of Remote Sensing Science, School of Geography, Beijing Normal University, Beijing, China
First published on 30th October 2014
Abstract
Nitrate pollution in aquatic systems caused by intensive agricultural activities is a serious problem in the Sanjiang Plain. In this study, a dual isotope approach (δ15N–NO3− and δ18O–NO3−) was employed to identify potential nitrate sources (atmospheric deposition, AD; NO3− derived from soil organic matter nitrification, NS; NO3− derived from chemical fertilizer nitrification, NF; and manure and sewage, M&S) and transformation processes occurring in the Abujiao River watershed located in the Sanjiang Plain. The Bayesian model (stable isotope analysis in R, SIAR) was utilized to apportion the contribution of the potential sources. In this watershed, the nitrate concentrations in the surface water were low (mean ± SD = 1.15 ± 0.84 mg L−1), and were greatly influenced by precipitation and land use conditions during the two sampling periods (the high flow period, September; the low flow period, November). On the contrary, in the ground water, high NO3− concentrations were observed (7.84 ± 5.83 mg L−1) and no significant temporal variation in NO3− was found during the sampling periods. The sampled water δ18O–NO3− values suggest that the nitrification process was not the main N cycling process, because most of the measured δ18O–NO3− values were above the expected δ18O–NO3− from nitrification throughout the sampling periods. Both the chemical and isotopic characteristics indicated that the signs of de-nitrification were absent in the surface water. However, significant de-nitrification processes were observed in the ground water for all sample periods. Results from the SIAR model showed that source contributions differed significantly during the two sampling periods. During the high flow period, chemical fertilizers and soil N fertilizer equally contributed to the major sources of nitrate in the surface water. In contrast, manure and sewage sources dominated the source contribution during the low flow period (November). This study suggested that with the assessment of the behaviors and sources of NO3−, effective nitrate reduction strategies and better management practices can be implemented to protect water quality.
Environmental impactNitrate pollution in aquatic systems caused by intensive agricultural activities is becoming an increasingly serious problem in the Sanjiang Plain, north eastern China. However, to control the main contamination remains challenging, partly because information on the nitrate behaviors and sources in aquatic systems is still limited. In this study, the transformation and origin features of nitrate in the aquatic systems of the Abujiao River watershed, Sanjiang Plain, are discussed. Water mixing and de-nitrification are recognized as the two major processes which cause a decrease in the nitrate in surface water and groundwater in this watershed. Chemical fertilizers and manure and sewage dominate the nitrate source contribution in the surface water during the high and low flow periods, respectively. |
1 Introduction
The Sanjiang Plain in northeastern China, which is one of the most important food bases in China, was once covered by wetlands and forests with precious black soil and abundant water resources. In order to address the food crisis, the Sanjiang Plain has experienced four large-scale agricultural development activities since the 1950s, and lots of wetland and forest areas have been exploited as crop land (which increased from 7870 km2 to 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 km2).1 Intensive agricultural activities have caused eco-hydrological changes, soil structure and function degradation, and a serious loss of black soil resources (decreasing from 60–100 cm to 16–72 cm), coupled with serious non-point nitrogen pollution in aquatic systems.2
330 km2).1 Intensive agricultural activities have caused eco-hydrological changes, soil structure and function degradation, and a serious loss of black soil resources (decreasing from 60–100 cm to 16–72 cm), coupled with serious non-point nitrogen pollution in aquatic systems.2
The soil structure and function degradation and serious non-point nitrogen pollution in the Sanjiang Plain have had a significant influence on food security and the biogeochemical cycles in the ecosystem. Researchers have reported that the use of fertilizers on farmland is the major non-point source contributing to the nitrate pollution of aquatic ecosystems.3 The land-use and soil property variation were suggested as the most sensitive factors to control the non-point nitrogen pollution output.4 However, those studies were mainly based on the combined interpretation of chemical data with statistical techniques, GIS and mathematical models, and the limited information on the nitrate sources and nitrogen (N) cycling process often caused a significant deficit in calculating the balance between the nitrogen loading and controlling the nitrate contamination in this high latitude watershed.5 Moreover, although some research on the source and fate of nitrate had been conducted in the other agricultural areas of China,6–8 the special black soil properties as well as seasonal freezing and thawing processes in the Sanjiang Plain inevitably resulted in a significantly different N cycling process.9 Meanwhile, due to agricultural activities, the original landscape and ecological structure have been changed, and single natural ecosystems were replaced by a hybrid ecosystem which was more complex for the identification of the sources and behaviors of nitrate.10 Therefore, the identification of how nitrate from various sources moves through the landscapes to water and which N cycling processes alter these sources during transport are critical in prioritizing effective nitrate reduction strategies.
The use of the isotope approach to identify potential pollutant sources is the current research trend and has been reported to be most diagnostic.11 Numerous researchers have documented that the isotopic compositions of nitrate (N and O) act as a useful tool to identify nitrate sources and to track the processes of the N cycle in aquatic ecosystems.12–14 δ15N–NO3− values have proven more useful for distinguishing NO3− derived from ammonium fertilizer, soil organic matter, and animal manure in aquatic ecosystems,15 whereas δ18O–NO3− values have proven more useful for identifying NO3− derived from nitrate-bearing fertilizer and atmospheric deposition of NO3−.15,16 The N cycling processes often involve coupled fractionation; for example, de-nitrification often results in a net change rate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in δ18O–NO3−
2 in δ18O–NO3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ15N–NO3− of the residual pool in water, suggesting that N fractionation is twice the amount of oxygen fractionation in many environments.12,17 The model of stable isotope analysis in R (SIAR) has been used successfully to predict the quantities of the relative contributions of different nitrate (NO3−) sources with uncertainties in the isotope fractionation.8,18,19 Additionally, hydro-chemical characteristics can provide some conventional information to help distinguish nitrate sources and N cycling processes.20 Thus, a combination of nitrate isotopes, SIAR, and hydro-chemical information may provide more detailed information regarding the sources and potential N cycling processes in intensive agricultural activities that impact on watersheds.
δ15N–NO3− of the residual pool in water, suggesting that N fractionation is twice the amount of oxygen fractionation in many environments.12,17 The model of stable isotope analysis in R (SIAR) has been used successfully to predict the quantities of the relative contributions of different nitrate (NO3−) sources with uncertainties in the isotope fractionation.8,18,19 Additionally, hydro-chemical characteristics can provide some conventional information to help distinguish nitrate sources and N cycling processes.20 Thus, a combination of nitrate isotopes, SIAR, and hydro-chemical information may provide more detailed information regarding the sources and potential N cycling processes in intensive agricultural activities that impact on watersheds.
In this study, we researched the nitrate sources and N cycles in aquatic systems from a watershed with intensive agricultural activities and mixed land use types in the Sanjiang Plain. By analyzing hydro-chemical characteristics (Cl−, NO3−, NH4+, and DO) and dual nitrate isotopic signatures, the potential nitrate sources and N cycling processes were examined systematically during the different sampling periods. The SIAR model was used to quantitatively apportion the contribution of potential sources. The objectives of this study were to (1) determine the factors which best explain the variability and isotope composition of nitrate in aquatic ecosystems, (2) identify the main N cycling processes in different water bodies and (3) apply the source apportionment model SIAR to estimate the proportion of each source of NO3− enrichment.
2 Materials and methods
2.1. Description of the study area
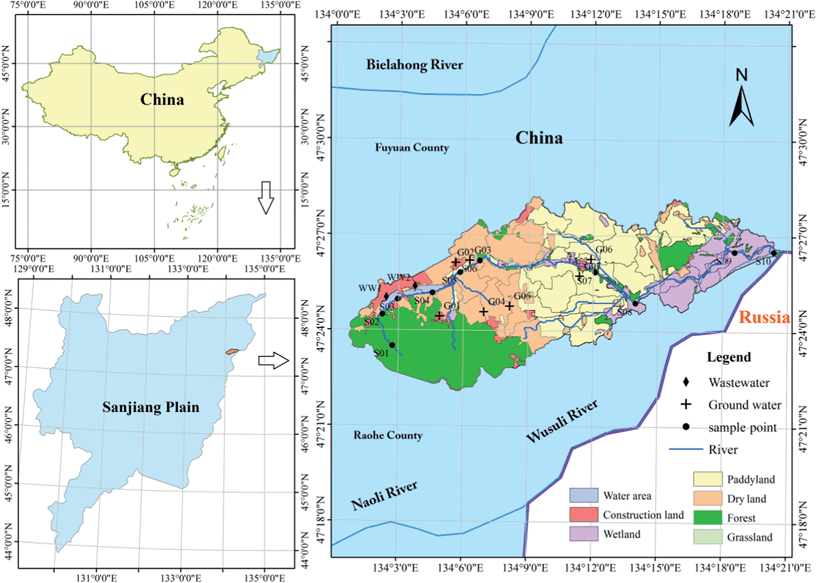
Our study focused on an intensive agriculture-impacted watershed (the Abujiao River catchment, latitude N 47°22′11′′–47°28′14′′, longitude E 134°0′45′′–134°20′47′′) that has mixed land use types and is a typical representative of the Sanjiang Plain of Northeast China (Fig. 1). The Abujiao River flows eastward and finally flows into the Wusuli River, with an elevation decline from 129 m to 38 m. This region is typically characterized as having temperate and continental climates, with a cold and long winter and a warm summer. In early spring and late winter, the temperature is low, with a lowest monthly average temperature of approximately −19.27 °C. Summers are short and muggy and the annual average temperature is 2.94 °C. After October, temperatures fall down to below 0 °C, with no rainfall occurring, and the ground is gradually covered by snow. The air temperature often rises above 0 °C in April, and the frozen topsoil begins to thaw from the surface downwards in late April.4 These freeze–thaw cycles have a strong influence on soil erosion in April. The average annual rainfall is 583.18 mm, most of which falls in summer (from July to September). According to the Genetic Soil Classification of China (GSCC), the primary soil types in this region include dark brown soil, bleached Baijiang soil, chernozem soil, meadow soil, and black soil.The Abujiao River Basin is a typical intensive agricultural activity-impacted watershed and its catchments cover 142.5 km2, with 56.12% for agriculture, 11.78% for wetland, 3.97% for construction land, 25.41% for forest, and 2.72% for other land use types (Fig. 1). Rice paddy (Oryza satiua L.) is the dominant crop (accounting for 57% of the total cropping area) in the river basin, which is planted in early May and harvested in October. The extensive application of fertilizer takes place from the end of April to July. Fertilizer N is almost all in the reduced form, i.e., urea (36.67%) and ammonium (63.33%), NH4HCO3 and NH4Cl. Nitrogen from manure (human and animal wastes) and sewage is directly poured into the river system, which makes an important contribution to the N input into the river basin.
2.2. Samples and analysis
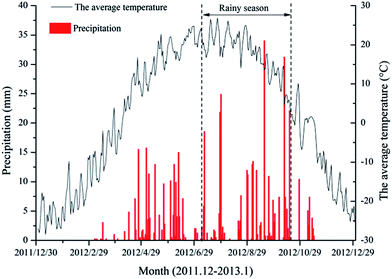
According to the information on temperature and rainfall in the study area (Fig. 2), we found a significant difference between September and November. Considering the convenience and safety of sampling campaigns, we selected those months as the study periods. In order to address the seasonal variability of the distribution and isotope composition of nitrate and the underlying causes, we investigated nitrate from surface water (two sampling campaigns: 18–20/9/2012; 7–9/11/2012) as well as ground water (three sampling campaigns: 19/9/2012; 28/9/2012; 10/11/2012) during the high flow period in September 2012 and the low flow period in November 2012 (Fig. 2). Water samples were collected from upstream to downstream of the Abujiao River along the main stream, comprising 20 surface water samples and 13 groundwater samples (all samples from wells for drinking or irrigation) (Fig. 1). Detailed information on the ground water samples can be found in Table 1.| Sample | Well depth (m) | Well purpose | Land use | Sampling times |
|---|---|---|---|---|
| G01 | 25 | Drinking | Urban | Three times (19/9/2012; 28/9/2012; 10/11/2012) |
| G02 | 78 | Drinking | Urban | Three times (19/9/2012; 28/9/2012; 10/11/2012) |
| G03 | 32 | Irrigation | Corn | Two times (19/9/2012; 10/11/2012) |
| G04 | 106 | Irrigation | Corn | One time (19/9/2012) |
| G05 | 30 | Irrigation | Soybean | Two times (19/9/2012; 10/11/2012) |
| G06 | 27 | Irrigation | Rice | Two times (19/9/2012; 10/11/2012) |
Wastewater, rainwater, soil and synthetic fertilizers were also sampled because they represented the primary sources of NO3− in the area. Untreated waste water samples (n = 6) were collected at the two urban sewage outfalls (Fig. 1) and three sampling campaigns were conducted (the sampling times were the same as for the groundwater sampling campaigns). All rain water samples (n = 4) were collected at an agricultural experiment station located in the studied watershed in September 2012 (from 9/9/2012 to 30/9/2012). Top soil samples (0–10 cm, n = 12) of different land use types (including 3 forest soils, 3 wetland soils, 2 unfertilized soybean soils, 2 unfertilized corn soils and 2 unfertilized rice soils) were obtained in the studied watershed in September and November 2012. Samples of urea and ammonium (n = 6), which were used widely in this watershed, were collected at the local market. Soil and fertilizer samples were preserved in polyvinyl chloride bags and frozen (−20 °C) until required for further analyses.
At each water sample site, pH, temperature (T) and dissolved oxygen (DO) were measured using portable equipment (YSI, USA). Then, three replicates of surface water samples at 0–20 cm depth at the center of the stream were collected using Teflon containers. All sampling equipment was pre-cleaned with non-phosphate detergent and rinsed thoroughly with deionized water. The collected water samples were filtered in situ through 0.45 μm cellulose-acetate filter paper for chemical and isotope analysis.
In the laboratory, anion concentrations in the water samples, including NH4+, NO3−, and Cl−, were determined using ion chromatography (Dionex, DX-600 and ICS-2100, USA), with a precision of 5%. Dissolved nitrate was converted to solid AgNO3 according to a reported approach.21 AgNO3 was then analyzed for δ15N using a DELTA plus XL mass spectrometer connected to a CE EA1112 C/N/Sanalyzer, and for δ18O using a MAT 253 mass spectrometer connected to a high-temperature conversion elemental analyzer. International standard IAEA-N3 was used for δ15N and δ18O calibration. The reproducibility of duplicate analyses was <0.9‰ for δ18O (averaging ±0.3‰), and <0.3‰ for δ15N (averaging ±0.2‰).
In the laboratory, plant debris and large stones were picked out from each soil sample, which was then ground, sieved and screened using 100 mesh before being freeze-dried again for use. A total of 0.5 g of soil samples was taken. Inorganic carbonates were removed using 0.5 mol L−1 HCl for acidification, and samples were then rinsed with deionized water until the filtrate was neutral. Silver nitrate solution was used to detect the presence or absence of Cl− residues, and then samples were freeze-dried and inorganic carbon was removed. All soil sample and fertilizer sample analysis was conducted in the Test Center of the Chinese Academy of Forestry, Key Laboratory of Stable Isotope, using a mass spectrometer (MAT253). The analytical precision of δ15N is ±0.2‰.
2.3. Calculations and statistical analysis
| Expected δ18Onitrate = 2/3δ18Owater + 1/3δ18OO2 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the different environments.16,18,23 However, previous studies have proved that under the limited data conditions, this theoretical estimation might offer a representative indicator for the local δ18O of nitrate oxidized from reduced N.7,13,24
1 in the different environments.16,18,23 However, previous studies have proved that under the limited data conditions, this theoretical estimation might offer a representative indicator for the local δ18O of nitrate oxidized from reduced N.7,13,24
δR = δR0 + ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(f) ln(f) | (2) |
where δR is the δ15N or δ18O value of the reactant nitrate at time t, δR0 is the initial δ15N or δ18O value of the nitrate, f is the remaining fraction of the nitrate, which equal to the remaining substrate concentration divided by the initial substrate concentration, and ε is the enrichment factor.
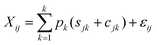 | (3) |
| Sjk ∼ N(μjk,ω2jk) | (4) |
| Cjk ∼ N(λjk,τ2jk) | (5) |
| εij ∼ N(0,σ2j) | (6) |
3 Results and discussion
3.1. The variation of dissolved nitrogen (DIN) and isotopic composition in surface water
The physicochemical properties and isotopic composition of nitrate of all surface water samples are depicted in Fig. 4. There were considerable differences in the N species and isotopic compositions in the surface water among various land use types. At urban sampling sites, the highest NH4+, Cl−, δ15N–NO3− and δ18O–NO3− levels in the surface water samples were observed (Fig. 4). These relatively high values appeared similar to the values observed for pollution of sewage origin, which indicates that untreated wastewater inputs were the principal factor impacting these sites. However, the isotopic composition of nitrate did not show a significant difference among the other sites (i.e., forest and wetland) and the lower δ15N–NO3− values of nitrate obtained at those sites were intermediate between the values measured for soil and NF (Fig. 6). For agricultural sampling sites, the highest value of NO3− (Fig. 4C) was observed during the high flow period. Meanwhile, the Cl− decreased dramatically from S04 (urban sampling sites) to S05 (agricultural sampling sites) (Fig. 4A). These results suggested that the loading of nitrate might be associated with agricultural activities. The spatial variation of nitrate contents demonstrated that there was a significant nutrient contribution from human activities and land use was a major influencing factor for nitrate concentration and isotopic composition in this watershed.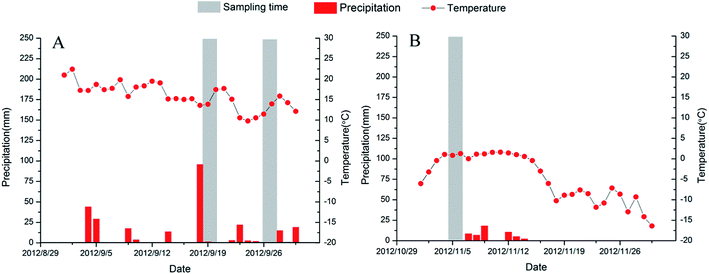 | ||
| Fig. 3 Records of daily precipitation and temperature at the Abujiao River watershed for the different sampling periods. | ||
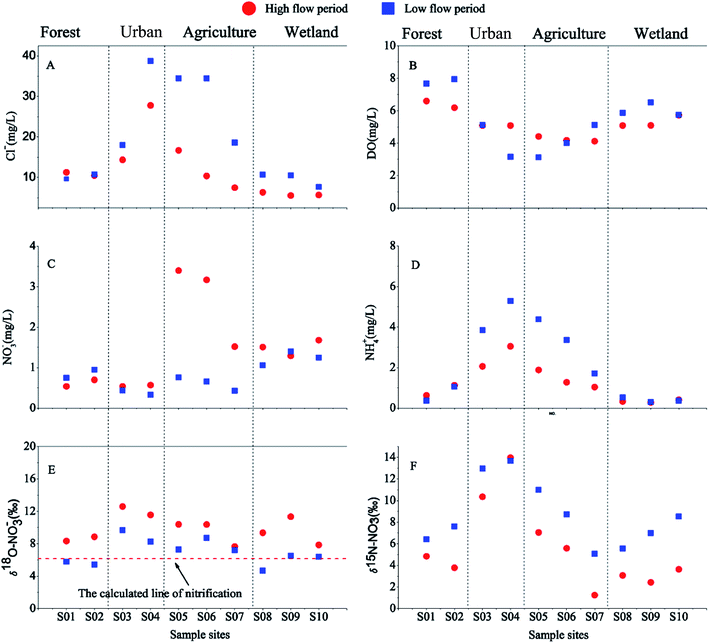 | ||
| Fig. 4 Varying trends of (A) Cl−, (B) DO, (C) NO3− (D) NH4+, (E) δ18O–NO3− and (F) δ15N–NO3− along the Abujiao river during the two sampling periods. | ||
A significant temporal variation in the isotopic composition of the surface water was also observed (Fig. 4). Compared with the high flow period, the samples of surface water were characterized by relatively high δ15N–NO3− values (increasing from 5.59 ± 3.91‰ to 8.65 ± 2.99‰) and low δ18O–NO3− values (decreasing from 9.83 ± 1.69‰ to 7.00 ± 1.56‰) during the low flow period. This finding could be attributed to the significant variation in precipitation between the samples collected during the two periods. During the two sampling periods, precipitation and temperature conditions were significantly different (Fig. 3). The high volumes of precipitation with low δ15N–NO3− and high δ18O–NO3− values in September might decrease the δ15N–NO3− and increase the δ18O–NO3− values in the river.28 Meanwhile, at the agricultural sites, the NO3− level changed dramatically between the two sampling periods. During the high flow period, the highest level of NO3− with a low isotopic composition was observed at sampling site S05 which was located in the agricultural area. These unusually high nitrate concentrations reflected that the NO3− might be mainly derived from chemical fertilizers and soil N, which was controlled by precipitation.29 In contrast, during the low flow period, the effects of precipitation were reduced, because there was little supplemental water (or material) from crop land. Simultaneously, sewage waste from up stream (the urban sites) had a serious impact on the sites in the agricultural area, which resulted in the NH4+ dominating the DIN with relatively high Cl− and δ15N–NO3−. These results indicate that precipitation may be another important factor that affects DIN and nitrate isotopic composition in surface water, especially in the rainy season.
3.2. The variation of DIN and isotopic composition in ground water
Compared with the surface water samples, in the ground water samples there were significant differences in the physicochemical properties and nitrate stable isotope ratios (Table 2). The NO3− in the ground water (7.84 ± 5.83 mg L−1, mean ± SD) was the dominant species (>95%) of the DIN, and the concentration was much higher than that in surface water (1.15 ± 0.84 mg L−1). Meanwhile, the δ15N–NO3− and δ18O–NO3− values in the ground water (18.95 ± 6.84‰ and 15.90 ± 5.62‰, respectively) were higher than those in the surface water (7.12 ± 3.74‰ and 8.42 ± 2.15‰, respectively). In contrast, NH4+ (0.13 ± 0.18 mg L−1) and DO (0.7 ± 0.2 mg L−1) were observed in ground water at much lower concentrations than those in surface water (NH4+ was 1.67 ± 1.53 mg L−1 and DO was 5.3 ± 1.3 mg L−1).| Samples | Variable | ||||||
|---|---|---|---|---|---|---|---|
| DO mg L−1 | Cl− mg L−1 | NH4+ mg L−1 | NO3− mg L−1 | δ15N–NO3− ‰ | δ18O–NO3− ‰ | ||
| SW | (Mean ± SD) | 5.3 ± 1.3 | 15.48 ± 10.29 | 1.67 ± 1.53 | 1.15 ± 0.84 | 7.12 ± 3.74 | 8.42 ± 2.15 |
| GW | (Mean ± SD) | 0.7 ± 0.2 | 23.21 ± 20.62 | 0.13 ± 0.18 | 7.84 ± 5.83 | 18.95 ± 6.84 | 15.90 ± 5.62 |
| Statistics | F | 132.45 | 2.00 | 12.26 | 21.14 | 38.41 | 30.08 |
| Sig. | <0.001 | P = 0.168 > 0.05 | 0.05 < P = 0.02 < 0.01 | <0.001 | <0.001 | <0.001 | |
Similar to the surface water samples, large spatial variations were in evidence for nitrate and nitrate stable isotope ratios in the ground water (Table 3). Compared to the agricultural sampling sites, higher Cl−, NO3−, δ15N–NO3− and δ18O–NO3− were observed at the urban sampling sites. The relatively high Cl−, δ15N–NO3− and δ18O–NO3− in those ground water samples suggests a higher proportion of isotopically enriched nitrate sources (i.e., manure waste sources). We also noticed that the highest δ15N–NO3− and δ18O–NO3− values were observed at sampling site G01 (26.27‰ and 22.28‰ for δ15N–NO3− and δ18O–NO3−, respectively) which are much higher than those in manure and sewage (15.32 ± 0.83‰ for δ15N–NO3− and 9.11 ± 0.93‰ for δ18O–NO3−). These results indicate that a nitrogen cycle process had happened and this process could lead to the δ15N–NO3− and δ18O–NO3− values increasing. The de-nitrification process, which was confirmed by the low DO concentrations and the linear relationship between δ15N–NO3− and δ18O–NO3− in the ground water (see Section 3.3), might explain these extraordinarily high nitrate stable isotope ratios.
| Sample site | Date | Cl− | NH4+ | NO3− | DO | δ15N–NO3− | δ18O–NO3− |
|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | mg L−1 | mg L−1 | ‰ | ‰ | ||
| a n.a. means below the detection limit. | |||||||
| G01 | 19/9/2012 | 52.48 | n.a. | 11.05 | 0.6 | 22.36 | 18.48 |
| 28/9/2012 | 47.24 | n.a. | 9.94 | 1.0 | 24.12 | 20.86 | |
| 10/11/2012 | 50.86 | n.a. | 7.43 | 0.8 | 26.27 | 22.28 | |
| G02 | 19/9/2012 | 37.08 | n.a. | 17.37 | 1.1 | 24.16 | 18.21 |
| 28/9/2012 | 37.11 | n.a. | 16.85 | 0.4 | 25.77 | 20.27 | |
| 10/11/2012 | 39.36 | n.a. | 15.98 | 0.8 | 26.47 | 21.88 | |
| G03 | 19/9/2012 | 9.75 | n.a. | 3.23 | 0.6 | 17.00 | 14.02 |
| 10/11/2012 | 6.37 | n.a. | 2.98 | 0.4 | 18.12 | 14.4 | |
| G04 | 19/9/2012 | 5.37 | 0.37 | 1.49 | 1.0 | 18.83 | 15.04 |
| G05 | 19/9/2012 | 3.62 | n.a. | 4.13 | 0.6 | 7.05 | 9.07 |
| 10/11/2012 | 3.37 | n.a. | 6.08 | 1.1 | 6.21 | 2.02 | |
| G06 | 19/9/2012 | 4.60 | 0.46 | 1.02 | 0.7 | 14.65 | 14.95 |
| 10/11/2012 | 4.50 | 0.68 | 0.97 | 0.7 | 15.34 | 15.22 | |
In contrast with the surface water samples, the effects of precipitation were not observed in the ground water, as can be seen from the following. Firstly, there were no observable variations (P > 0.05, two-way ANOVA tests) in the nitrate and nitrate stable isotope ratios in the ground water during the two sampling periods. If the ground water samples were affected by precipitation, relatively high δ18O–NO3− and low δ15N–NO3− would be observed. Furthermore, only a slight fluctuation of Cl− was observed between the different sampling periods. Due to the dilution effect of the precipitation, Cl− in the ground water would be changed dramatically under its influence. It should be noted that the de-nitrification process was also observed in those samples, and the isotopic fractionation effects might conceal the effects of precipitation.
3.3. Transport and transformation of nitrate
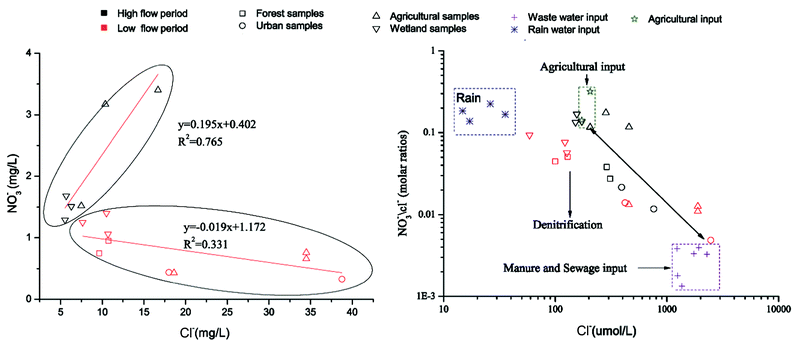 | ||
| Fig. 5 (A) Relationship between Cl− and NO3− concentrations, and (B) relationship between Cl− μmolar concentration and NO3−/Cl− molar ratio in the Abujiao River. | ||
The Cl− sources in water from various areas generally include natural sources (dissolution of minerals), agricultural chemicals (i.e., KCl), animal waste, septic effluent, and road salt. Chemical fertilizers generally have high nitrogen contents with low chloride contents. Sewage effluents had high Cl− contents and low ratios of NO3−/Cl−. Elevated nitrate contents were observed in water samples with agricultural inputs. The values of NO3−/Cl− will decrease if de-nitrification removes nitrate or if plants take up the nutrients.31 Thus, the ratio of NO3−/Cl− and the Cl− concentration can provide additional information to help distinguish the different input sources.
In this study, we did not collect the runoff and drainage from farmland directly. However, we noticed that during the high flow period, extremely high NO3− levels were found at agricultural sampling sites. Meanwhile, the Cl− decreased from sampling sites S05 to S07 (in the agricultural areas) and gradually stabilized at 5–7 mg L−1. Therefore, the min and max of NO3− at those sites and the stabilized Cl− were used as the end values of agricultural input. Some untreated wastewater samples were collected at the two sewage outfalls. These data were invoked as the end values of manure and sewage inputs. Meanwhile, some precipitation samples were also collected at this watershed, which were regarded as giving the end values of rainfall. However, it was difficult to define the accurate range of the end value of the de-nitrification process. Because the de-nitrification process could remove nitrate, which causes the values of NO3−/Cl− to decrease, the end value of the de-nitrification effect might exist under the end values of agricultural input and manure and sewage input.
The plot of NO3−/Cl− molar ratios versus Cl− concentration is illustrated in Fig. 5B. The results in Fig. 5B show the extreme variation in nitrate sources during the two sampling periods. During the high flow period, agricultural inputs heavily influenced the distribution of nitrate in the river. In contrast, during the low flow period, manure and sewage inputs and the de-nitrification process may dominate the nitrate distribution. These results also suggest that anthropogenic activities were a leading contributor to nitrate pollution in the whole watershed.
At forest and wetland sites, the values of δ18O–NO3− in the surface water were within the range of calculated values during all sampling periods. This result indicates that microbial nitrification might play a significant role at those sites. At urban sites, untreated wastewater containing high levels of NH4+ was poured into the river directly. NH4+ decreased down the river (from sampling site S05 to S07) with NO3− increasing and δ15N–NO3− decreasing. To our knowledge, two processes might explain this phenomenon in the surface water: one is the nitrification process, and the other is mixing with other sources (often containing higher NO3−, and lower NH4+ and δ15N–NO3−). The chemical behavior of chloride in natural water is conservative and its concentration can change only by mixing within the river system. Therefore, we combined the information of water chemistry (such as Cl−) and tried to explore the reduction mechanism of NH4+ at those sampling sites.
Supposing that there was only a nitrification process in the river, the Cl− concentration should not vary when the NH4+ changes. However, our data at those sites did not support this hypothesis. As can be seen in (Fig. 4A and D), the trend and extent of Cl− were consistent with those of NH4+. This indicates that some nitrate sources might flow into this river. It should be noted that those sites were located in agricultural areas, and the runoff and drainage from farmland (compared with untreated wastewater, this water often contained lower NH4+, Cl−, δ18O–NO3− and δ15N–NO3−) might flow into the surface water through some small ditches. Moreover, a similar decrease in δ18O–NO3− was found at those sites, which also suggests that the surface water might mix with runoff and drainage from farmland. Finally, nor did the values of δ18O–NO3− support the above hypothesis, because the δ18O–NO3− values at the other sites were all above the typical upper limit of δ18O–NO3− from nitrification of reduced N (Fig. 4E). Three processes (mixing with precipitation, addition of synthetic NO3− fertilizer, and de-nitrification) might produce the heavier δ18O–NO3− in water. The contribution of synthetic NO3− could be readily eliminated, because few of the synthetic N fertilizers were applied in this watershed. Precipitation might be one of the most important factors contributing to the relatively high values of δ18O–NO3− in the surface water, especially for the high flow period. Thus, these results suggest that nitrification might not play an important role in reducing NH4+ levels at those sites compared with other processes (such as dilution effects and precipitation).
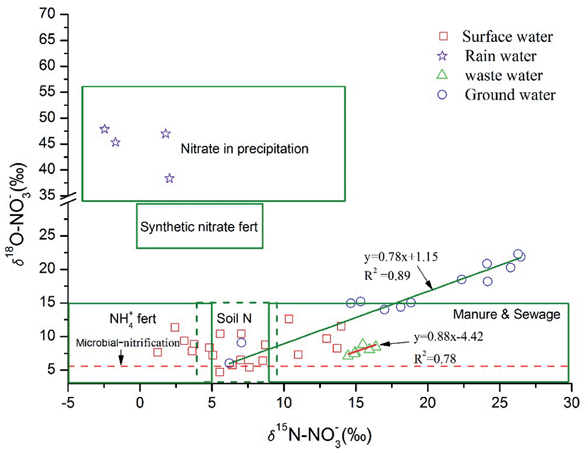 | ||
| Fig. 6 Ranges of δ18O and δ15N values for potential nitrate sources and values measured in Abujiao River. | ||
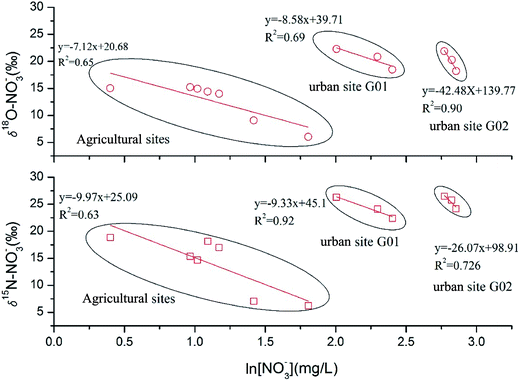
To better understand the de-nitrification process in ground water, cross plots of δ15N–NO3− and δ18O–NO3−versus ln[NO3−] were used to evaluate the de-nitrification process, which could be described by eqn (2). Plotting nitrogen isotope ratios versus ln[nitrate concentration] could offer some useful information on the de-nitrification process. Microbial de-nitrification typically results in progressively increasing δ15N nitrate values as nitrate concentrations decrease. A significant negative linear correlation was expected between nitrogen isotope ratios and ln[nitrate concentration] when the de-nitrification process occurred.12 Therefore, we checked our data and hoped to find this pattern by plotting nitrogen isotope ratios versus ln[nitrate concentration].
The results were interesting. When we put our data together, a weak positive correlation was observed, which suggested no significant de-nitrification process in the ground water. This result was not consistent with the dual isotope results. However, we noticed two obvious discontinuity points in Fig. 7 and those discontinuity points happened to coincide with the boundary of the sampling sites. Moreover, when we checked the data from each sampling site, a significant negative correlation (R2 > 0.6, as expected) was observed between those data. These results indicate that de-nitrification might occur in the ground water, but the different land uses determined the sources of nitrate and their different original δ15N–NO3− and δ18O–NO3− values, which conceal the de-nitrification effect when using the δ15N–NO3− and δ18O–NO3−versus ln[NO3−] method, when those samples mixed in this study area. It is worth noting that the ε values in eqn (2) for δ15N and δ18O at the G02 site were much higher than for other sites (Fig. 7). This suggested that a higher degree of de-nitrification had taken place at this site. However, due to inadequate sampling numbers and hydro-chemical information, the reasons for this result need further study.
3.4. Nitrate source apportionment using SIAR
To estimate the contributions of different NO3− sources, two types of isotope δ15N–NO3− and δ18O–NO3− (j = 2) from four major NO3− sources (atmospheric deposition, AD; NO3− derived from soil organic matter nitrification, NS; NO3− derived from chemical fertilizer nitrification, NF; and manure and sewage, M&S) were measured over two periods (the high flow period in September and the low flow period in November). The δ15N and δ18O values of different sources were used as site specific isotope values. All sources were normally distributed (Kolmogorov–Smirnoff test, Table 4). As expected, all data were consistent with those reported in the literature. There were observable variations in nitrate isotope compositions in precipitation (n = 4; −0.70 ± 2.41‰ for δ15N–NO3− and 43.85 ± 4.93‰ for δ18O–NO3−). In contrast, the values of the nitrate isotope compositions in sewage (the untreated wastewater) showed little change during the sampling period (n = 6; 15.32 ± 0.83‰ for δ15N–NO3− and 9.11 ± 0.93‰ for δ18O–NO3−). Additionally, the N isotopic composition of soil samples across land use types ranged from +2.91‰ to +7.24‰ (n = 12; 5.81 ± 1.51‰). Chemical fertilizers (n = 6) were measured, and found to have a value of −1.12 ± 1.41‰. However, the δ18O values of the soil N and synthetic fertilizer sources were not directly measured. The nitrate from “NS” and “NF” sources were mainly produced by microbial nitrification. Therefore, in this study, the δ18O values of the soil N and synthetic fertilizer sources were set as the same as the δ18O values from nitrification. This calculation yielded a range of values from −4.04‰ to +6.15‰ for δ18O–NO3− produced via nitrification (as mentioned in Section 3.3). The calculation data were time-series data, and after statistical analysis, the mean ± SD of those data (1.24 ± 3.13‰) could be obtained. Therefore, the value of 1.24 ± 3.13‰ represented the δ18O–NO3− value of nitrification sources in the study.| Sources | N | δ15N–NO3− (‰) | δ18O–NO3− (‰) | Statisticsc | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Referenced rangeb | Mean ± SD | Referenced rangeb | δ15N (sig.) | δ18O (sig.) | ||
| a Atmospheric deposition, AD; NO3− derived from soil organic matter nitrification, NS; NO3− derived from chemical fertilizer nitrification, NF; manure and sewage, M&S; N, number of samples; SD, standard deviation. b Cited from ref. 37. c Kolmogorov–Smirnoff test; if sig. > 0.05, test distribution is normal. | |||||||
| AD | 4 | −0.70 ± 2.41 | −13–13 | 43.85 ± 4.93 | 25–75 | 0.894 | 0.82 |
| M&S | 6 | 15.32 ± 0.83 | 4–25 | 9.11 ± 0.93 | −5–10 | 0.952 | 0.992 |
| NS | 12 | 5.81 ± 1.51 | −3–8 | 1.24 ± 3.13 | −10–10 | 0.792 | 0.877 |
| NF | 6 | −1.12 ± 1.41 | −6–6 | 1.24 ± 3.13 | −10–10 | 0.683 | 0.877 |
Because a significant de-nitrification process was observed in the ground water, which altered the original isotopic composition by fractionation, to determine the degree of de-nitrification and to reproduce the value of the initial isotopic composition in ground water which was employed in the SIAR model, further research was needed. Therefore, in this study, the SIAR model was utilized only for the surface water. The fractionation factors for all sources [i.e., cjk in eqn (5)] were set as zero, because no significant signs of de-nitrification were observed in the surface water (as described in Section 3.3). Measured isotope data from the surface water samples were used as inputs in SIAR. In order to identify the sources of nitrate in different land use areas, the studied watershed was divided into four sections: forest areas (including sampling sites S01 and S02), urban areas (including sampling sites S03 and S04), agricultural areas (including sampling sites S05, S06 and S07) and wetland areas (including sampling sites S08, S09 and S10). Two different runs of the SIAR model were performed. In the first run, for the forest areas, NF (fertilizer nitrification) and M&S (manure and sewage) as potential NO3− sources were excluded since in this area there were no fertilizer and sewage inputs, because this area was the headwater of the Abujiao River, and the human activities were limited. In the second, for other areas, all four sources were included in the calculation.
The ranges of contributions of each NO3− source to the surface water in the two periods are presented in Fig. 8. The SIAR mixing model outputs revealed a high variability in the contribution of the four potential NO3− sources. The surface water showed distinctly different source apportionment patterns during the two sampling periods. During low flow period, the contribution of “M&S” is the highest (mean probability estimate (MPE) 36.17%), followed by “NS” (MPE 35.69%), “NF” (MPE 22.20%), and “AD” (MPE 5.94%). Compared to the low flow period, a decrease in the “M&S” source contribution occurred (MPE from 36.17% to 22.88%), which at the same time resulted in an increased contribution of “AD” and “NF” (MPE from 5.94% to 15.46% and from 22.20% to 30.10%, respectively). Between the two sampling periods, the contribution of the “NS” sources was similar and the contribution of the source slightly decreased from 35.69 to 31.55. The SIAR outputs were in accordance with the results obtained using Cl− concentration (Section 3.3) and the dual plot approach (Section 3.3), which suggests that the agricultural activities and urban wastewater dominated the nitrate distribution for the high and low flow periods. In general, the results from the SIAR model indicate that the sources from human activities (“M&S” and “NF”) contributed the most nitrate to the aquatic system in the Abujiao watershed (MPE 58.37% and 52.98% for low and high flow periods, respectively).
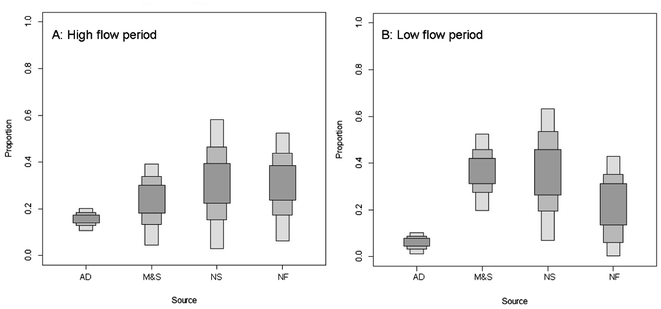 | ||
| Fig. 8 Proportional contributions of four potential nitrate sources estimated by SIAR for different sampling periods in the Abujiao River watershed. | ||
The ranges of contributions of each NO3− source to the surface water in four areas are presented in Fig. 9. According to the SIAR mixing model outputs, the contribution of the four potential NO3− sources showed high variability. As expected, the surface water showed distinctly different source apportionment patterns between the different areas. In forest areas, the contribution of “NS” is the highest (MPE 86.9%) and the contribution of “AD” only accounted for 13.1% (MPE). This source allocation pattern was similar to previous findings in other regions (the nitrification of soil organic matter as the dominant source for the stream in forest areas).10,34,35 The highest contribution of “M&S” (MPE 64.43%) was observed in the urban areas. This can be explained by the fact that a large number of untreated wastewaters were poured into the river directly, which made an important contribution to the N input into the river basin. In agricultural areas, with other sources of water input (such as runoff and drainage from farmland), the contribution of “M&S” decreased quickly (MPE from 64.43% in urban areas to 34.91% in agricultural areas) while the contribution of the “NF” and “NS” showed rapid growth (MPE from 10.13% in urban areas to 31.81% in agricultural areas and MPE from 16.55% in urban areas to 22.95% in agricultural areas, respectively).
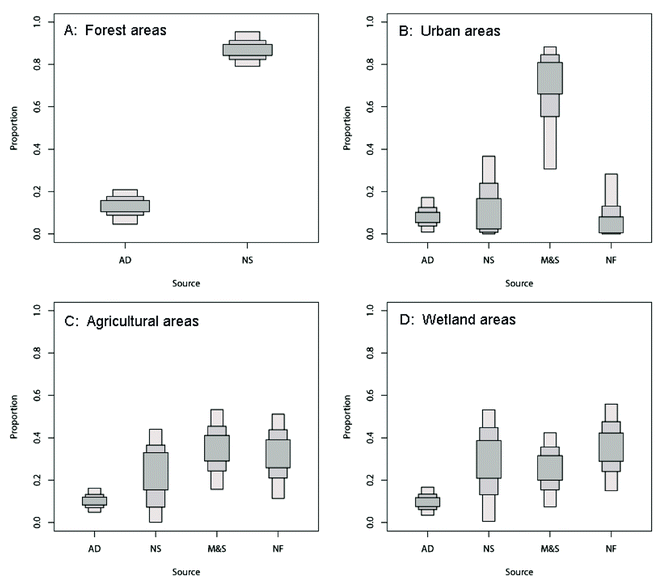 | ||
| Fig. 9 Proportional contributions of four potential nitrate sources estimated by SIAR for different land use areas in the Abujiao River watershed. | ||
In wetland areas, the proportion of “NF” and “NS” continued to increase. The highest contribution of “NF” (MEP 35.43%) was observed, followed by “NS” (MEP 29.27%). This was an interesting result, because the agricultural activities were limited (no direct anthropogenic input), so why did the contribution of “NF” continue to increase? Two possible reasons might explain this result. One reason is the input of the drainage from farmland through ditches. As shown in Fig. 1, the sampling site S08 was located at the junction of an agricultural area and wetland. Meanwhile, a tributary (a ditch for farmland drainage) directly flowed into the river, and the interchange site was located between the sampling sites S08 and S09. Therefore, the input of the drainage from farmland might cause the contribution of “NF” to increase. The other reason is the uncertainty of the calculation. Previous studies suggested that small variations in the isotope values of samples and the nitrogen cycle might result in significant changes in the source apportionment as estimated using SIAR.8,36 Therefore, this might be another reason for this unexpected result.
It should be noted that in this study, NO3− sources, particularly AD and M&S, had relatively wide ranges of δ15N–NO3−, δ18O–NO3− values, which caused the contribution of the estimated sources from “NF” and “NS” to show a high degree of uncertainty. Therefore, the uncertainty of the potential NO3− sources for this watershed should be given special attention and additional sources and water samples may be required to reduce uncertainty.
4 Conclusions
Owing to the food crisis, nitrate pollution in aquatic systems caused by intensive human activities is becoming an increasingly serious problem in the Sanjiang Plain in north-eastern China. However, to control the main source of contamination remains challenging, partly because the information on the nitrate sources and behaviors in aquatic systems is still limited. In this study, an intense human activity-impacted watershed with mixed land use (Abujiao River watershed) was selected as the study area. There were considerable differences in the physicochemical properties between the surface water and ground water, except for Cl− levels. The NO3− in the ground water (7.84 ± 5.83 mg L−1, mean ± SD) was the dominant species (>95%) of DIN during both studied periods, and the concentration was much higher than in the river (1.15 ± 0.84 mg L−1) for all sample areas. Nevertheless, no water sample in the study area exceeds the WHO drinking water limit of 50 mg L−1 NO3−. The nitrate concentrations in the surface water were greatly influenced by precipitation and land use conditions during the two sampling periods (high flow period, September; low flow period, November). In contrast, there was no significant temporal variation in NO3− in the ground water.The transformation processes were identified using Cl− concentration and dual isotope approach (δ15N–NO3− and δ18O–NO3−). The variation of Cl− suggested that during the high flow period, the dilution process (the surface water mixing with runoff caused by precipitation and drainage from farmland) was dominant and other processes such as de-nitrification occurring in the rivers contributed little to the variation of NO3− in the surface water. However, this dilution process decreased during the low flow period due to the limited water supplement. The sampled water δ18O–NO3− values suggest that the nitrification process was not the main N cycling process in the study area, because most measured δ18O–NO3− values were above the expected δ18O–NO3− from nitrification throughout the sampling period. Both chemical and isotopic characteristics indicated that the signs of de-nitrification were absent in the surface water. However, significant de-nitrification was observed in the ground water for all sample periods in the study area.
The Bayesian model (stable isotope analysis in R, SIAR) was used to apportion the contribution of potential sources. The results from SIAR software show that source contributions differ significantly between the high flow period (September) and low flow period (November). In the high flow period, “NF” and “NS” equally contributed as the major sources in the surface water. In contrast, during the low flow (November) period, “M&S” and “NS” dominated the source contribution along the downstream part of the river. Meanwhile, the contribution of the four potential NO3− sources also showed high variability between the different land use areas. The uncertainty of the potential NO3− sources for this watershed should be given special attention and additional sources and water samples may be required to reduce uncertainty.
This study proved that agricultural and residents' activities controlled the NO3− distribution during the high and low flow period, respectively, and with the assessment of temporal variation and sources of NO3−, better agricultural management practices and sewage disposal programs can be implemented to protect water quality in this high latitude watershed.
Acknowledgements
This research work was funded by the National Natural Science Foundation of China (no. 41171384; 41301529) and National Science & Technology Pillar Program of China during the Twelfth Five-year Plan Period (no. 216027).References
- K. Song, D. Liu, Z. Wang, B. Zhang, C. Jin, F. Li and H. Liu, Acta Geograph. Sin., 2008, 63, 93 Search PubMed.
- F. Hao, X. Lai, W. Ouyang, Y. Xu, X. Wei and K. Song, Environ. Manage., 2012, 50, 888–899 CrossRef PubMed.
- Y. Cao, C. Tang, X. Song, C. Liu and Y. Zhang, J. Environ. Monit., 2012, 14, 2624–2633 RSC.
- W. Ouyang, H. Huang, F. Hao and B. Guo, J. Hydrol., 2013, 495, 126–134 CrossRef CAS PubMed.
- M. B. David, L. E. Gentry, D. A. Kovacic and K. M. Smith, J. Environ. Qual., 1997, 26, 1038–1048 CrossRef CAS.
- T. Liu, F. Wang, G. Michalski, X. Xia and S. Liu, Environ. Sci. Technol., 2013, 47, 13412–13421 CrossRef CAS PubMed.
- J. Ding, B. Xi, R. Gao, L. He, H. Liu, X. Dai and Y. Yu, Sci. Total Environ., 2014, 484, 10–18 CrossRef CAS PubMed.
- L. Yang, J. Han, J. Xue, L. Zeng, J. Shi, L. Wu and Y. Jiang, Sci. Total Environ., 2013, 463, 340–347 CrossRef PubMed.
- X. Pu, H. Cheng, Y. Shan, S. Chen, Z. Ding and F. Hao, Acta Agric. Scand., Sect. B, 2012, 62, 749–764 CAS.
- D. A. Burns, E. W. Boyer, E. M. Elliott and C. Kendall, J. Environ. Qual., 2009, 38, 1149–1159 CrossRef CAS PubMed.
- C. Kendall, M. B. Young and S. R. Silva, Applications of stable isotopes for regional to national-scale water quality and environmental monitoring programs, in Isoscapes, ed. J. B. West, G. J. Bowen, T. E. Dawson and K. P. Tu, Springer, 2010, pp. 89–111 Search PubMed.
- C. Kendall, E. M. Elliott and S. D. Wankel, Stable Isot. Ecol. Environ. Sci., 2007, 2, 375–449 Search PubMed.
- F. Chen, G. Jia and J. Chen, Biogeochemistry, 2009, 94, 163–174 CrossRef CAS.
- S. D. Wankel, C. Kendall and A. Paytan, J. Geophys. Res.: Biogeosci., 2005–2012, 2009, 114 Search PubMed.
- C. Kendall and J. J. McDonnell, Isotope tracers in catchment hydrology, Elsevier, 1998 Search PubMed.
- B. Mayer, S. M. Bollwerk, T. Mansfeldt, B. Hütter and J. Veizer, Geochim. Cosmochim. Acta, 2001, 65, 2743–2756 CrossRef CAS.
- R. Aravena and W. D. Robertson, Ground Water, 1998, 36, 975–982 CrossRef CAS PubMed.
- D. Xue, J. Botte, B. De Baets, F. Accoe, A. Nestler, P. Taylor, O. Van Cleemput, M. Berglund and P. Boeckx, Water Res., 2009, 43, 1159–1170 CrossRef CAS PubMed.
- D. Xue, B. De Baets, O. Van Cleemput, C. Hennessy, M. Berglund and P. Boeckx, Environ. Pollut., 2012, 161, 43–49 CrossRef CAS PubMed.
- S. Bernal, A. Butturini and F. Sabater, Biogeochemistry, 2006, 81, 269–289 CrossRef CAS PubMed.
- S. Silva, C. Kendall, D. Wilkison, A. Ziegler, C. Chang and R. Avanzino, J. Hydrol., 2000, 228, 22–36 CrossRef CAS.
- K. K. Andersson and A. B. Hooper, FEBS Lett., 1983, 164, 236–240 CrossRef CAS.
- D. A. Burns and C. Kendall, Water Resour. Res., 2002, 38, 9-1–9-11 CrossRef.
- D. Kaown, D.-C. Koh, B. Mayer and K.-K. Lee, Agric., Ecosyst. Environ., 2009, 132, 223–231 CrossRef CAS PubMed.
- A. C. Parnell, R. Inger, S. Bearhop and A. L. Jackson, PLoS One, 2010, 5, e9672 Search PubMed.
- J. B. Hopkins III and J. M. Ferguson, PLoS One, 2012, 7, e28478 Search PubMed.
- A. L. Jackson, R. Inger, S. Bearhop and A. Parnell, Ecol. Lett., 2009, 12, E1–E5 CrossRef PubMed.
- S. Silva, P. Ging, R. Lee, J. Ebbert, A. Tesoriero and E. Inkpen, Environ. Forensics, 2002, 3, 125–130 CrossRef CAS.
- F.-J. Yue, C.-Q. Liu, S.-L. Li, Z.-Q. Zhao, X.-L. Liu, H. Ding, B.-J. Liu and J. Zhong, J. Hydrol., 2014, 519, 329–339 CrossRef CAS PubMed.
- D. Widory, E. Petelet-Giraud, P. Négrel and B. Ladouche, Environ. Sci. Technol., 2005, 39, 539–548 CrossRef CAS.
- C.-Q. Liu, S.-L. Li, Y.-C. Lang and H.-Y. Xiao, Environ. Sci. Technol., 2006, 40, 6928–6933 CrossRef CAS.
- δ18O values in precipitation water for 1989 were taken from Water Isotope System for Data Analysis, Visualization and Electronic Retrieval (WISER), GNIP – Global Network of Isotopes in Precipitation, International Atomic Energy Agency, Vienna, Austria, http://www.univie.ac.at/cartography/project2/wiser/index.php Search PubMed.
- M. O. Rivett, S. R. Buss, P. Morgan, J. W. Smith and C. D. Bemment, Water Res., 2008, 42, 4215–4232 CrossRef CAS PubMed.
- R. T. Barnes and P. A. Raymond, Ecol. Appl., 2010, 20, 1961–1978 CrossRef.
- K. B. Piatek, M. J. Mitchell, S. R. Silva and C. Kendall, Water, Air, Soil Pollut., 2005, 165, 13–35 CrossRef CAS.
- D. Xue, B. De Baets, O. Van Cleemput, C. Hennessy, M. Berglund and P. Boeckx, Environ. Pollut., 2012, 161, 43–49 CrossRef CAS PubMed.
- D. Xue, J. Botte, B. De Baets, F. Accoe, A. Nestler, P. Taylor, O. Van Cleemput, M. Berglund and P. Boeckx, Water Res., 2009, 43, 1159–1170 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |