Partial nitritation ANAMMOX in submerged attached growth bioreactors with smart aeration at 20 °C
James M.
Shannon
,
Lee W.
Hauser
,
Xikun
Liu
,
Gene F.
Parkin
,
Timothy E.
Mattes
and
Craig L.
Just
*
Department of Civil and Environmental Engineering, University of Iowa, Iowa City, IA 52242, USA
First published on 11th November 2014
Abstract
Submerged attached growth bioreactors (SAGBs) were operated at 20 °C for 30 weeks in smart-aerated, partial nitritation ANAMMOX mode and in a timer-controlled, cyclic aeration mode. The smart-aerated SAGBs removed 48–53% of total nitrogen (TN) compared to 45% for SAGBs with timed aeration. Low dissolved oxygen concentrations and cyclic pH patterns in the smart-aerated SAGBs suggested conditions favorable to partial nitritation ANAMMOX and stoichiometrically-derived and numerically modeled estimations attributed 63–68% and 14–44% of TN removal to partial nitritation ANAMMOX in these bioreactors, respectively. Ammonia removals of 36–67% in the smart-aerated SAGBs, with measured oxygen and organic carbon limitations, further suggest partial nitritation ANAMMOX. The smart-aerated SAGBs required substantially less aeration to achieve TN removals similar to SAGBs with timer-controlled aeration. Genomic DNA testing confirmed that the dominant ANAMMOX seed bacteria, received from a treatment plant utilizing the DEMON® sidestream deammonification process, was a Candidatus Brocadia sp. (of the Planctomycetales order). The DNA from these bacteria was also present in the SAGBs at the conclusion of the study providing evidence for attached growth and limited biomass washout.
Environmental impactLimited infrastructure improvement budgets for small, rural communities have left countless streams and rivers under threat by discharges of inadequately treated domestic wastewater. Acute ammonia toxicity can kill organisms at the local scale and chronic nutrient releases contribute to local, regional and coastal hypoxia attributable to undesirable, and often toxic, algal blooms. Therefore, to protect the environment and to better ensure the long-term viability of rural communities, relatively inexpensive, easy to maintain and increasingly energy efficient wastewater treatment systems with nutrient removal capabilities are needed. This study investigated how submerged attached growth bioreactors equipped with “smart”, pH-controlled aeration could remove nitrogen from domestic wastewater via the partial nitritation ANAMMOX process at 20 °C. |
Introduction
Effective and affordable treatment of municipal wastewater flows of less than 3.8 × 103 m3 per day (1 million gallons per day, MGD) poses significant design challenges for small communities, especially in cooler climates. Increasingly stringent standards and guidelines for discharges of ammonium (NH4+) and total nitrogen (TN) have driven the exploration of treatment systems that maximize TN removal while minimizing aeration requirements. Submerged attached growth bioreactors (SAGBs) have been successfully utilized for smaller wastewater flows (<1 MGD) due to relative ease of operation and robustness. Underground placement of SAGBs also facilitates cold-climate treatment and improves aesthetics.1 SAGBs can be continuously aerated to maximize treatment of carbonaceous biological oxygen demand (cBOD) and total Kjeldahl nitrogen (TKN) with greater than 95% cBOD and 90% and TKN removal.2 A planted, vertical-flow SAGB with continuous aeration achieved a loading-rate dependent NH4+ removal of 65–87%.3 A similar system with continuous aeration removed 97%, 99% and 29% chemical oxygen demand (COD), NH4+ and TN, respectively.4 Additionally, planted horizontal-flow SAGBs removed up to 96.3% TN in summer (Montreal, Canada) and nearly 60% in a 5 °C winter, greenhouse temperature.5 A similar system removed 4–42% TKN in Montreal summer and 13–29% TKN in winter.6Other SAGBs utilize intermittent aeration to facilitate nitrification and denitrification which reduces aeration costs as compared to constantly aerated systems. Our previous work demonstrated that intermittently aerated, horizontal-flow SAGBs dosed with municipal primary effluent removed 84–93% cBOD and 65–95% TN in planted and unplanted cells.7 A planted, intermittently aerated vertical flow SAGB removed 54–78% of NH4+ and 29–57% of TN as a function of hydraulic loading.3 A similar system achieved 96% COD, 99% NH4+ and 90% TN removal8 and another achieved 96% COD, 97% NH4+, and 74% TN removal.4
The Amphidrome® SAGB treats domestic wastewater flows of up to 0.5 MGD to less than 30 mg L−1 cBOD and less than 10 mg L−1 TN.9,10 With methanol addition, a similar system removed over 94% cBOD and 52–72% TN from municipal wastewater.11 Another study, performing sidestream treatment of dewatering centrate, achieved an average TN removal of 85% when methanol was added.12 A similar SAGB with limited aeration favored nitrite (NO2−) formation from NH4+ while minimizing nitrate (NO3−) production and with the addition of methanol and sodium bicarbonate, 25% of the TN was removed.13
Partial nitritation (i.e. the fractional conversion of available NH4+ to NO2−) coupled with anaerobic ammonium oxidation (ANAMMOX) is a relatively new wastewater treatment approach that can treat TN while decreasing aeration needs.14,15 Partial nitritation requires precision dissolved oxygen (DO) control (typically <0.5 mg L−1) to select for ammonium oxidizing archaea/bacteria (AOB) activity while limiting nitrite oxidizing bacteria (NOB). Under these conditions, NH4+ is oxidized to NO2− as alkalinity is consumed and pH (typical range, 6.5–8) is driven lower:15
| NH4+ + 1.38O2 + 1.98HCO3− → 0.018C5H7NO2 + 0.98NO2− + 1.04H2O + 1.89H2CO3 | (1) |
With a desired level of NH4+ oxidation reached, aeration is turned off and ANAMMOX activity commences as DO tends toward zero. ANAMMOX bacteria recover alkalinity as NH4+ and NO2− are converted to nitrogen gas (N2) and NO3− as described by Strous:14
| NH4+ + 1.32NO2− + 0.066HCO3− + 0.13H+ → 1.02N2 + 0.26NO3− + 0.066CH2O0.5N0.15 + 2.03H2O | (2) |
Combining these equations produces a nitrogen balance useful for inferring partial nitritation ANAMMOX activity from water chemistry. Modifying eqn (2) to include the same empirical formula for biomass (C5H7NO2) that is used in eqn (1) and rebalancing the stoichiometric coefficients for HCO3−, H2O, and biomass yields:
| NH4+ + 1.32NO2− + 0.07HCO3− + 0.13H+ → 1.02N2 + 0.26NO3− + 0.014C5H7NO2 + 2.05H2O | (3) |
Combining eqn (1) and (3) reveals the net stoichiometry for the complete partial nitritation ANAMMOX process that occurs over two, rate independent steps:
| 2NH4+ + 0.34NO2− + 1.38O2 + 2.05HCO3− + 0.13H+ → 1.02N2 + 0.26NO3− + 0.032C5H7NO2 + 3.09H2O + 1.89H2CO3 | (4) |
Converting N-containing species in eqn (4) to “as N” equivalents and normalizing to NH4–N+ yields the nitrogen balance for the partial nitritation ANAMMOX process:
| NH4–N+ + 0.063NO2–N− → 0.33N2–N + 0.037NO3–N− + 0.0026C5H7NO2–N | (5) |
Similar to the nitrification–denitrification process, the consumption and subsequent recovery of alkalinity across the two stage partial nitritation ANAMMOX cycle results in a characteristic “saw tooth” pH pattern over time. This pH signal can serve as an aeration control parameter toward the creation of “smart-aerated” systems. The most common current application for smart-aerated, partial nitritation ANAMMOX is for large treatment systems without attached growth media that are operated at 25–40 °C.16–20 One planted SAGB with controlled DO of 0.2–0.6 mg L−1 achieved 87.2% cBOD removal and 68.7–85.1% TN removal.21 A SAGB using hydrophilic acryl fiber netting as the growth medium treated high NH4+ wastewater via partial nitritation ANAMMOX at 35 °C with an aerobic zone at 2–3 mg L−1 DO for AOB activity and an anoxic zone for ANAMMOX achieved 60–80% NH4+ removal.22
Partial nitritation ANAMMOX theoretically requires 0.7 moles of O2 to yield 0.5 mole of N2 compared to 1.8 moles required for nitrification–denitrification and 1.3 moles required for nitritation–denitritation (Fig. 1).23 Smart-aerated SAGBs operating in partial nitritation ANAMMOX mode hold promise to remove nitrogen from wastewater using less aeration and thus reducing treatment costs for small communities. But, many of these communities experience wastewater temperatures below the generally accepted 25 °C minimum for effective ANAMMOX-based nitrogen removal.24 Therefore, this study is what we understand to be the first report of partial nitritation ANAMMOX below the 25 °C threshold in pilot-scale, smart-aerated SAGBs operated at 20 °C.
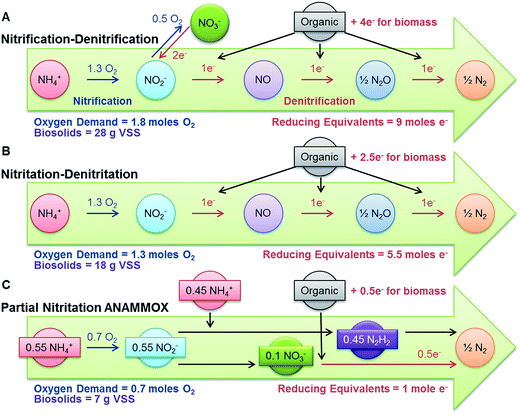 | ||
| Fig. 1 Comparison of oxygen demand, reducing equivalents and biosolids production for (A) nitrification–denitrification, (B) nitritation–denitritation, and (C) partial nitritation ANAMMOX. Adapted from Gao et al.23 | ||
Materials and methods
ANAMMOX seed reactor activity and DNA analysis
ANAMMOX seed material (1.6 L) from the Hampton Roads Sanitation District, York River, Virginia, treatment plant that was recently retrofitted to be the first DEMON® sidestream deammonification system in North America.25 The seed was maintained in a 2 L, glass container that was table shaken at 100 rpm at 38.5 °C. A nitrogen purge line with diffuser stone was inserted to maintain anaerobic conditions and the seed reactor was periodically fed NH4+ (∼25 mg L−1, variable), NO2− (∼25 mg L−1, variable), NaHCO3 (100 mg L−1), NaH2PO4 (25 mg L−1), K2HPO4 (30 mg L−1), MgCl2 (40 mg L−1), and CaCl2 (60 mg L−1). ANAMMOX activity was measured during a 72 hour NH4+ and NO2− (initial concentrations of 25 mg L−1) utilization experiment with analysis by ion chromatographs (AS2000 and AS900, Thermo Scientific Dionex, Sunnyvale, CA) equipped for cations (CS15 column) and anions (AS22 column) with software control and data processing (Chromeleon, version 7). Dissolved oxygen was measured by luminescence with an electronic probe and meter (IntelliCAL™ LDO101 probe, HQ40d meter, Hach Company).Presence of ANAMMOX bacteria was determined by DNA extraction, polymerase chain reaction (PCR) amplification of the 16S rRNA gene, cloning, sequencing and phylogenetic analysis. Genomic DNA was extracted (PowerWater® Sterivex™ DNA Isolation Kit, MO BIO Laboratories, Carlsbad, CA) from 200 mL of 20-fold diluted (RNase-free water, Qiagen, Germantown, MA) ANAMMOX seed and PCR inhibiting substances were removed (QIAquick PCR Purification Kit, Qiagen). A 25 μL PCR reaction mix containing 12.5 μL of Taq PCR Master Mix (Qiagen), 600 nM each of the forward and reverse primers (A438f/A684r; specific for the ANAMMOX 16S rRNA gene26) and 450 ng of DNA template was used. Partial 16S rRNA genes (246 base pair (bp) expected product size) were amplified (Eppendorf MasterCycler EP S, Hamburg, Germany) and the products were purified (MinElute PCR Purification Kit, Qiagen). The purified PCR products were ligated overnight at 4 °C into the pCR®2.1 vector using the TA Cloning® Kit (Invitrogen, Carlsbad, CA) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar insert to vector ratio. Ligations were transformed into One Shot® TOP10 Chemically Competent E. coli (Invitrogen) and transformants were analyzed according to the cloning kit instructions. Plasmids were extracted using QIAprep Spin Miniprep Kit (Qiagen) and clones were PCR-screened with M13 primers (both F and R). Appropriately sized inserts were Sanger-sequenced with the M13F primer (5′-GTAAAACGACGGCCAG-3′) at the Iowa Institute of Human Genetics, Genomics Division. The 16S rRNA gene sequences were analyzed for nearest neighbor sequences via the SimRank function in Greengenes (http://greengenes.lbl.gov), a chimera-checked 16S rRNA database.27 SimRank estimates the similarity between two sequences with respect to how many unique 7 nucleotide sequence runs (7-mers) they share. SimRank similarity scores do not necessarily equate with % identities that are obtained by sequence alignment. Nucleotide sequences derived from this study have been deposited in Genbank (accession no. KM401817-KM401837).
1 molar insert to vector ratio. Ligations were transformed into One Shot® TOP10 Chemically Competent E. coli (Invitrogen) and transformants were analyzed according to the cloning kit instructions. Plasmids were extracted using QIAprep Spin Miniprep Kit (Qiagen) and clones were PCR-screened with M13 primers (both F and R). Appropriately sized inserts were Sanger-sequenced with the M13F primer (5′-GTAAAACGACGGCCAG-3′) at the Iowa Institute of Human Genetics, Genomics Division. The 16S rRNA gene sequences were analyzed for nearest neighbor sequences via the SimRank function in Greengenes (http://greengenes.lbl.gov), a chimera-checked 16S rRNA database.27 SimRank estimates the similarity between two sequences with respect to how many unique 7 nucleotide sequence runs (7-mers) they share. SimRank similarity scores do not necessarily equate with % identities that are obtained by sequence alignment. Nucleotide sequences derived from this study have been deposited in Genbank (accession no. KM401817-KM401837).
Pilot-scale SAGB setup and operation
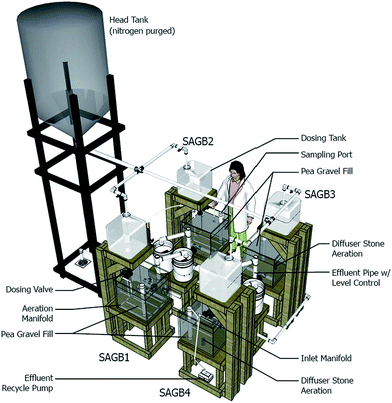
Four pilot-scale SAGBs (Fig. 2) were constructed within a temperature controlled chamber operated at 20 °C. Synthetic wastewater was stored in a 950 L polypropylene head tank positioned 4 m above floor level. The head tank was connected via 3.8 cm diameter PVC pipe to four 45 L polypropylene dosing tanks positioned above the inlet of each 61 cm × 61 cm × 46 cm SAGB. The dosing tank outlets were connected to an electronic valve and that to an inlet manifold. The inlet manifold was a 1.3 cm diameter PVC down pipe connected to a 1.3 cm diameter, 55 cm long, horizontal pipe with eight 2.4 mm dosing holes. Treated wastewater exited each SAGB via a horizontal, 3.8 cm diameter PVC pipe, 55 cm long with sixteen 3.6 mm diameter holes. The effluent manifold piping penetrated the SAGB wall before connecting to a water level control apparatus. The level control apparatus was a vertical 3.8 cm diameter, 30.5 cm tall PVC pipe open to atmosphere. The level control apparatus was contained in a 19 L bucket that drained to the sanitary sewer via 3.8 cm diameter pipe. SAGB1 and SAGB2 contained aeration manifolds consisting of four, 1.3 cm diameter PVC pipes, 50 cm long, with 3.2 mm outlet holes connected via 61 cm long distribution piping including a 76 cm tall inlet pipe. SAGB3 and SAGB4 were aerated via a 2 cm diameter diffuser stone placed 13 cm from the inlet, 2.5 cm above bottom. Compressed air was provided by a pump (Pondmaster AP 100, Danner Manufacturing, Islandia, NY) connected to a distribution manifold with adjustable needle flow valves. Additionally, SAGB4 was equipped with a recirculation pump that delivered effluent water to the sampling port at 0.1 LPM.Washed pea gravel (∼0.1 m3) was added to each SAGB before inoculation with 45 L of municipal primary effluent (Iowa City, IA). A synthetic wastewater, comprised of yeast extract (10 mg L−1), casamino acids (10 mg L−1), NaHCO3 (100 mg L−1), NaH2PO4 (25 mg L−1), K2HPO4 (30 mg L−1), MgCl2 (40 mg L−1), and CaCl2 (60 mg L−1), sodium acetate (110 mg L−1), glucose (100 mg L−1) and glycine (67 mg L−1) (modified from Klatt28), was added gradually to each SAGB over 7 days to minimize bacterial washout. The head tank was filled with synthetic wastewater that was continuously sparged with nitrogen to slow microbial degradation prior to dosing. The dosing valves were microcontroller (Arduino UNO R3, SmartProjects, Italy) programmed to deliver 3 L of synthetic wastewater every 6 hours to achieve a mean hydraulic residence time of approximately 4 days. All SAGBs were operated for 19 weeks with SAGB1 and SAGB2 aerated at 1.2 LPM on a 6 hour on, 6 hour off cycle to mimic conditions from our previous work.7 SAGB3 and SAGB4 received no mechanical aeration during this period.
Synthetic wastewater dosing was then halted, and 0.25 L of the ANAMMOX seed was added to each SAGB followed by a 7 day attachment period. At this point, SAGB3 and SAGB4 were each equipped with pH-controlled (IntelliCAL™ PHC101 probe and SC200 universal controller, Hach Company, Loveland, CO) air flow meters (FMA5518, Omega Engineering, Stamford, Connecticut) that delivered 2.0 LPM when on. Given the established pH range of 6.5–8.0 for partial nitritation ANAMMOX processes, two pH ranges between 7.0 and 7.5 were chosen for this study. The pH-control for SAGB3 was programmed to begin aeration at pH 7.25 and to cease aeration at pH 7.05 and the pH-control for SAGB4 had set points of 7.45 and 7.25, respectively. The SAGBs were inoculated again with primary effluent 3 weeks and 7 weeks into the 10 week partial nitritation ANAMMOX operational period that culminated in a 48 hour intensive sampling event.
SAGB nitrogen removal assessment and ANAMMOX analysis
The transient nitrogen-species behavior and overall nitrogen removal of each SAGB was assessed during an intensively-sampled 48 hour period in the 30th week of operation. During this time, the influent NH4+ (n = 1), TN (n = 4), COD (n = 4), total organic carbon (TOC, n = 4), NO2− (n = 1) and NO3− (n = 1) concentrations and effluent NH4+ (n = 21), TN (n = 4), COD (n = 4), TOC (n = 4), NO2− (n = 21) and NO3− (n = 21) concentrations were determined. Dissolved oxygen was directly measured and SAGB samples were collected for NH4+, NO2− and NO3− analyses every 2 hours from a 3.8 cm diameter sample port (Fig. 2) installed 13 cm from the inlet. The port spanned the depth of the pea gravel and contained several 1 cm diameter holes along its length and was wrapped with porous fabric. Samples for NH4+, NO2− and NO3− were filtered, stored at 4 °C and analyzed by ion chromatography within 48 hours. Total nitrogen was measured by persulfate digestion method 4500-N C (ref. 29) and COD was determined using the dichromate method 5220D.29 Dissolved oxygen was measured as described previously and the pH was measured either by the continuously operating probes already described or by a glass electrode and meter (AR15, Fisher Scientific, Pittsburg, PA). Total organic carbon was measured by direct method 415.3 (ref. 30) and alkalinity was measured via Hach Method 10239 (Hach Company). At the conclusion of the experiment, pea gravel (700 grams) from each SAGB was collected near the inlet at approximately 20 cm below surface for ANAMMOX DNA analysis. The samples were collected in sterile, glass containers and 100 mL of autoclaved deionized water was added. The samples were shaken vigorously to dislodge biomass which was analyzed using the DNA protocol previously described.Dynamic kinetic modeling of partial nitritation ANAMMOX associated TN removal
A numerical stock and flow model, with dynamic coupling to aeration events, was built and utilized to explore the linked N-transformations performed by AOBs, NOBs, denitrifiers, and ANAMMOX bacteria in SAGB3 and SAGB4 over time (Fig. 3). When aeration was on, AOB and NOB activity was allowed. When aeration was off, denitrification and ANAMMOX activity was allowed. The ANAMMOX rates for NO2− utilization, NO3− formation and N2 formation were stoichiometrically-coupled to the ANAMMOX rate for NH4+ utilization according to eqn (3). Production of ANAMMOX biomass is relatively small in comparison to transformations of other N stocks and was, therefore, lumped with N2 production (N removal). The differential equations that comprise the model were solved numerically using Euler's method with 1 hour time steps (STELLA version 8.0, ISEE Systems, Inc., Lebanon, New Hampshire).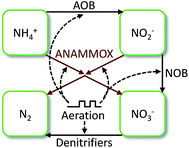 | ||
| Fig. 3 Graphical representation of the aeration-coupled, numerical stock and flow model used to describe the partial nitritation ANAMMOX kinetics in SAGB3 and SAGB4. | ||
Results and discussion
ANAMMOX seed reactor activity and bacterial identification
At the onset of the ANAMMOX activity experiment, NH4+ and NO2− concentrations in the seed reactor were 25.5 and 23.9 mg-N per L, respectively. After 72 hours, the NH4+ concentration was 5 mg-N per L and NO2− was 8.7 mg-N per L with first-order decay coefficients 0.54 per day and 0.34 per day, respectively. Dissolved oxygen was <0.1 mg L−1. The removal of NH4+ under anaerobic conditions was viewed as one line of evidence that ANAMMOX bacteria were active in the seed reactor. Furthermore, SimRank analysis of the partial 16S rRNA gene (246 bp) showed top hits as a Candidatus Brocadia sp. (of the Planctomycetales order) with a 75–97% SimRank identity. Candidatus Brocadia and Candidatus Kuenenia were found in biomass samples from the wastewater treatment plant in Strauss, Austria,31 which provided the ANAMMOX seed for the York River plant that supplied our seed material. Other bacteria identified as possibly present for the seed reactor and for the SAGBs included Candidatus Anammoxoglobus, Candidatus Jettenia, and Candidatus Scalindua which are all known ANAMMOX bacteria.SAGBs with timer-controlled aeration (SAGB1 and SAGB2)
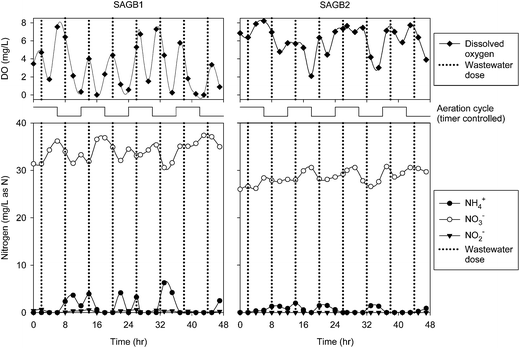
The sample port results for SAGB1 (Fig. 4) showed that DO concentrations varied between zero and approximately 8 mg L−1 with DO being utilized with each dosing of synthetic wastewater. SAGB2 (Fig. 4) showed slightly higher overall DO concentrations within the sample port, but less synchronicity between wastewater dosing time and DO consumption than for SAGB1. Nitrate was the dominant nitrogen form measured in the sample ports for SAGB1 and SAGB2 which, considered with the DO data, indicates that nitrification occurred during the aerated phases of the operational cycle. Periods of anaerobic conditions, suggested by DO data for SAGB1, and periods of low DO measured in SAGB2 indicated that denitrification (to N2 and/or N2O) was possible in these bioreactors when aeration was off. A total of 1728 L of air was delivered to SAGB1 and SAGB2 during the four, 6 hour aeration cycles that occurred during the 48 hour intensive measurement period.The 45% reduction of TN concentration from average influent values to average effluent values in SAGB1 and SAGB2 (Table 1) suggests that nitrification–denitrification was indeed occurring. The nitrification phase suggested activity by AOBs and by NOBs as effluent nitrate concentrations reached 36 ± 4 mg-N per L. But, the denitrification potential would have been limited by organic carbon availability (1.4 ± 0.3 mg L−1 in the effluent, Table 1) in SAGB1 and SAGB2 remaining from a dosed amount of 16 mg L−1. Compared to our previous work,7 these SAGBs underperformed on TN removal (65–95% previously) and performed similarly with respect to oxygen demand reduction (84–93% cBOD removal previously). These results were expected given that mean DO concentrations were quite high (4.6 ± 2.6 mg L−1) and that these SAGBs were operated as controls for the partial nitritation ANAMMOX SAGBs. DNA results from pea gravel samples collected at the conclusion of the 30 week experiment confirmed the presence of Candidatus Brocadia with a SimRank of >91% for the four samples analyzed. But, ANAMMOX activity was assumed to be negligible given the periods of high DO and given that conditions clearly favored NOB growth and activity.
| SAGB | Influent concentration (mg L−1) average ± S.D. | Effluent concentration (mg L−1) average ± S.D. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N | NH4+ | COD | TOC | Alk | NO3− | NO2− | Total N (removal) | NH4+ (removal) | COD (removal) | TOC (removal) | Alk (removal) | NO3− | NO2− | |
| a Estimated from values obtained from the SAGB3 and SAGB4 dosing tanks. | ||||||||||||||
| 1 & 2 | 58a | 30 | 77a | 16a | 143a | <0.1 | <0.1 | 32 ± 3 (45%) | <0.1 (100%) | 2 ± 4 (97%) | 1.4 ± 0.3 (91%) | N.A. | 36 ± 4 | <0.1 |
| 3 | 55 ± 3 | 34 | 67 ± 6 | 15 ± 4 | 141 | <0.1 | <0.1 | 28 ± 0.7 (48%) | 11 ± 1.5 (67%) | 16 ± 10 (76%) | 2.0 ± 2.4 (87%) | 64 (55%) | 17 ± 1.5 | 0.8 ± 0.3 |
| 4 | 61 ± 9 | 33 | 86 ± 34 | 17 ± 2 | 145 | <0.1 | <0.1 | 29 ± 2 (53%) | 21 ± 2 (36%) | 14 ± 5 (83%) | 1.4 ± 0.9 (92%) | 105 (28%) | <0.1 | <0.1 |
Partial nitritation ANAMMOX SAGB without recirculation (SAGB3)
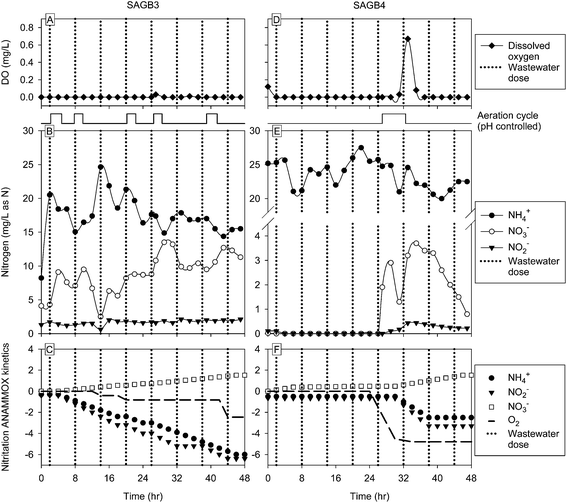
Sample port results for SAGB3 (Fig. 5A & B) indicated that DO concentrations were at or near zero throughout the 48 hour sampling period. The pH-controlled aeration cycle was triggered five times (Fig. 5) and the presence of NO2− (∼1 mg-N per L) and NO3− (∼10 mg-N per L) in the sample port was an indication that available oxygen was consumed, at least partially, by AOB and NOB activity. With additional oxygen, the NOBs would have converted all NO2− to NO3− as demonstrated in the more fully aerated SAGB1 and SAGB2. Therefore, SAGB3 was shown capable of performing partial nitritation – the first step in the partial nitritation ANAMMOX process. A total of 1344 L of air was delivered to SAGB3 from the 5 aeration cycles during the 48 hour intensive measurement period.In the effluent, total nitrogen was reduced 48% and the NH4+ concentration was reduced 67% as compared to the influent (Table 1) in SAGB3. The removal of NH4+ in an anaerobic bioreactor that contains NO2− is strong evidence for ANAMMOX activity. Assuming the entire 55 mg-N per L influent TN in SAGB3 was available as NH4+ for partial nitritation ANAMMOX, eqn (5) predicts 3.5 mg-N per L NO2− would be produced and consumed, 2.1 mg-N per L NO3− and 18.2 mg L−1 N2 would be formed and 0.14 mg-N per L would accumulate into ANAMMOX biomass. Total nitrogen removal predicted by partial nitritation ANAMMOX would therefore be 18.3 mg-N per L (N2 plus biomass-N) and would account for 68% of the 27 mg-N per L TN removed from SAGB3 (Table 1).
The kinetic modeling results (Fig. 5C) showed dynamic coupling to aeration events through stepwise utilization of NH4+, NO2− and dissolved oxygen. The production of partial nitritation ANAMMOX associated NO3− (as opposed to NOB-associated production) was modeled as a steady increase over the 48 hour period since NO2− was constantly present (Fig. 5B) and, therefore, did not limit the ANAMMOX reaction. The rate coefficients for AOBs, NOBs, denitrification, ANAMMOX NH4+ utilization and ANAMMOX NO2− utilization were used in the model to generate partial nitritation ANAMMOX associated conversion rates for NH4+, NO2−, NO3−, and O2 (Table 2). Again, assuming the entire 55 mg-N per L influent TN in SAGB3 was available as NH4+ for partial nitritation ANAMMOX, the modeled NH4+ conversation rate of 3 mg per L per day over a 4 day retention time would indicate a loss of 12 mg L−1, or 44.4%, of the 27 mg-N per L removed. This result, coupled with the analysis above, suggests that partial nitritation ANAMMOX associated TN removal was between 44 and 68% in SAGB3.
| SAGB | Rate coefficients (h−1) | Partial nitritation ANAMMOX associated conversion rates (mg per L per day) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AOB | NOB | Denitrification | ANAMMOX NH4+ | ANAMMOX NO2− | NH4+ | NO2− | NO3− | O2 | Total N% removal | |
| 3 | 1.2 | 0.1 | 0.01 | 0.3 | 0.4 | −3.0 | −3.2 | 0.76 | −0.82 | 44 |
| 4 | 1.0 | 0.1 | 0.01 | 0.5 | 0.66 | −1.3 | −1.7 | 0.34 | −2.4 | 16 |
Nitrogen removal may have alternatively occurred via denitrification and/or denitritation. Theoretical removals were estimated assuming 100% of the COD removal (51 mg L−1) occurred as a result of these processes. Given that 2.86 mg COD is required to convert 1 mg-N NO3− and 1.71 mg COD is required to convert 1 mg-N NO2− (calculated using methods described in Rittmann and McCarty32) to N2, up to 17.8 mg-N per L NO3− and up to 29.8 mg-N per L NO2− could have been removed via denitrification or denitritation, respectively. Additionally, using a net biomass yield of 0.4 and assuming that biomass was 12.4% nitrogen,32 an estimated 2.5 mg-N per L was incorporated into biomass. Collectively, these quantities represent 75% and over 100% of the TN removal measured in SAGB3, respectively. However, it is highly unlikely that 100% of the COD removal occurred via anaerobic processes since faster growing aerobic heterotrophs would have consumed some of the DO during times of aeration. For example, if 50% of the DO was consumed by heterotrophs, the denitrification/denitritation potential would have been reduced to a level where significant partial nitritation ANAMMOX would be required to close the nitrogen mass balance. Furthermore, effluent NO3− concentrations of 17 ± 1.5 mg-N per L suggest additional oxygen was consumed by autotrophic NOBs. The elevated NO3− concentrations also indicate that denitrification potential was limited by carbon scarcity, providing further indirect evidence for significant partial nitritation ANAMMOX. DNA results from pea gravel samples collected in SAGB3 at the conclusion of the 10 week nitritation ANAMMOX operational phase had top hits for Candidatus Brocadia with a SimRank of 75–79% for three samples.
Partial nitritation ANAMMOX SAGB with recirculation (SAGB4)
Sample port results for SAGB4 (Fig. 5D & E) showed that a single, pH-controlled aeration cycle was triggered during the sampling period which caused a momentary DO increase from <0.1 mg L−1 to ∼0.7 mg L−1. The NH4+ concentrations were slightly greater than those measured in the SAGB3 sampling port, but the NO3− and NO2− concentrations were substantially lower than SAGB3. Collectively, these results indicate that the DO concentrations were lower overall in SAGB4 compared to SAGB3 and that NOB activity was consequently much lower in SAGB4 as well. The lack of measureable NO2− in the SAGB4 sample port indicates low AOB activity and/or rapid NO2− utilization by ANAMMOX bacteria. A total of 660 L of air was provided to SAGB4 during the 48 hour intensive measurement period.In the effluent, the total nitrogen was reduced by 53% in SAGB4 (Table 1). If the entire 61 mg-N per L of influent TN were transformed through partial nitritation ANAMMOX, 3.8 mg-N per L NO2− would have been formed and utilized, 20.1 mg-N per L N2 would have been emitted, 2.3 mg-N per L NO3− would have been produced and 0.16 mg-N per L of biomass would have grown (based on eqn (5)). If this were the case, 63% of the 32 mg-N per L TN removed (Table 1) by SAGB4 would be attributable to partial nitritation ANAMMOX. Effluent COD was reduced by 83% and NO3− and NO2− were not detected (Table 1). The kinetic modeling results (Fig. 5F) revealed the oxygen dependence of NO2− formation by AOBs and the lack of partial nitritation ANAMMOX associated NH4+ utilization when NO2− is absent (Fig. 5E). Again, various rate coefficients were used in the model to generate associated conversion rates (Table 2) and the TN removal attributed to partial nitritation ANAMMOX was 16% using this approach. Therefore, a range of 16 to 63% of partial nitritation ANAMMOX associated TN removal was achieved for SAGB4.
Using the same approach as described for SAGB3, up to 25.2 mg-N per L NO3− or up to 42.2 mg-N per L NO2− could have been removed via denitrification or denitritation if 100% of the COD was removed during those processes. Incorporation of N into biomass would have removed approximately 3.6 mg-N per L. These values represent 90% and over 100% of the 32 mg-N per L TN removed. However, the low DO conditions and lack of NO3− production measured in the sample port suggests that denitrification was not a significant removal mechanism. Nitritation–denitritation could have been a significant nitrogen loss mechanism in SAGB4, but partial nitritation ANAMMOX would have been a viable removal mechanism as well. And, the unlikely scenario that 100% of the COD removal was a consequence of denitritation increases the likelihood of significant partial nitritation ANAMMOX removal being required to close the nitrogen mass balance. DNA analysis of the attached growth at the end of the experiment again suggested the presence of Candidatus Brocadia with a SimRank of 78–97% for the four samples analyzed.
This study provides compelling evidence that SAGBs with pH-controlled aeration can remove similar amounts of TN via the partial nitritation ANAMMOX process at 20 °C while utilizing substantially less aeration than timer-controlled SAGBs that perform nitrification–denitrification. This result is significant since only recently has a stable nitritation ANAMMOX culture been reported to operate below 25 °C at the lab-scale.24,33 De Clippeleir's work utilized a lab-scale, rotating biological contactor at 15 °C. Hu's experiment was done in a lab-scale sequencing batch reactor initially at 30 °C followed by a gradual temperature reduction to as low as 12 °C. Both studies showed partial nitritation ANAMMOX to be feasible at these lower temperatures while treating relatively low-ammonia wastewater. Our data also supports the feasibility of utilizing one-stage bioreactors34 for partial nitritation ANAMMOX at a larger, pilot-scale and with autonomous, pH-controlled aeration. Our partial nitritation ANAMMOX associated NO2− utilization rates of 1.7–3.2 mg-N per L per day were much lower than reported rates for suspended cell cultures (400–1100 mg per L per day).34 But, this was expected since the cell cultures were studied at ideal temperatures and at a much higher cell densities than can be expected in SAGBs. The relatively high NH4+ concentrations in the effluent of the smart-aerated SAGBs is an indication that more research is needed to optimize AOB and ANAMMOX activity while minimizing NOB activity. Nonetheless, this study expands our understanding of the promise and current limitations of smart-aerated, partial nitritation ANAMMOX SAGBs for biological nitrogen removal.
Conclusions
The SAGBs with smart-aeration, operating in partial nitritation ANAMMOX mode, required less aeration (1344 L and 660 L) than the timer-controlled SAGBs (1728 L) during the 48 hour intensive sampling period while achieving a similar level of TN removal at 20 °C. This represents an aeration-associated energy efficiency benefit of over 50%. But, high effluent NH4+ concentrations (11–21 mg L−1) in the smart-aerated SAGBs indicated that research is needed to optimize the operational parameters to maximize TN removal, meet NH4+ discharge limits and gain the reduced aeration benefit from partial nitritation ANAMMOX SAGBs.Acknowledgements
The authors acknowledge the financial support of Donald Bently and Dick Konzen and thank Dr Charles Bott for graciously providing ANAMMOX bacteria. Research support was provided by Jonathan Durst, Brandon Barquist and Katie Langenfeld.References
- S. Schlegel and H. Koeser, Water Sci. Technol., 2007, 55, 83–89 CrossRef CAS
.
-
DWA/ATV, Plants with Submerged Fixed Beds – ATV Manual for Biological and Advanced Wastewater Treatment (in German), Berlin, 1997 Search PubMed
.
- H. Dong, Z. Qiang, T. Li, H. Jin and W. Chen, J. Environ. Sci., 2012, 24, 596–601 CrossRef CAS
.
- J. Fan, S. Liang, B. Zhang and J. Zhang, Environ. Sci. Pollut. Res. Int., 2013, 20, 2448–2455 CrossRef CAS PubMed
.
- G. Maltais-Landry, R. Maranger, J. Brisson and F. Chazarenc, Water Res., 2009, 43, 535–545 CrossRef CAS PubMed
.
- C. Ouellet-Plamondon, F. Chazarenc, Y. Comeau and J. Brisson, Ecol. Eng., 2006, 27, 258–264 CrossRef PubMed
.
- E. D. Redmond, C. L. Just and G. F. Parkin, Water Environ. Res., 2014, 86, 305–313 CrossRef CAS
.
- J. Fan, W. Wang, B. Zhang, Y. Guo, H. H. Ngo, W. Guo, J. Zhang and H. Wu, Bioresour. Technol., 2013, 143, 461–466 CrossRef CAS PubMed
.
-
P. B. Pedros and W. K. Dobie, WEFTEC, 2006 Search PubMed
.
- F. R. Mahony Associates, Amphidrome, http://www.amphidrome.com, accessed web page.
- P. B. Pedros, J. Y. Wang and H. Metghalchi, J. Environ. Eng., 2007, 133, 191–197 CrossRef CAS
.
- P. B. Pedros, A. Onnis-Hayden and C. Tyler, Water Environ. Res., 2008, 80, 222–228 CrossRef CAS
.
-
P. B. Pedros, C. Cherchi, A. Onnis-Hayden and E. Wenger, WEFTEC, 2007 Search PubMed
.
- M. Strous, J. J. Heijnen, J. G. Kuenen and M. S. M. Jetten, Appl. Microbiol. Biotechnol., 1998, 50, 589–596 CrossRef CAS
.
-
Water Environment Federation, Biofilm Reactors, WEF Manual of Practice No. 35, WEF Press, Alexandria, Virginia, 2010 Search PubMed
.
-
M. O'Shaughnessy, J. Sizemore, M. Musabyimana, P. Sanjines, S. Murthy, B. Wett, I. Takacs, D. Houweling, N. G. Love and K. Pallansch, WEFTEC, 2008 Search PubMed
.
- K. A. Third, A. O. Sliekers, J. G. Kuenen and M. S. Jetten, Syst. Appl. Microbiol., 2001, 24, 588–596 CrossRef CAS PubMed
.
- W. Zeng, Y. Zhang, L. Li, Y.-z. Peng and S.-y. Wang, Enzyme Microb. Technol., 2009, 45, 226–232 CrossRef CAS PubMed
.
- H. Zhao, R. Lemaire, M. Christensson, G. Thesing, F. Veuillet, J. Ochoa, D. Lamarre and A. Gadbois, Single-Stage Deammonification Process Performance-MBBR Versus IFAS Configurations, WEF Nutrient Removal and Recovery, 2013.
- B. Wett, Water Sci. Technol., 2006, 53, 121–128 CrossRef CAS
.
- L. Y. Zhang, L. Zhang, Y. D. Liu, Y. W. Shen, H. Liu and Y. Xiong, Desalination, 2010, 250, 915–920 CrossRef CAS PubMed
.
- K. Furukawa, P. K. Lieu, H. Tokitoh and T. Fujii, Water Sci. Technol., 2006, 53, 83 CrossRef CAS
.
- H. Gao, Y. D. Scherson and G. F. Wells, Environ. Sci.: Processes Impacts, 2014, 16, 1223–1246 CAS
.
- Z. Hu, T. Lotti, M. de Kreuk, R. Kleerebezem, M. van Loosdrecht, J. Kruit, M. S. Jetten and B. Kartal, Appl. Environ. Microbiol., 2013, 79, 2807–2812 CrossRef CAS PubMed
.
- Worldwide Water, North America's first full-scale DEMON® deammonification system recognized, http://www.worldwaterworks.com/resources/press-releases/aaees-honor-award, accessed web page.
- S. Humbert, J. Zopfi and S.-E. Tarnawski, Environ. Microbiol. Rep., 2012, 4, 484–490 CrossRef CAS PubMed
.
- T. Z. DeSantis, P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu and G. L. Andersen, Appl. Environ. Microbiol., 2006, 72, 5069–5072 CrossRef CAS PubMed
.
- C. G. Klatt and T. M. LaPara, Biotechnol. Bioeng., 2003, 82, 313–320 CrossRef CAS PubMed
.
-
APHA, AWWA and WPCF, Standard methods for the examination of water and wastewater, 22nd edn, Washington, DC, 2012 Search PubMed
.
- Code Federal Regulations, Title 40—Protection of Environment, parts 136–149, US Government Printing Office, Washington, DC, 2012.
- B. Wett, G. Nyhuis, S. Podmirseg, M. Gómez - Brandón, T. Puempel, M. Hell, W. Kirchler, M. Cesconi and S. Murthy, Population dynamics at the limits of DEMON plant operations, http://www.essdemon.com/libraries.files/Wett_DEMON_plant_operations.pdf, accessed web page.
-
B. Rittmann and P. McCarty, Environmental Biotechnology: Principles and Applications, McGraw-Hill, New York, NY, 2001 Search PubMed
.
- H. De Clippeleir, S. E. Vlaeminck, F. De Wilde, K. Daeninck, M. Mosquera, P. Boeckx, W. Verstraete and N. Boon, Appl. Microbiol. Biotechnol., 2013, 97, 10199–10210 CrossRef CAS PubMed
.
- T. Lotti, R. Kleerebezem, C. Lubello and M. C. M. van Loosdrecht, Water Res., 2014, 60, 1–14 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |