Facilitated transport of small molecules and ions for energy-efficient membranes†
Yifan
Li
ab,
Shaofei
Wang
ab,
Guangwei
He
ab,
Hong
Wu
ab,
Fusheng
Pan
ab and
Zhongyi
Jiang
*ab
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: zhyjiang@tju.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
First published on 9th October 2014
Abstract
In nature, the biological membrane can selectively transport essential small molecules/ions through facilitated diffusion via carrier proteins. Intrigued by this phenomenon and principle, membrane researchers have successfully employed synthetic carriers and carrier-mediated reversible reactions to enhance the separation performance of synthetic membranes. However, the existing facilitated transport membranes as well as the relevant facilitated transport theories have scarcely been comprehensively reviewed in the literature. This tutorial review primarily covers the two aspects of facilitated transport theories: carrier-mediated transport mechanisms and facilitated transport chemistries, including the design and fabrication of facilitated transport membranes. The applications of facilitated transport membranes in energy-intensive membrane processes (gas separation, pervaporation, and proton exchange membrane fuel cells) have also been discussed. Hopefully, this review will provide guidelines for the future research and development of facilitated transport membranes with high energy efficiency.
Key learning points(1) The panorama of facilitated transport and its important implications.(2) Chemistries and reactions involved in facilitated transport. (3) Approaches to exploring advanced functional materials to facilitate the transport of molecules and ions. (4) Application paradigms of facilitated transport in membrane processes. (5) Design of energy-efficient, high-performance membranes with a facilitated transport feature through biomimetic and bioinspired strategies. |
1. Introduction
A high-performance membrane which allows fast and selective transmembrane permeation of small molecules/ions is essential for triggering revolutionary changes in many significant chemical processes.1 As the pore size of the membrane falls below 1 nm, the relevant membrane processes can easily inherit the benefits from equilibrium-governed separation and rate-governed separation, thus acquiring high energy efficiency. The permeation of small molecules through the membrane is usually described by the well-known “solution–diffusion” mechanism, where solubility and diffusivity are governed by thermodynamic and kinetic/transport factors, respectively. Therefore, the ideal membranes should render an appropriate chemical microenvironment to ensure high solubility, and also possess a well-tailored microstructure to ensure high diffusivity. In this regard, molecular sieve membranes seem to be the preferred choice for molecular transport, which have great potential for simultaneous enhancement of permeability and selectivity. However, molecular sieve membranes may not be the best choice for ion transport, which strongly relies on electrochemical interactions. Also, the difficulties in fabricating molecular sieves into defect-free thin membranes impede the broad applications of the molecular sieving membrane.2 Consequently, interest has been growing in the selective transport mechanisms which allow efficient enrichment of the desired permeant based on broader material chemistries and more specific interactions.If we take a look at nature, we can easily find an ideal model-biological membrane, which can selectively transport essential small molecules/ions through facilitated diffusion via carrier protein. Early evidence of carrier-mediated facilitated diffusion was traced back to half a century ago.3 As one important type of structure proteins of the cell membrane, a carrier protein can specifically and reversibly bind small molecules (e.g. sugar, amino acid, and nucleotide) or ions (e.g. Na+, K+, Mg2+, Ca2+, Cl−, HCO3−), and transport them to the other side of the cell membrane via conformational variation. The specificity of facilitated diffusion arises from the ingenious integration of multiple types of interactions between carrier protein and the target permeant, e.g. electrostatic interactions, hydrogen bond interactions, hydrophobic interactions, cation–π interactions, etc. Herein the biological term “facilitated diffusion” highlights the contribution of molecular recognition based on specific interactions to the overall transmembrane permeability. According to the function of carrier protein, “facilitated transport” is also often used as a substitute of facilitated diffusion, and carrier protein is also called “transport protein” or a “transporter”.4
Intrigued by the carrier-involved model developed for the cell membrane, we can design a “carrier” for synthetic membranes by mimicking the function of carrier protein, so as to enable facilitated transport of small molecules or ions through membranes. Considering the complexity of molecular recognition and conformational variation for carrier protein, the simplified carrier for “in vitro” facilitated transport is expected to reversibly react with the target species/permeants followed by the formation of a transient complex. The transport of the target permeant is thus enabled by the motion of the complex (mobile carrier) or the hopping of the target permeant from one carrier to another (fixed carrier). Compared to the solubility-selective and diffusivity-selective transport, reactivity-selective transport mediated by carriers appears to be a much more specific transport manner. From a thermodynamics viewpoint, the introduction of reversible reactions is favorable for acquiring high energy efficiency. In this way, an excellent paradigm for overcoming the “tradeoff” effect between permeability and selectivity can be portrayed by borrowing reactivity selectivity from carriers.5 For example, facilitated transport membranes have been successfully explored for diverse membrane systems: (1) CO2 removal from diverse sources (e.g. natural gas, flue gas, and shift gas, where CO2/CH4, CO2/N2, CO2/H2 separation are the common tasks, respectively), typically using amino group containing membranes;5 (2) oxygen enrichment from air (O2/N2 separation), typically using cobalt porphyrin containing membranes;6 (3) olefin/paraffin separation (ethylene/ethane, propylene/propane separation), typically using Ag+-containing membranes;7 (4) gasoline desulphurization (thiophene/octane separation), typically using transition metal ion-containing membranes;8 (5) heavy metal ion recycling, typically using extractive membranes.9 Although the term “facilitated transport membrane” has rarely been employed to describe the proton exchange membrane in the literature, facilitated transport of proton with acidic groups as donors and basic groups as acceptors has already been widely acknowledged because the rapid transfer of proton relies heavily on a fundamental proton transfer reaction.10 Furthermore, the well-known vehicle mechanism and Grotthus mechanism proposed for the proton exchange membrane is akin to the facilitated transport mechanism mentioned above.11 In this sense, integrating the proton exchange membrane into the topic of the “facilitated transport membrane” may be helpful to understand the common features of molecule transport and ion transport, and acquire the whole picture of facilitated transport membranes.
Facilitated transport theories play pivotal roles in the rational design and tunable fabrication of facilitated transport membranes. On one hand, carrier-mediated transport mechanisms should be included, which are closely related to the carrier mobility and the physicochemical properties of the membrane matrix. On the other hand, facilitated transport theories should encompass the various types of carriers and the corresponding reversible carrier–permeant reactions, which can be merged in “facilitated transport chemistries”. In summary, facilitated transport theories are comprised of carrier-mediated transport mechanisms and facilitated transport chemistries, which reflect the physical and chemical aspects of facilitated transport, respectively.
This tutorial review focuses on the design and fabrication of high-performance dense membranes for energy-intensive processes with deep insights into facilitated transport theories, encompassing carrier-mediated transport mechanisms and facilitated transport chemistries. The potential applications of facilitated transport membranes are also summarized. Due to the limitation of the length of this article as well as the scope of energy-intensive processes, the membrane processes are confined to gas separation, pervaporation and proton exchange membrane fuel cells. More comprehensive review articles concerning other membrane processes can be found elsewhere.4,9
2. Facilitated transport membranes
According to the mobility of carriers, facilitated transport membranes can be classified into three types: (1) a mobile carrier membrane, through which the carrier can diffuse freely; (2) a semi-mobile carrier membrane, in which the carrier can migrate elsewhere at a cost of high diffusional activation energy; (3) a fixed-site carrier membrane, in which the carrier can only vibrate within a confined nanospace, rather than migrate elsewhere. Correspondingly, these three types of facilitated transport membranes exhibit distinct differences in phase states, materials types, and especially, transport mechanisms. Mobile carrier membranes are usually liquid membranes, of which the major ingredient is often carrier solution or carrier-bearing liquid compounds, e.g. ionic liquid. The transport of the target permeant through mobile carrier membranes obeys the vehicle mechanism, in which the carrier serves as a “ferryboat” plying between the two sides of membrane (Fig. 1a). Both the semi-mobile carrier and fixed-site carrier membranes are solid-state membranes, of which polymer materials are often utilized as the hosting matrix. For fixed-site carrier membranes, the target molecule/ion has to pass through the membrane via carrier-to-carrier hopping (Fig. 1c). In a general sense, this mechanism is called the “hopping mechanism”, as proposed by Cussler and co-workers.12 Semi-mobile carrier membranes are usually composed of a plasticized polymer matrix and physically restricted carriers whose mobility lies in between a mobile carrier and a fixed-site carrier. As a consequence, both the vehicle mechanism and the hopping mechanism dominate the facilitated transport process (Fig. 1b). Such a definition of a semi-mobile carrier membrane allows a novel survey of facilitated transport membranes with different carrier types and two basic transport mechanisms: vehicle mechanism and hopping mechanism.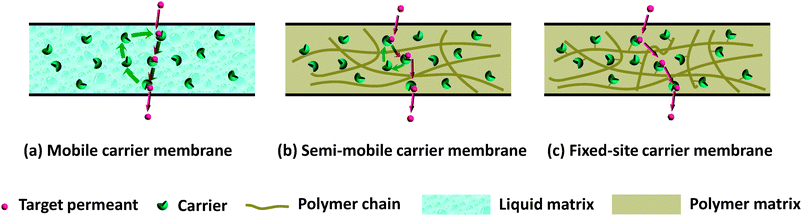 | ||
| Fig. 1 Schematic illustration of the carrier-mediated transport mechanisms for (a) a mobile carrier membrane; (b) a semi-mobile carrier membrane; and (c) a fixed-site carrier membrane. | ||
2.1 Facilitated transport membranes with mobile carrier
Mobile carrier membrane is the simplest type of facilitated transport membrane. With the active carrier distributed within the liquid matrix, the mobile carrier membrane is akin to the cell membrane in view of both structures and facilitated transport properties. The liquid matrix as a continuous phase manipulates higher permeability than the solid polymer matrix, and the vehicle-like carriers further facilitate the transport of the target permeant. Presently, mobile carrier membranes have been utilized to facilitate the transport of CO2, O2, olefins, metal ions, and biomolecules. Generally, the total flux of the target permeant, JA, can be illustrated as the sum of Fickian diffusion and carrier-mediated diffusion, as shown in eqn (1):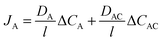 | (1) |
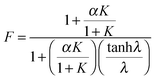 | (2) |
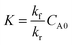 | (3) |
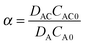 | (4) |
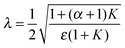 | (5) |
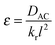 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λ)/λ. A maximum F is reached when λ → ∞ and thus (tanh
λ)/λ. A maximum F is reached when λ → ∞ and thus (tanh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λ)/λ → 0, while F decreases to 1 when λ → 0 and thus (tanh
λ)/λ → 0, while F decreases to 1 when λ → 0 and thus (tanh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λ)/λ → 1. According to eqn (5), λ monotonically increases with K and α, and therefore high K and α values are desired. Since kr and DAC also affect the value of ε, high kf and CAC0 as well as moderate kr and DAC are favored, which highlights the importance of both reaction kinetics and thermodynamics.
λ)/λ → 1. According to eqn (5), λ monotonically increases with K and α, and therefore high K and α values are desired. Since kr and DAC also affect the value of ε, high kf and CAC0 as well as moderate kr and DAC are favored, which highlights the importance of both reaction kinetics and thermodynamics.
A mobile carrier membrane is usually fabricated into supported liquid membrane (SLM) to maintain sufficient mechanical stability. However, the development of practical SLMs requires the prudent consideration of the tricky liquid/carrier loss issue, because volatile or low-viscosity liquid is subject to being expelled out under elevated temperature or cross-membrane pressure difference. A supported ionic liquid membrane (SILM) might be a promising alternative to suppressing the liquid loss, owing to its negligible vapour pressure and high viscosity. Besides, the high flexibility of ionic liquid chemistry allows elaborate design and synthesis of appropriate SILMs. The carrier can be either a solute or a portion of the ionic liquid, and both charged and uncharged carriers are available. Although carrier mobility will be somewhat restricted by the high viscosity of ionic liquid, the highly ionic matrix has already allowed hopping of the ionic-state complex, especially when facilitating the transport of CO2 or olefins.7,14 In this sense, SILM has been rendered some characteristics of semi-mobile carrier membranes.
2.2 Facilitated transport membranes with a semi-mobile carrier
The term “semi-mobile carrier membrane” is new but essential because a large quantity of facilitated transport membranes cannot be simply classified into a mobile carrier membrane or a fixed-site carrier membrane. For example, water is an important carrier for CO2 and proton in a hydrated polymer matrix. If the polymer matrix is fully swollen by water, this type of membrane is no more a fixed-site membrane in the strict sense because the water-mediated vehicle mechanism may also play a pivotal role in facilitated transport. In a broader sense, when small molecules with active carriers are doped into polymer membranes, the carriers might migrate if the polymer matrix is plasticized or swollen by a solvent. This type of membrane was initially called a “polymer inclusion membrane” or a “polymeric plasticized membrane” for selectively extracting heavy metal ions or small biomolecules from aqueous sources.9 Actually, such semi-mobile carrier membranes have been also reported for CO2 separation.15Obviously, if there is a continuous liquid mesophase or macrophase spanning the entire membrane thickness, a semi-mobile carrier membrane will become analogous to the mobile carrier membrane due to the reduced contribution from the hopping mechanism. Therefore, it can be deduced that the liquid or aqueous phase which usually exists within the semi-mobile carrier membrane is required to simultaneously plasticize the polymer matrix and accommodate the carriers. With the integration of the liquid phase and the solid polymer matrix, the semi-mobile carrier membrane is expected to combine the merits of both a mobile carrier membrane and a fixed-site carrier membrane by compromising permeability and stability. Unfortunately, few efforts have been devoted to elucidating the relevant “mixed-matrix” facilitated transport mechanism. The mixed Grotthuss and vehicle transport mechanism in proton conducting polymers revealed by ab initio molecular dynamics simulations may aid in further elucidating the transport mechanism of semi-mobile carrier membranes at the molecular level.
2.3 Facilitated transport membranes with a fixed-site carrier
A fixed-site carrier membrane was originally developed due to the instability of the liquid mobile carrier membrane. Traditional fixed-site carrier membranes consist of merely polymeric materials, and the carriers must be covalently bound to polymer chains. In recent years, the rapid development of the mixed matrix membrane or polymer composite membrane has created new opportunities to design diverse fixed-site carrier membranes.16 Alternatively, a carrier can be immobilized by either the polymer matrix or the filler phase of the mixed matrix membrane. In this sense, the carrier does not necessarily come from the side-chain of the polymer, which significantly broadens the range of screening suitable matrix polymers.Actually, in the past five years the scope of fixed-site carrier membranes has been dramatically enlarged in the field of CO2 separation. Since carrier mobility depends on not only the connection mode of the carrier but also the plasticization or swelling state of the polymer matrix, a membrane containing both covalently bound carrier and physically doped carrier may be also classified into the fixed-site carrier membrane. Nevertheless, the majority of carriers are not permitted to migrate in an ordinary working state. With the definition of the semi-mobile carrier membrane, it is explicit that a fixed-site carrier membrane containing physically doped carrier may switch to the semi-mobile carrier membrane or even mobile carrier membrane with the increase of the swelling degree of the polymer matrix.
For fixed-site carrier membranes, hopping mechanism is the dominant facilitated transport mechanism. Despite this, the present hopping mechanism assuming a carrier-to-carrier hopping might be too simple. For example, facilitated transport was thought to occur only when the distance between two carriers was shorter than the diffusional jump distance, as obtained from the diffusional activation energy. However, even at extremely low carrier concentration, facilitated transport of O2 was also observed,17 which might be attributed to the carrier-mediated increase in the chemical potential of O2. Considering the weak polarity of O2, its concentration around a carrier should be much higher than that in the matrix, and therefore the “hopping” model needs modification at low carrier concentrations. Another important issue is the effect of diffusion resistance on carrier-to-carrier hopping. Theoretically, a carrier cannot show high activity unless it is accessible for the target permeant. A high free volume matrix caused by swelling or the intrinsic porosity favors hopping, while a dense matrix may hinder hopping, even resulting in “starvation” of the carrier. Based on the insufficient exploitation of carrier activity caused by the diffusion resistance of the membrane, Li et al.5 proposed the concept of “undesired membrane structure”, which may be solved by isolating the polar carrier groups from each other by rigid aromatic groups and by packing the polymer chains in disordered manners. As a consequence, a deep insight into hopping mechanism for fixed-site carrier membranes is essential to comprehensively consider carrier properties and the effect of its surroundings.
3. Facilitated transport chemistries
An in-depth understanding of facilitated transport theories based on facilitated transport chemistries is essential to the rational design of facilitated transport membranes. The carriers are traditionally classified simply based on several specific target permeants, e.g. O2, CO2, olefin, and sugar, etc. Actually, facilitated transport theories are applicable to a broader range of molecules and ions, therefore it is necessary to re-arrange the knowledge points about facilitated transport chemistries based on the limited types of reversible reactions. Herein, four basic types of reversible reactions are included, namely proton transfer reaction, nucleophilic addition reaction, π-complexation reaction and electrochemical reaction. Carriers and the corresponding target permeants are therefore closely connected with reversible reactions as the ligament (Fig. 2).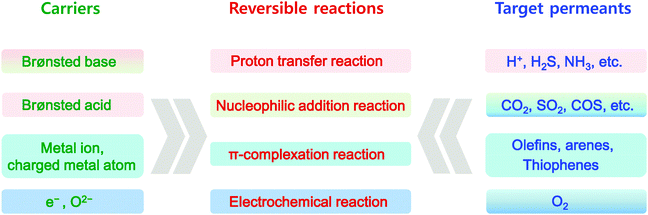 | ||
| Fig. 2 The four typical types of reversible reactions for facilitated transport membranes together with their corresponding carriers and target permeants. | ||
3.1 Facilitated transport chemistries in terms of proton transfer reaction
Proton transfer reaction is one type of acid–base reaction that can be described by Brønsted–Lowry acid–base theory. Proton transfer reaction can facilitate proton transport itself, as well as the transport of some small molecules with Brønsted acidity or basicity, e.g. H2S, NH3, etc. In other cases, for example, facilitated CO2 transport, although proton transfer reaction is not the first-step reaction between the carrier and the target permeant, it remains important to complete the cycle of multi-step reactions and release uncomplexed carriers.Facilitated proton transport is primarily mediated by acid–base pairs. Strictly, proton transfer between a conjugate acid–base pair (generally expressed as HA/A− or BH+/B) does not belong to the proton transfer reaction because of no production of new intermediates. Only if a second Brønsted base (B′) serves as the proton acceptor from HA or a second Brønsted acid (HA′) serves as the proton donor towards B, proton transfer reaction will occur following one formula of eqn (7) and (8):
| HA + B′ ⇌ B′H+ + A− | (7) |
| B + HA′ ⇌ BH+ + A′− | (8) |
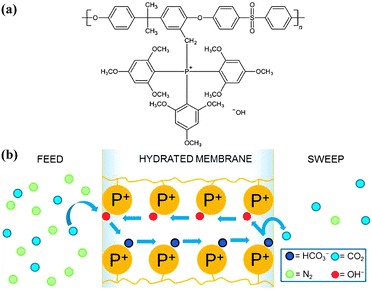
Considering that most proton exchange membrane materials bear −SO3H or −PO3H2 groups, Brønsted base as a carrier has attracted more attention. As mentioned above, water is an important proton carrier which is capable of facilitating both vehicle and Grotthus mechanisms. Actually, water as a Brønsted base provides a conjugate acid–base pair as H3O+/H2O, triggering a proton transfer reaction as eqn (9):
| HA + H2O ⇌ H3O+ + A− | (9) |
Consequently, water often functions as a proton transfer bridge between proton donating groups and proton accepting groups when the distance between them exceeds the mean free path of proton hopping. Furthermore, water plays critical roles in dissociation of acid groups and construction of a continuous hydrogen-bonding network for successive proton hopping.
Water is an essential proton carrier in the overwhelming majority of cases, while other Brønsted bases may be also available as a proton carrier based on water-independent proton transfer. Kreuer and co-workers stated that carrier-mediated proton conductivity was simultaneously determined by proton donating and proton accepting capacities.18 A relatively stronger Brønsted base than water can form an acid–base pair with an acid group through proton transfer reaction. Taking the typical –NH2 group as an example, it can be closely linked to a proton donating group (typically –SO3H or –PO3H2), and the protonation/deprotonation of each group would be facilitated by the electrostatic attraction of the other groups, thus greatly reducing the energy barrier for proton hopping.19,20 Azole groups (e.g. imidazole, triazole, tetrazole) as another important family of Brønsted bases were also reported to form acid–base pairs with –SO3H or –PO3H2 groups, which drastically enhanced proton conduction.21–23
There are still some proton exchange membrane materials based on basic groups, e.g. chitosan, polybenzimidazole. In particular, those containing azole groups are expected to be operated at anhydrous state, which is required for high-temperature, low-humidity fuel cell membrane.24 Without the acid groups, azole rings have to flit to enable long-range proton hopping between adjacent azole rings, and therefore the construction of acid–base pairs would dramatically facilitate such a rate-limiting step of long-range proton hopping. Since phosphoric acids are also anhydrous proton conductors, the integration of azole-based polymer matrix and phosphoric acid is expected to facilitate proton transport in the absence of water.24,25
3.2 Facilitated transport chemistries in terms of nucleophilic addition reaction
Nucleophilic addition reaction often occurs at the C atom of an asymmetric double bond (e.g. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, S
O, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The displacement of the electron cloud towards the O atom occurs, and therefore the positively charged C atom is prone to be attacked by nucleophilic agents, e.g. water, OH−, amino groups, etc. In this manner, nucleophilic addition reaction allows facilitated transport of small molecules C
O). The displacement of the electron cloud towards the O atom occurs, and therefore the positively charged C atom is prone to be attacked by nucleophilic agents, e.g. water, OH−, amino groups, etc. In this manner, nucleophilic addition reaction allows facilitated transport of small molecules C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, e.g. CO2, SO2, COS, etc. Since the common nucleophilic agents belong to Brønsted base, herein the major carrier for nucleophilic addition reaction is concluded as a Brønsted base in Fig. 2. On the other hand, facilitated transport of CO2 has become one of the most important and representative topics about facilitated transport membranes. Various types of Brønsted bases, such as OH−, CO32−, F−, PO43−, –COO−, amino groups, and even water, can be designed as CO2 carriers, which bring about difficulty in understanding the common facilitated transport mechanism. From the viewpoint of nucleophilic addition reaction, it is possible to categorize different types of CO2 carriers and systematically compare their facilitating effects.
O bond, e.g. CO2, SO2, COS, etc. Since the common nucleophilic agents belong to Brønsted base, herein the major carrier for nucleophilic addition reaction is concluded as a Brønsted base in Fig. 2. On the other hand, facilitated transport of CO2 has become one of the most important and representative topics about facilitated transport membranes. Various types of Brønsted bases, such as OH−, CO32−, F−, PO43−, –COO−, amino groups, and even water, can be designed as CO2 carriers, which bring about difficulty in understanding the common facilitated transport mechanism. From the viewpoint of nucleophilic addition reaction, it is possible to categorize different types of CO2 carriers and systematically compare their facilitating effects.
Based on the theory of nucleophilic addition reaction, the reaction rate is determined by the nucleophilicity of the nucleophilic agent, which is further determined by both the basicity and polarizability of the nucleophilic atom. For the most Brønsted bases utilized as a CO2 carrier, the nucleophilic atoms (N, O, F atoms) are localized in the same row of Periodic Table of the Elements, and hence the basicity of carrier is the major factor that determines nucleophilicity. Consequently, a strong Brønsted base is preferred to acquire high carrier reactivity.
The conjugative base of a weak acid is usually a strong base. These anion-type carriers are better nucleophilic agents than non-ionic carriers. In theory, OH− should have been the best choice because of its strong basicity and easy availability. The direct addition of OH− onto CO2 results in HCO3−:
| CO2 + OH− ⇌ HCO3− | (10) |
 | ||
| Fig. 3 (a) Chemical structure of tris(2,4,6-trimethoxyphenyl)polysulfone-methylene quaternary phosphonium hydroxide; and (b) the facilitated-transport pathway. Reprinted with permission from ref. 27. Copyright 2014 Wiley-VCH. | ||
Another representative anion-type carrier is carboxylate group (–COO−). Despite weaker basicity than OH−, –COO− as an organic carrier can be covalently bound to polymer chains, resulting in a more stable fixed-site carrier membrane. When utilizing –COO− as a carrier, H2O not only assists in the dissociation of the carboxylate salt but also acts as a direct carrier that triggers another nucleophilic addition reaction:
| CO2 + H2O ⇌ H2CO3 | (11) |
The carboxylate group as an indirect carrier can further react with H2CO3 and shift the equilibrium of eqn (11) to the right:
| –COO− + H2CO3 ⇌ –COOH + HCO3− | (12) |
Actually, many anion carriers facilitate CO2 transport following the similar reaction mechanism as eqn (11) and (12). Few of them have been explored because they exhibit weaker basicity than OH− and cannot be covalently immobilized to polymer chains like –COO−.
Amino groups are the typical non-ionic CO2 carriers which can be covalently connected to polymer chains. Despite the non-ionic characteristics, amino groups usually display stronger basicity than –COO−, as supported by the higher pKa value of NH4+ (9.26) than CH3COOH (4.76). The reaction between CO2 and primary or secondary amino groups was reported as the following formulae:
| CO2 + RR′NH ⇌ RR′NH+COO− | (13) |
| RR′NH+COO− + RR′NH ⇌ RR′NCOO− + RR′NH2+ | (14) |
| RR′NH+COO− + H2O ⇌ RR′NCOO− + H3O+ | (15) |
| RR′NCOO− + H2O ⇌ RR′NH + HCO3− | (16) |
For tertiary amino group, the lack of active H atom does not permit the zwitterion mechanism. Instead, H2O acts as a carrier to firstly react with CO2 like eqn (11), and the tertiary amino group as another carrier can further react with H2CO3 and shift the equilibrium of eqn (11) to the right:
| RR′R′′N + H2CO3 ⇌ RR′R′′NH+ + HCO3− | (17) |
| RR′R′′N + CO2 + H2O ⇌ RR′R′′NH+ + HCO3− | (18) |
Intrigued by the development of amine-based chemical adsorption agents, Ho's group developed sterically hindered amine to increase CO2 loading capacity and accelerate the amine–CO2 reactions.28 A sterically hindered amine is defined as either a primary amine in which the N atom is attached to a tertiary C atom or a secondary amine in which the N atom is attached to at least one secondary or tertiary C atom. The alkyl group inhibits the formation of a carbamate ion as shown in eqn (14), and thus facilitates the formation of HCO3− as shown in eqn (15)–(17). Zhao and Ho firstly reported the effect of amine steric hindrance in a solid phase and demonstrated encouraging facilitation effects.28 They synthesized a series of sterically hindered polyamines by grafting alkyl groups to the primary amine group of polyallylamine (PAAm). This work also implies the possibility of further understanding facilitated transport by distinguishing the contributions from the steric hindrance effect, electron donating effect and carrier content.
As mentioned above, water is an important non-ionic CO2 carrier, while it is a weak Brønsted base. Without another stronger base as a carrier, water can also efficiently convert CO2 into HCO3− by stabilizing the deprotonated intermediate, OH−, and transporting the synchronously generated H+ elsewhere. In biological organisms, carbonic anhydrase can catalyze the hydration of CO2, and the active center is comprised of a zinc ion coordinated by three imidazole groups of histidine residues. Considering the following equilibrium:
| (His)3Zn(OH2) ⇌ (His)3Zn(OH−) + H+ | (19) |
| (His)3Zn(OH−) + CO2 + H2O ⇌ (His)3Zn(OH2) + HCO3− | (20) |
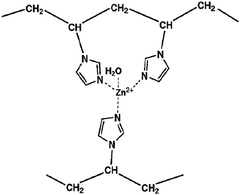 | ||
| Fig. 4 Chemical structure of a poly(N-vinylimidazole)–zinc complex. Reprinted with permission from ref. 29. Copyright 2012 the Royal of Social Chemistry. | ||
3.3 Facilitated transport chemistries in terms of π-complexation
Facilitated transport based on π-complexation is mainly limited to the transport of unsaturated small molecules, e.g. olefins, aromatic compounds, thiophenes, O2 and CO2. Majority of the carriers for π-complexation are transition metal ions, while the positively charged transition metal atoms have been also explored as an olefin carrier. The fundamental interactions of π-complexation consist of cation–π and π–d interactions. From the perspective of acid–base reaction, π-complexation can be perceived as another type of acid–base reaction on the basis of Lewis acid–base theory and hard–soft–acid–base (HSAB) theory. According to the polarizability of the electron-donating atom of an unsaturated bond, olefins, aromatic compounds, thiophenes are categorized into soft base (C or S atom provides electron), while O2 and CO2 belong to hard base (O atom provides electron).Olefins are the most representative target permeants as soft bases. Theoretically, any metal ion that belongs to soft acids can be selected as an olefin carrier, e.g. Ag+, Pd2+, Hg2+, Cd2+, Cu+, Au+, etc. Ag+ as an olefin carrier has been intensively investigated because of its high activity, low toxicity and moderate chemical stability. The high activity of Ag+ can be explained as follows: Ag+ is a highly polarizable and deformable metal ion due to the unoccupied 5s orbitals and the activatable 4d-orbital electrons. The 5s orbitals of Ag+ can accept the π electrons donated from the occupied 2p orbitals of olefin molecules to form σ-bonds, and back-donation of electrons from the occupied 4d orbitals of Ag+ into the empty π*–2p antibonding orbitals of olefin molecules results in π-bonds. The synergic effect of σ-bonds and π-bonds strengthens the complexation bond, enabling the high specificity of Ag+–olefin complexation. One Ag+ can reversibly bind one or two olefin molecules, while the latter case corresponds to an intermediate state during the exchange of the complexed olefin molecule by a new one. That is, Ag+ usually forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with olefins:
1 complex with olefins:
| Ag+ + olefin ⇌ [Ag(olefin)]+ | (21) |
Among the Ag+-containing compounds, soluble silver salts are usually utilized to provide dissociated Ag+. These silver salts are usually added into a polar polymer matrix to form solid-stated polymer electrolyte membranes, where the activity of Ag+ is mainly determined by the counter ion and the available coordinating sites. The large anions such as BF4−, CF3SO3−, ClO4−, and SbF6− are preferred choices because of their low lattice energy and relatively weak Ag+–anion interactions. Among the available silver salts, AgBF4 and AgCF3SO3 show relatively high activity but insufficient stability. On the other hand, Ag+ coordinates with the polar ligand of the polymer, and a threshold concentration of Ag+ was observed because the coordination number by the ligands of the polymer should be less than 3 to ensure the reaction activity with olefins.17
Silver salts can be also added into ionic liquid to prepare SILMs, where Ag+ is immobilized within membrane via electrostatic attraction and its activity is no longer affected by the competitive coordinating effect. It becomes possible to investigate the effect of a large number of counter ions based on the platform of SILMs. What's more, new silver salts from room temperature ionic liquid have also been reported, in which Ag+ was the only cation. Such ionic liquids could maximize the concentration of Ag+ within SILMs.7
A positively charged metal atom has become another critical type of olefin carrier in recent years.31–33 By replacing Ag+ with silver nanoparticles (AgNP) of which the surface was positively charged by an organic electron-acceptor molecule, the Ag atoms at the surface of AgNP were found capable of facilitating olefin transport as efficient carriers. As shown in Fig. 5, a strong electron acceptor, 7,7,8,8-tetracyanoquinodimethane (TCNQ), was employed to tune the surface positive charge of a silver nanoparticle. When TCNQ was in contact with AgNP, the interface dipole was induced, so that high positive charge appeared on the AgNP surface. Such charged Ag atoms performed even better than Ag+ as olefin carriers within the membrane.33 More importantly, this judicious exploration provided great opportunities to well address the concerns on carrier stability. Other metal nanoparticles made of Au or Cu with charged surface atoms were also available in facilitating olefin transport.
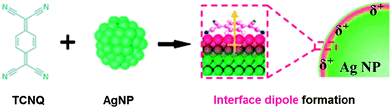 | ||
| Fig. 5 A schematic illustration of the surface positive charge on Ag nanoparticles induced by an electron acceptor. Reprinted with permission from ref. 33. Copyright 2011 Wiley-VCH. | ||
The carriers for O2 are quite different from those for soft bases. Considering the high electronegativity of the O atom and the unshared pair electrons in O2, O2 is a hard base with good affinity towards softer acids such as Co2+, Fe2+ and Mn2+. On the other hand, due to the electron withdrawing effect of O2, the center metal ions need higher electron density to lower the energy of metal–O2 complexes. Consequently, other Lewis bases such as electron-donating ligands are necessary to activate the O2 carrier. In biological organisms, haemoglobin smartly employs ferrous porphyrin (FeIIP) with an axial-direction histidyl residue as a fifth ligand to reversibly bind O2 (Fig. 6).17 Planner porphyrin ensures the stability of a metal complex, and the fifth ligand plays key roles in donating external electrons and inhibiting the irreversible formation of peroxido-bridged dimers. Intrigued by the structure of FeIIP, many researchers have attempted to design artificial carrier systems using different metal complexes. Considering the in vitro instability of FeII complexes in the presence of O2, the major efforts were devoted to developing CoII complexes. Among the various types of Co complexes, cobalt porphyrin (CoP) derivatives and cobalt–Shiff base complexes are perceived as highly active and stable carrier systems for O2-selective transmembrane permeation. However, in most relevant works the complexes were directly doped into liquid membranes or polymeric membranes. Examples about the covalent connection of such complexes to polymer chains have rarely been reported.
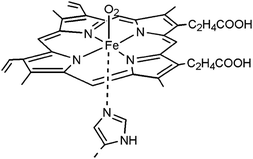 | ||
| Fig. 6 The complex between ferrous porphyrin and O2 with the aid of histidyl residue as a fifth ligand in haemoglobin. Reprinted with permission from ref. 17. Copyright 2006 Wiley-VCH. | ||
Metal ions or charged metal atoms as CO2 carriers were not reported until several years ago. It is notable that CO2 is a Lewis base rather than a Lewis acid owing to the lack of unoccupied orbitals. Analogous to O2, CO2 is also a hard base, while it is softer than O2 due to the delocalized π bond. Based on HSAB theory, hard acid and junction acid should be the better choices than Ag+, yet recently reported results imply that things are not so simple. Chung and co-workers embedded Zn2+ complexes into a glassy polymer membrane, and firstly verified the facilitation effect.34 Both O2/N2 and CO2/CH4 selectivities showed remarkable increment compared to the control membrane, indicative of the similar facilitated transport mechanisms. K+ was also reported as a CO2 carrier in the polyvinyl pyrrolidone (PVP) membrane, and it was the first time that a salt-doped solid-state membrane exhibited much higher CO2 permeance than the control membrane in the dry state.35 This result is surprising because the metal ions usually lead to higher polymer rigidity and lower free volume. The authors attributed the increase of CO2 permeance to the favorable interactions between K+ and CO2, whereas other possibilities such as non-selective cracks could not be excluded. In another impressive example, positively polarized Cu nanoparticles dispersed in ionic liquid simultaneously enhanced CO2/gas selectivity and CO2 permeance. The ionic liquid not only dissociated micro-sized Cu flakes into nanoparticles, but also induced surface positive charges like the case of TCNQ (Fig. 7). However, the facilitation effect was weaker than the case of olefin.36 We deduce that the uneven charge distribution and the linear molecule shape of CO2 would inevitably reduce the overlapping of electron clouds between Cu and CO2, resulting in weaker binding strength.
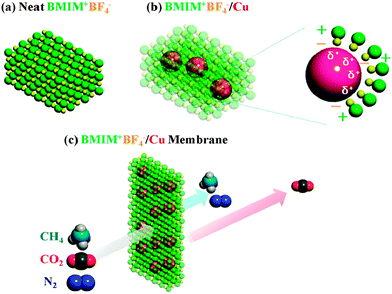 | ||
| Fig. 7 Dissociated and surface charged Cu nanoparticles by ionic liquid and complexation with CO2 molecules. Reprinted with permission from ref. 36. Copyright 2012 the Royal of Social Chemistry. | ||
3.4 Facilitated transport chemistries in terms of electrochemical reaction
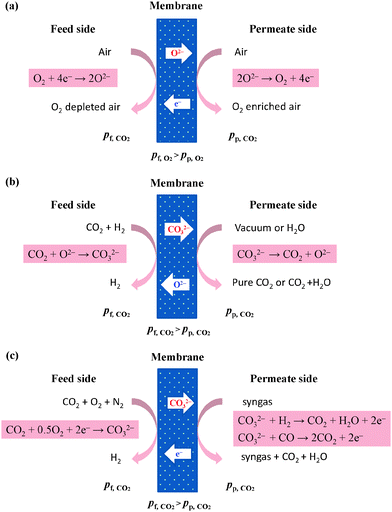
All the aforementioned reactions occur within the entire membrane. If a membrane is both ion conductive and electron conductive, it is feasible to trigger facilitated transport by electrochemical reactions, which typically occur at the two sides of membranes. This concept can be realized for O2 transport with a ceramic-based mixed conductor membrane as both an ion conductor and an electron conductor at high operation temperature.37 As shown in Fig. 8a, O2 molecules are firstly ionized by electrons at the feed side, and then are transported through the membrane in the form of O2−, followed by deionization at the permeate side. Synchronously, the released electrons are transported back to the feed side. A high temperature above 600 °C is usually required to activate these ion conducting processes. If the membrane material permits, the operation temperature ought to be as high as possible to reduce the ion conducting resistance. Sometimes the surface reaction may be the rate-controlling step, and a thin catalytic surface layer (usually consists of perovskite, with a general formula of ABO3) is required to accelerate the conversion of O2 to O2−. From the viewpoint of facilitated transport, electron can be regarded as the carrier, and the mixed conductor membranes for selective O2 transport can be regarded as a semi-mobile carrier membrane.Applying similar principles, two types of mixed conductors can be utilized to design CO2 separation membrane: mixed carbonate-ion and oxide-ion conductor (MOCC, Fig. 8b) and mixed carbonate-ion and electron conductor (MECC, Fig. 8c), which are suitable for pre-combustion and post-combustion CO2 capture, respectively.38 O2− is the carrier for the former case, while the latter case is actually an integration of an O2 selective membrane and a CO2 selective membrane. Unlike the case of a crystalline and solid oxide-ion conductor, molten carbonate is often filled into the membrane pores to conduct carbonate ion. Consequently, the membrane framework should be highly porous to ensure continuous distribution of a carbonate-ion conductor. According to the definition of three types of facilitated transport membranes, mixed conductor membranes for selective CO2 transport can be treated as mobile-carrier membranes.
A facilitated transport membrane involving an electrochemical reaction is also applicable for separating other gas mixtures with the assistance of membrane catalysis. Due to the possibility of integrating O2 permeation and catalytic oxidation reaction, an O2-selective mixed conductor membrane is expected to separate the two components of redox conjugate pairs, e.g. CO/CO2, C2H4/C2H6, C3H6/C3H8, etc. As shown in Fig. 9, the periodically placed dehydrogenation membrane module and selective H2 oxidation module can break the thermodynamic limitation of oxidative dehydrogenation process, and thus propane is likely to be continuously converted to propylene, resulting in good separation and highly efficient propylene production.
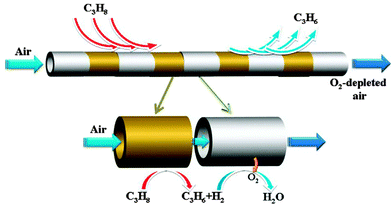 | ||
| Fig. 9 Stepwise oxidative dehydrogenation of propane with a sequence of dehydrogenation and hydrogen combustion with the aid of a mixed conductor membrane. Reprinted with permission from ref. 37. Copyright 2013 Elsevier. | ||
4. Applications of facilitated transport membranes in energy-intensive processes
This section describes the applications of facilitated transport membranes in energy-intensive processes, especially gas separation, pervaporation and fuel cells. The engineering-relevant issues such as scale-up difficulty, investment, membrane stability are also included.4.1 Gas separation
Facilitated transport membranes are mostly designed for gas separation due to the weak interactions between gas molecules and membrane materials. Reversible reactions prove very useful for enriching the target permeants without irreversible binding. Three typical examples are CO2 capture, air separation and olefin/paraffin separation, respectively.Nowadays, CO2 capture has become globally concerned due to the anthropogenically forced carbon emission and climate change. Fortunately, CO2 transport can be facilitated due to the abundant facilitated transport chemistries as described in Section 3. That is, facilitated CO2 transport is possible based on different reversible reactions, namely nucleophilic addition reaction, π-complexation and electrochemical reaction, providing researchers with more opportunities.
Among the three types of reactions, nucleophilic addition reaction represents the main stream for facilitating CO2 transport. In recent years, great progresses have been achieved for facilitated CO2 transport based on nucleophilic addition reaction. The typical examples about such type of facilitated CO2 transport membranes are summarized in Table S1 (ESI†). Firstly, thanks to the fascinating carrier-involved chemistry, the separation performance of CO2 separation membrane in terms of permeability and selectivity has been elevated to an unprecedentedly high level. Besides the aforementioned biomimetic poly(N-vinylimidazole)–zinc complex membranes, Wang and coworkers39 designed and fabricated mixed matrix membranes filled with nanosized hydrotalcite (HT), an anionic pillared layered material with basicity. Owing to the mobile ionic carriers within the interlayer gap of HT with the aid of water condensation, high-speed CO2 transport channels were successfully constructed, as shown in Fig. 10. With a polyethyleinimine-based copolymer as a polymer matrix, the mixed matrix membrane exhibited a high CO2 permeance up to 5693 GPU (1 GPU = 10−6 cm3 (STP) cm−2 s−1 cmHg−1) and a CO2/N2 selectivity of 268 at 0.11 MPa. Although carriers tended to be saturated at elevated pressures, a CO2 permeance above 500 GPU and a CO2/N2 selectivity above 40 was maintained at 1.0 MPa. Next, the importance of increasing effective carrier content has been highlighted. Qiao et al.40 found that doping piperazine (PIP) into PVAm could yield a physically crosslinked structure without increasing crystallinity. The multiple hydrogen bonds between PVAm and PIP allowed very high PIP loading (as high as 230 wt% of PVAm) without loss of mechanical stability. As such, a substantial increase of effective carrier content was acquired, resulting in an extremely high CO2 permeance of 6500 GPU and a CO2/N2 selectivity of 277 at 0.11 MPa. To the best of our knowledge, this study reported the highest CO2 permeance at low pressures so far. Last but not the least, more carriers have been designed considering the carrier stability under different conditions. For example, carboxylate-type carriers have proved effective in acquiring the desired carrier stability in an oxidative atmosphere. Aromatic carboxylate groups as carriers could also avert the severe compact packing of matrix polymer chains, yielding high CO2 permeance41.
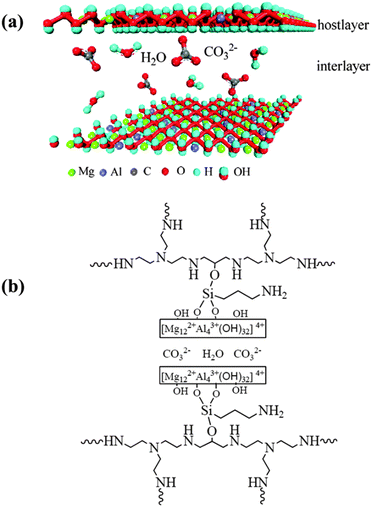 | ||
| Fig. 10 (a) The anionic pillared layered structure of hydrotalcite; (b) the mixed matrix structure of a polyethyleinimine-based copolymer and hydrotalcite with 3-aminopropyltriethoxysilane as a interfacial linker. Reprinted with permission from ref. 39. Copyright 2014 the Royal of Social Chemistry. | ||
The aforementioned facilitated CO2 transport membranes rely heavily on water, which allows appropriate degree of swelling of the membrane matrix and thus high CO2 permeability. Kasahara et al. developed amino acid ionic liquid-based facilitated transport membranes to promote CO2 transport without the aid of water. Since the liquid membrane matrix rendered low diffusion resistance, the membranes achieved high CO2 permeability up to 8300 Barrer and high CO2/N2 selectivity up to 146 at 100 °C under dry conditions.42 The effect of carrier saturation at elevated pressures proved the occurrence of facilitated transport. The authors also designed polymeric ion-gel membranes to enhance membrane stability while maintaining high CO2 permeability.43 More information about the development of amino acid ionic liquid-based facilitated transport membranes can be found in Table S2 (ESI†).
Another notable breakthrough was reported by Izak and coworkers.44 They proposed the concept of separating CO2, H2S and impurities from biogas by a “condensing-liquid membrane”, based on the different solubility of components in a very thin continuously refreshed water layer supported by a hydrophobic porous substrate. Actually the thin water layer also acted as facilitated transport carriers for CO2, while the lack of proton acceptors restricts the dissociation of H2CO3. By further modifying the surface of substrate with basic groups or long chains, better separation performance can be expected.
Facilitated CO2 transport based on π-complexation is a burgeoning research field. Since metal ions can also complex with the polymer matrix and lead to chain rigidification, it remains difficult to evaluate the contribution of π-complexation to CO2/CH4 selectivity.34 Also, considering the role of water as a nucleophilic agent, it is also challenging to distinguish the facilitation effect due to nucleophilic addition reaction from that due to π-complexation. Consequently, the existing studies on metal ion-mediated facilitated CO2 transport were conducted under anhydrous conditions, and the corresponding CO2 permeances were much lower than the values mentioned above.34–36 We speculate that water is an indispensible ingredient for high CO2 permeance, because water-induced swelling on polymeric frameworks may aid in increasing effective carrier content and taking the full advantages of carriers. Accordingly, it is recommended that the future work on polymer-based facilitated transport membranes for CO2 separation should include the comparison of dry-state and humidified-state gas separation performances at different carrier loadings, which may be very helpful to more deeply understand the differences in membrane structures and transport mechanisms.
Facilitated CO2 transport based on electrochemical reaction requires a mixed conductor membrane, which is usually an inorganic ceramic membrane. The electrochemical reactions described in Section 3 are quite simple, while the overall CO2 permeance through a mixed conductor membrane is determined by not only the intrinsic carbonate and oxygen ionic conductivities of the carbonate and ceramic phases, but also the pore and solid microstructure of the ceramic support. In particular, the latter is often the controlling factor due to the difficulty in controlling the connectivity and tortuosity of the ion conducting channels. Zhang et al.38 reported a high-flux mixed carbonate-ion and oxide-ion conductor (MOCC) membrane consisting of highly and efficiently interconnected three-dimensional ionic channels. A combined “co-precipitation” and “sacrificial template” method was used to synthesize a solid oxide porous matrix with highly interconnected solid and uniformly distributed pores. The molten carbonate phase then fills into these pores to form a dense MOCC membrane. Such type of membrane shows a CO2 flux density two orders of magnitude higher than the existing ceramic–carbonate systems fabricated by other techniques. Therefore, this type of membrane is a promising alternative to implement high-temperature CO2 capture from flue gases or shift gases.
Olefin/paraffin separation is another typical process of which the energy efficiency is expected to be enhanced by a facilitated transport membrane. In the petrochemical industry, separation olefin from paraffin is typically one of the most expensive and most energy-consuming separation processes because of the high cooling capacity and large number of theoretical plates required for cryogenic distillation. Membrane separation of olefin/paraffin is advantageous over cryogenic distillation in energy and investment cost, while the conventional membranes based on solution–diffusion mechanism are insufficient to produce high-purity olefin. Facilitated transport membranes involving π-complexation prove to be promising candidates because of the expected high separation specificity. According to the manners of introducing metal ion carriers into a membrane, facilitated transport membranes for olefin/paraffin separation can be divided into four types: SILM, ion exchange membrane, polymer electrolyte membrane, and nanocomposite membrane. All the three basic types of facilitated transport membranes mentioned in Section 2 are encompassed.
SILMs are often compared with ion exchange membranes because of the strong binding of Ag+ by electrostatic force. However, the high separation performance of ion exchange membrane relies on the dissociation of Ag+ and swelling of the polymer matrix, which requires introduction of water. Herein the introduction of water is actually not a good choice like the case for CO2 capture because of the strict limitation of water content for olefin polymerization. The ionic and liquid like SILMs exclude the necessity of water, and hence SILMs have witnessed a rapid progress in these years. As one example, Pitsch et al.7 reported an ionic liquid nanocomposite membrane comprising a multi-layer support structure hosting the ionic salt [Ag]+[Tf2N]−. The ionic salt renders liquid like upon complexation with propylene, resulting in facilitated transport of propylene over propane at benchmark-setting selectivity and permeance levels. The membrane also showed good resistance to C2H2 poisoning.
As for the polymer electrolyte membrane, Ag+ is immobilized by relatively weak coordinating interactions, and hence water is also unnecessary to promote Ag+ dissociation. Large amount of Ag+ is permitted to be doped into the membrane due to the multiple coordinating interaction sites. At high Ag+ loading, the strong plasticizing ability of olefin molecules allows high fractional free volume to reduce the effect of diffusion resistance, which as well as the enrichment of hopping site for olefin leads to remarkable increment of olefin permeance, as mentioned as a threshold concentration effect of carrier loading in Section 3.3. Therefore, achieving high olefin/paraffin separation performance of polymer electrolyte membrane is already not a challenge in laboratory. Nevertheless, maintaining the stability of Ag+ in the real multi-component gas feed remains a great challenge. One possible solution is to add strong oxidant into the membrane as a stabilizing agent, so as to prevent Ag+ from being reduced. However, a cyclic process is required after the stabilizing agent is exhausted. Merkel et al.45 presented a baseline study describing the scope of addressing carrier instability. They developed an in situ regeneration method by using peroxide/acid liquid or vapor phase treatment to oxidize the reduced Ag+ carriers within the polymer electrolyte membrane. However, the poisoned Ag+ by H2S cannot be regenerated through this method because the oxidation process may produce Ag2SO4, which is also not effective in facilitating olefin transport. Doping the membrane with nitrate salts has also proven effective in suppressing the reduction of silver ion. For example, Kang et al. found that doping Al(NO3)3 into a poly(2-ethyl-2-oxazoline)–AgBF4 complex could prolong the lifetime of Ag+ for more than 14 days, which was attributed to the ionic aggregation between the Ag+ of AgBF4 and NO3− of Al(NO3)3. The ionic aggregation phenomena could be further interpreted by the favorable interactions between BF4− and Al3+ and the weakened interactions between Al3+ and NO3−.46 More examples can be found in Table S3 of ESI.†
Nanocomposite membranes containing positively charged metal nanoparticles have been mentioned in Section 3. Like the case of Ag+, AgNP was identified as one of the best choices. As a typical example, an AgNP-containing nanocomposite membrane was successfully fabricated by a reflux method using the casting solution of a PVP–AgBF4 polymer electrolyte membrane. In this way, the average size of AgNPs was controlled to 20 nm with a standard deviation of 3 nm, and a homogeneous dispersion of AgNPs within the membrane was observed. By selecting an appropriate electron acceptor, positive charge was induced onto the AgNP surface, and the resultant facilitated transport membrane exhibited a high mixed-gas propylene/propane selectivity up to 50, with a moderate propylene permeance of 3.5 GPU.33 Although such performance could not rival the best results for polymer electrolyte membranes, the as-prepared PVP–AgNP nanocomposite membrane is expected to show much better stability against reductive atmosphere than most polymer electrolyte membranes.
Air separation is also a tough task for conventional membranes because of the similar dynamic diameters and low boiling points of O2 and N2. In chemical industries, membrane air separation is already regarded as a cost-effective process to produce moderately pure streams containing >95% N2 or 60–80% O2. The production of O2 or N2 with higher purity requires higher separation efficiency enabled by facilitated transport membranes. Actually, the earliest understanding on facilitated transport phenomena and principles arose from the naturally-occurring O2 carriers. Despite a long history of synthetic O2 carrier development, the application prospect of facilitated transport in air separation is not as promising as that in CO2 capture and olefin/paraffin separation. The main reasons lie in the lack of stability of the O2–carrier coordinating bonds and the insufficient physical adsorption capacity of O2 in dense polymer membranes. Also, the essential multidentate ligands for the center metal ion of the O2 carrier may sterically hinder the motion of the unique active site, lowering the reaction rate. One typical representation is the carrier saturation problem. Most O2 carriers are only effective at very low pressure and become quickly saturated below 1 bar. As such, both O2 permeability and O2/N2 selectivity decrease to rather low values with limited advantages over common polymer membranes. There are two strategies to solve this problem: (1) enhancing the intrinsic carrier activity, which is extremely challenging since a huge number of Co complexes have already been attempted; (2) increasing the carrier content, which is relatively more feasible. Given the most active carrier systems such as CoP derivatives, the main challenge is to maintain adequate mechanical strength at high carrier loading, because the planar and rigid ligands often aggregate in the membrane and result in brittleness. Chikushi et al. synthesized porphyrin network polymers via a Michael addition-type click reaction with acetoacetate-substituted CoP (CoPac) as the Michael addition donor and tri/tetra-acrylate as the acceptor (Fig. 11). The as-synthesized polymer allowed high CoPac content up to 70 wt%, rendering the membrane with both high O2 permeability (10–100 Barrer) and O2/N2 selectivity (above 30).47
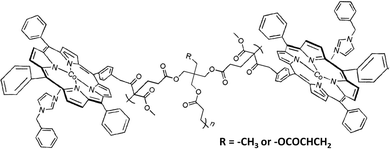 | ||
| Fig. 11 The chemical structure of the porphyrin network polymers synthesized by Chikushi et al. Reprinted with permission from ref. 47. Copyright 2014 Wiley-VCH. | ||
A mixed conductor membrane comprising an electron conductor and an O2− conductor has shown great potential in air separation as a special type of facilitated transport membrane. On account of the transport of O2− through oxygen vacancies, a mixed conductor membrane is theoretically expected to produce 100% pure O2. Sunarso et al.48 reported a high performance BaBiScCo hollow fiber membrane, which combined the merits of high O2 flux from barium–cobalt in tandem with the stability and electrical conductivity enhancement by scandium oxide. The resultant membrane delivered high flux up to 11.4 ml cm−2 min−1 at 950 °C, which has exceeded the target value of 10 ml cm−2 min−1 sought by the research community. Whereas, from the viewpoint of engineering, the high temperature required for producing high concentration of oxygen vacancies is not beneficial for acquiring high energy efficiency. A combined strategy using the mixed conductor membrane as a membrane reactor for high-temperature oxidation/reduction reactions might be appropriate to make better use of the high-grade energy. Also, the long term stability of the mixed conductor membrane and the related membrane configuration should be carefully considered to evaluate the future of the mixed conductor membrane in air separation.
4.2 Pervaporation
Facilitated transport membranes are also effective in pervaporation, especially for aromatic compound separation. Most aromatic compounds exhibit weak polarity and therefore low solubility in conventional membranes. Facilitated transport based on π-complexation is expected to significantly increase the concentration of the target permeant within the membrane. One of the most representative examples is gasoline desulfurization, which is an essential tache in clean energy production. The combustion of gasoline with high sulphur content would enable excessive emission of SO2, which is one of the main air pollutants. The removal of thiophenes from gasoline is the most challenging work for gasoline desulfurization because of the similar physical properties between thiophene and the hydrocarbon components of gasoline, heptane and octane. Hydrodesulfurization (HDS) processes constitute the most effective method presently, while it requires large quantity of H2. Although it is often impossible to deeply remove thiophenes from gasoline solely by membrane technologies, rough removal of the majority of thiophene by membrane technologies is also attractive because the enrichment of sulphur in the permeate means remarkable reduction of the treatment quantity of gasoline for the HDS process.The facilitated transport chemistries for thiophene are analogous to the case of olefins, while thiophene allows a wider range of metal ions as carriers, e.g. Ni2+, Cu2+, and Ce4+.8 The major difference lies in the low content of thiophene in gasoline, indicating that thiophene cannot be overwhelmingly enriched in a membrane. In order to obtain sufficiently high flux when abundant heptane or octane exists within the membrane, hydrophobic polysiloxane is often employed as the matrix polymer, which can be sufficiently swollen by heptane and octane. Due to the lack of carrier loading capacity of polysiloxane, the carriers are often loaded by micro-/nano-sized particles, which are further embedded in polysiloxane as a filler. Liu et al.49 found that a thin dopamine layer on TiO2 microspheres allowed high Ag+ loading (37.6 wt% of Ag+/TiO2 microspheres) via electron donor–acceptor coordination bonds (Fig. 12). The Ag+-loaded fillers embedded into the rubbery polysiloxane membrane brought about additional free volume at the polymer–filler interface, which further lowered the negative effect of diffusion resistance on facilitated transport. The optimum membrane presented a permeation flux of 4.14 kg m−2 h−1 (1.97 times as much as polysiloxane control membrane) and an enrichment factor of 8.56 (1.95 times as much as polysiloxane control membrane).
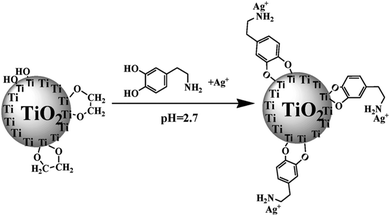 | ||
| Fig. 12 The synthetic route of a Ag+/TiO2 microsphere for membrane desulfurization. Reprinted with permission from ref. 49. Copyright 2011 Elsevier. | ||
4.3 Proton exchange membrane fuel cell (PEMFC)
Proton exchange membrane fuel cell (PEMFC) is another representative application of the facilitated transport membrane. The key component of PEMFC is proton exchange membrane (PEM), which functions as an electrolyte for transporting protons from the anode to the cathode as well as blocking the passage of electrons and fuel between the electrodes. Designing and optimizing the proton carriers is important for high performance PEMs. The primary proton carriers include water, which facilitates both vehicle and hopping mechanisms, and other Brønsted acids–bases, which facilitate the hopping mechanism. As for water, the major objective is to achieve high water retention under low humidity and elevated temperature, affording significantly enhanced system efficiency. For the latter case, the incorporated proton carrier should balance the proton accepting/donating properties of the existing proton conducting group. One attractive solution is to design acid–base pairs via proton transfer reaction.Microcapsules as fillers in PEM have been reported recently as promising alternatives to increasing the water retention capacity of membrane at low humidity. Jiang and co-workers utilized polymeric microcapsules as water reservoirs due to the possibility of integrating elastic storage and capillary storage mechanisms.50 The highly hydrophilic and crosslinked capsule shell allowed steady retention of water in the bound-water state, yielding dramatically enhanced water retention properties and interconnected proton transfer pathways. However, it remains a tough work to completely impede water release at low humidity.
Constructing acid–base pairs has rapidly emerged at the forefront of PEM research. Given an acidic sulfonated poly(ether ether ketone) (SPEEK) matrix, basic amino groups were anchored to halloysite nanotube (HNT) via dopamine chemistry, so as to form acid–base pairs with the sulfonate group of SPEEK (Fig. 13). The incorporation of dopamine-modified HNTs (DHNTs) reduced the channel size, water uptake, and area swelling of the SPEEK membranes, while the acid–base pairs created continuous pathways for a 30% increase of proton conductivity and a 52% increase of power density of a single cell. By comparison, only slight increase of proton conductivity was observed when unmodified HNT was embedded into SPEEK, because of the lower proton accepting properties of hydroxyl groups and the reduced possibility of proton transfer reaction.20 On the other hand, a basic matrix requires acidic carriers to form acid–base pairs. Song et al. developed a tetrazole-based polymer as the matrix of PEM, and the –PO3H2 group was connected onto the polymer chains to construct acid–base pairs. Considering that both tetrazoles and phosphoric acids are capable of transferring proton under anhydrous conditions, the acid–base pair afforded enhanced proton conductivity and peak power density at 120 °C.25
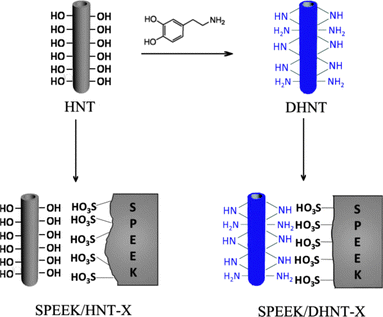 | ||
| Fig. 13 Preparation of dopamine-modified halloysite nanotubes and the interfacial chemical structures between SPEEK bulk and nanotube. Reprinted with permission from ref. 20. Copyright 2013 Wiley-VCH. | ||
It is also possible to achieve two goals of retaining water and designing acid–base pairs by incorporating multi-functional microcapsules. Wang et al.23 designed and fabricated imidazole microcapsules (IMCs)-embedded SPEEK composite membranes. The hollow structure and hydrophilicity of the IMCs endowed the composite membranes with significantly improved water retention properties under low humidity, thus facilitating proton transport within the well-connected water channels (Fig. 14). The concentrated imidazole groups in IMC shells facilitated proton hopping via proton exchange. Moreover, the formation of acid–base pairs (bioinspired proton transport highway) between the sulfonic acid group on SPEEK and the imidazole group on IMCs, mimicking the aspartic acid–Schiff base complex in bacteriorhodopsin, allowed ultrafast proton transport with low energy barrier.
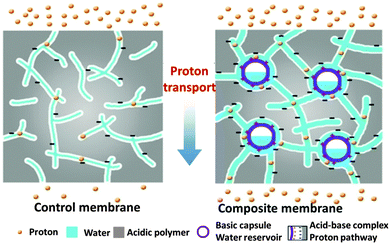 | ||
| Fig. 14 A combined strategy involving water retaining and acid–base pair designing by a composite SPEEK–imidazole capsule membrane. Reprinted with permission from ref. 23. Copyright 2012 Wiley-VCH. | ||
5. Concluding remarks
Facilitated transport phenomena and theories have opened up novel avenues for fast and selective transport of specific small molecules or ions. With deep insights into the carrier-mediated transport mechanisms and facilitated transport chemistries, great progresses in designing and fabricating facilitated transport membranes have been achieved in the past decade. Given the classification of the available carriers and the relevant reversible reactions for facilitated transport in this tutorial review, facilitated transport membranes can be fabricated using a variety of materials, e.g. polymers, polymer composites or inorganic materials, and have shown attractive performance in a broad range of energy-intensive processes, especially CO2 capture and olefin/paraffin separation. Moreover, some polymer-based membrane materials (e.g. polyvinylamine) with excellent solution processing characteristics have been considered for kilogram-scale synthesis and fabrication into large-area asymmetric composite membranes. The stability problems for CO2 carriers and olefin carriers have also been partially solved.Despite the achievements mentioned above, there are still many emerging challenges and opportunities for both the scientific community and engineers, and some of them are highlighted as follows. (1) The facilitated transport chemistries need further diversification for higher carrier reactivity. In particular, metal ion-involved facilitated transport widely exists in biological organisms. Many proteins with catalytic functions allow high specificity reverse binding towards a guest molecule/ion, which heavily relies on a synergy of multi-site interactions, including reversible chemical bonds and physical interactions. That is, the active center atom of a carrier cannot normally take effects without the aid of the ambient microenvironments. Moreover, like the cases of carbonic anhydrase and haemoglobin, appropriate amino acid residues are required to tune the HSAB acidity/basicity of the centre metal ions to the desired level. In this way, more attentions should be paid to the unique and common features of different acid–base reactions where the acids and bases are defined by Brønsted theory, Lewis theory, and HSAB theory, respectively. (2) The complex transport mechanisms need deeper elucidation. Firstly, the relationship between the facilitated transport mechanism and conventional solution–diffusion mechanism remains elusive. Obviously, the facilitated transport mechanism is not an independent mechanism that can be separately investigated experimentally. The involvement of a carrier would inevitably interfere with the physical structure of the membrane matrix and therefore the transport properties follow the solution–diffusion mechanism. Secondly, taking ion transport into considerations, whether and to what extent the diffusivity resistance of the membrane matrix affects hopping mechanism remains unknown, since the carrier-to-carrier hopping does not belong to Fickian diffusion. The existing facilitated transport membranes employing hopping mechanism are mostly swollen by water or plasticized by the target permeant, and therefore this effect was often ignored. The mixed conductor membrane using oxygen vacancies or molten carbonates may help to understand the related mechanism. Since O2 and CO2 cannot plasticize the polymer matrix at relatively low pressure without the aid of other plasticization agents, whether the facilitation effect of π-complexation on their permeabilities can be enhanced by adding a plasticization agent would be an interesting topic. (3) The membrane fabrication methods need more alternatives. In view of the manners of introducing carriers, the concentration and distribution of carriers should be better controlled to enhance the facilitation transport effect. A soluble carrier ingredient is permitted to achieve high carrier concentration within the membrane, whereas an insoluble carrier ingredient suffers from the insufficient carrier concentration. Also, the carriers are expected to distribute at the accessible regions, rather than self-rigidified regions. That is, the interactions of carriers may cause chain rigidification or carrier aggregation, which would drastically lower the effective carrier concentration. In view of material selection, organic polymers are selected most of the time, while mixed matrix polymer composites composed of polymer and functional filler seem better alternatives. Recently, nanocomposite membranes with facilitated transport carriers have become a promising direction to further enhance gas permeance, especially for CO2 capture and olefin/parafin separation. With the aid of an adsorptive filler, the carrier saturation problem is also expected to be partially addressed, which is important to maintain the high performance in the cases where elevated feed pressure is required, e.g. natural gas sweetening, post-combustion CO2 capture and air separation. On one hand, the carriers can be loaded onto the fillers, which expands the selection range of available matrix polymers. On the other hand, the filler phase is believed to interrupt the crystalline or rigidified region caused by strong cohesive interactions, producing higher fractional free volume and effective carrier concentration. Inorganic mixed conductors now represent a relatively independent branch, while it illumines the possibility of converting molecular transport into ion conduction. (4) The membrane stability problems need smarter solutions. Nowadays, the efforts devoted to enhance carrier stability are mainly for addressing the stability of Ag+ against light, reductive and sulphur-containing impurities. The development of anti-oxidation carriers for CO2 capture has also been reported. It should be mentioned that the instability facilitated transport membranes should be also considered in the following two cases: (1) water is necessary as a carrier or plasticization agent, and in this case the water retention and management in the membrane process will be very important to maintain membrane stability; (2) mobile carriers are dissolved in liquid membranes, and therefore ionic liquid-based facilitated transport may be more appropriate for membrane contactors.
Altogether, incorporating facilitated transport into membranes enriches the multiple selectivity mechanism, and significantly intensifies the mass transfer and application efficiency of membrane processes. Although there is still a long road ahead, we believe that the literature published to date donate excellent examples for future exploitation of facilitated transport membranes. With the rapid advancement in chemistry and materials science, facilitated transport membranes with superior transport properties and operation stability are expected to be developed in the near future. In particular, we should bear in mind that the inspiration models of facilitated transport arise from nature, and therefore the advances in protein chemistry (especially for carrier proteins, channel proteins, and enzymes) and synthetic biology might be of great values to pursue new breakthroughs in designing the next generation of facilitated transport membranes.
Acknowledgements
The authors are grateful for the financial support from National Science Fund for Distinguished Young Scholars (21125627), the National High Technology Research and Development Program of China (2012AA03A611), and the Programme of Introducing Talents of Discipline to Universities (No. B06006).Notes and references
- D. L. Gin and R. D. Noble, Science, 2011, 332, 674–676 CrossRef CAS PubMed.
- S. Basu, A. L. Khan, A. Cano-Odena, C. Q. Liu and I. F. J. Vankelecom, Chem. Soc. Rev., 2010, 39, 750–768 RSC.
- T. Rosenberg and W. Wilbrandt, J. Gen. Physiol., 1957, 41, 289–296 CrossRef CAS.
- P. A. Gale, R. Pérez-Tomás and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed.
- S. Li, Z. Wang, X. Yu, J. Wang and S. Wang, Adv. Mater., 2012, 24, 3196–3200 CrossRef CAS PubMed.
- B. Belaissaoui, Y. Le Moullec, H. Hagi and E. Favre, Sep. Purif. Technol., 2014, 125, 142–150 CrossRef CAS PubMed.
- F. Pitsch, F. F. Krull, F. Agel, P. Schulz, P. Wasserscheid, T. Melin and M. Wessling, Adv. Mater., 2012, 24, 4306–4310 CrossRef CAS PubMed.
- W. Liu, Y. Li, X. Meng, G. Liu, S. Hu, F. Pan, H. Wu, Z. Jiang, B. Wang, Z.-X. Li and X.-Z. Cao, J. Mater. Chem. A, 2013, 1, 3713–3723 CAS.
- M. Almeida, R. W. Cattrall and S. D. Kolev, J. Membr. Sci., 2012, 415, 9–23 CrossRef PubMed.
- O. F. Mohammed, D. Pines, J. Dreyer, E. Pines and E. T. Nibbering, Science, 2005, 310, 83–86 CrossRef CAS PubMed.
- C. Laberty-Robert, K. Valle, F. Pereira and C. Sanchez, Chem. Soc. Rev., 2011, 40, 961–1005 RSC.
- E. L. Cussler, R. Aris and A. Bhown, J. Membr. Sci., 1989, 43, 149–164 CrossRef CAS.
- R. D. Noble and C. A. Koval, in Materials Science of Membranes for Gas and Vapor Separation, ed. Y. Yampolskii, I. Pinnau and B. Freeman, John Wiley & Sons, Ltd, Chichester, 2006, pp. 411–435 Search PubMed.
- S. Kasahara, E. Kamio, T. Ishigami and H. Matsuyama, J. Membr. Sci., 2012, 415, 168–175 CrossRef PubMed.
- K. Ramasubramanian, Y. Zhao and W. S. Winston Ho, AIChE J., 2013, 59, 1033–1045 CrossRef CAS.
- Y. Li, G. He, S. Wang, S. Yu, F. Pan, H. Wu and Z. Jiang, J. Mater. Chem. A, 2013, 1, 10058–10077 CAS.
- Y. S. Kang, J. H. Kim, J. Won and H. S. Kim, in Materials Science of Membranes for Gas and Vapor Separation, ed. Y. Yampolskii, I. Pinnau and B. Freeman, John Wiley & Sons, Ltd, Chichester, 2006, pp. 391–410 Search PubMed.
- M. Schuster, T. Rager, A. Noda, K. D. Kreuer and J. Maier, Fuel Cells, 2005, 5, 355–365 CrossRef CAS.
- H. Wu, X. Shen, T. Xu, W. Hou and Z. Jiang, J. Power Sources, 2012, 213, 83–92 CrossRef CAS PubMed.
- H. Zhang, T. Zhang, J. Wang, F. Pei, Y. He and J. Liu, Fuel Cells, 2013, 13, 1155–1165 CrossRef CAS.
- R. Subbaraman, H. Ghassemi and T. A. Zawodzinski, J. Am. Chem. Soc., 2007, 129, 2238–2239 CrossRef CAS PubMed.
- M. Yamada and I. Honma, Angew. Chem., Int. Ed., 2004, 43, 3688–3691 CrossRef CAS PubMed.
- J. Wang, X. Yue, Z. Zhang, Z. Yang, Y. Li, H. Zhang, X. Yang, H. Wu and Z. Jiang, Adv. Funct. Mater., 2012, 22, 4539–4546 CrossRef CAS.
- A. Sannigrahi, S. Ghosh, S. Maity and T. Jana, Polymer, 2011, 52, 4319–4330 CrossRef CAS PubMed.
- M.-K. Song, H. Li, J. Li, D. Zhao, J. Wang and M. Liu, Adv. Mater., 2014, 26, 1277–1282 CrossRef CAS PubMed.
- T.-J. Kim, H. Vrålstad, M. Sandru and M.-B. Hägg, J. Membr. Sci., 2013, 428, 218–224 CrossRef CAS PubMed.
- L. Xiong, S. Gu, K. O. Jensen and Y. S. Yan, ChemSusChem, 2014, 7, 114–116 CrossRef CAS PubMed.
- Y. Zhao and W. S. Winston Ho, J. Membr. Sci., 2012, 415–416 Search PubMed , 132–138.
- K. Yao, Z. Wang, J. Wang and S. Wang, Chem. Commun., 2012, 48, 1766–1768 RSC.
- Y. Li, Q. Xin, H. Wu, R. Guo, Z. Tian, Y. Liu, S. Wang, G. He, F. Pan and Z. Jiang, Energy Environ. Sci., 2014, 7, 1489–1499 CAS.
- Y. S. Kang, S. W. Kang, H. Kim, J. H. Kim, J. Won, C. K. Kim and K. Char, Adv. Mater., 2007, 19, 475–479 CrossRef CAS.
- S. W. Kang, K. Char and Y. S. Kang, Chem. Mater., 2008, 20, 1308–1311 CrossRef CAS.
- I. S. Chae, S. W. Kang, J. Y. Park, Y.-G. Lee, J. H. Lee, J. Won and Y. S. Kang, Angew. Chem., Int. Ed., 2011, 50, 2982–2985 CrossRef CAS PubMed.
- F. Y. Li, Y. Li, T. S. Chung and S. Kawi, J. Membr. Sci., 2010, 356, 14–21 CrossRef CAS PubMed.
- J. H. Oh, Y. S. Kang and S. W. Kang, Chem. Commun., 2013, 49, 10181–10183 RSC.
- J. H. Lee, J. Hong, J. H. Kim, Y. S. Kang and S. W. Kang, Chem. Commun., 2012, 48, 5298–5300 RSC.
- Y. Wei, W. Yang, J. Caro and H. Wang, Chem. Eng. J., 2013, 220, 185–203 CrossRef CAS PubMed.
- L. Zhang, N. Xu, X. Li, S. Wang, K. Huang, W. H. Harris and W. K. S. Chiu, Energy Environ. Sci., 2012, 5, 8310–8317 CAS.
- J. Liao, Z. Wang, C. Gao, S. Li, Z. Qiao, M. Wang, S. Zhao, X. Xie, J. Wang and S. Wang, Chem. Sci., 2014, 5, 2843–2849 RSC.
- Z. Qiao, Z. Wang, C. Zhang, S. Yuan, Y. Zhu, J. Wang and S. Wang, AIChE J., 2013, 59, 215–228 CrossRef CAS.
- M. Wang, Z. Wang, S. Li, C. Zhang, J. Wang and S. Wang, Energy Environ. Sci., 2013, 6, 539–551 CAS.
- S. Kasahara, E. Kamio, T. Ishigami and H. Matsuyama, Chem. Commun., 2012, 48, 6903 RSC.
- S. Kasahara, E. Kamio, A. Yoshizumi and H. Matsuyama, Chem. Commun., 2014, 50, 2996–2999 RSC.
- M. Poloncarzova, J. Vejrazka, V. Vesely and P. Izak, Angew. Chem., Int. Ed., 2011, 50, 669–671 CrossRef CAS PubMed.
- T. C. Merkel, R. Blanc, I. Ciobanu, B. Firat, A. Suwarlim and J. Zeid, J. Membr. Sci., 2013, 447, 177–189 CrossRef CAS PubMed.
- S. W. Kang, J. H. Kim, J. Won and Y. S. Kang, J. Membr. Sci., 2013, 445, 156–159 CrossRef CAS PubMed.
- N. Chikushi, E. Ohara, A. Hisama and H. Nishide, Macromol. Rapid Commun., 2014, 35, 976–980 CrossRef CAS PubMed.
- J. Sunarso, S. Liu, Y. S. Lin and J. C. Diniz da Costa, Energy Environ. Sci., 2011, 4, 2516–2519 CAS.
- W. Liu, B. Li, R. Cao, Z. Jiang, S. Yu, G. Liu and H. Wu, J. Membr. Sci., 2011, 378, 382–392 CrossRef CAS PubMed.
- J. Wang, H. Zhang, X. Yang, S. Jiang, W. Lv, Z. Jiang and S. Qiao, Adv. Funct. Mater., 2011, 21, 971–978 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information and references for the facilitated transport membranes discussed. See DOI: 10.1039/c4cs00215f |
| This journal is © The Royal Society of Chemistry 2015 |