Magnetically recoverable Ni/C catalysts with hierarchical structure and high-stability for selective hydrogenation of nitroarenes†
Peng
Zhang
a,
Chang
Yu
a,
Xiaoming
Fan
a,
Xiuna
Wang
a,
Zheng
Ling
a,
Zonghua
Wang
b and
Jieshan
Qiu
*a
aCarbon Research Laboratory, Liaoning Key Lab for Energy Materials and Chemical Engineering, State Key Lab of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: jqiu@dlut.edu.cn
bDepartment of Chemistry, Qingdao University, Qingdao 266071, Shandong, China
First published on 10th November 2014
Abstract
Here we report that magnetic Ni/C catalysts with hierarchical structure can be fabricated from a mixture of nickel acetate, polyethylene glycol-200 and furfural by a one-step hydrothermal method, followed by calcination. It has been found that the calcination temperature is the key factor affecting the structure, morphology and the catalytic performance of the Ni/C catalysts. Of the as-made catalysts, the Ni/C sample calcined at 300 °C features small-size metallic Ni particles with high dispersion in the carbon matrix and a unique hierarchical structure, and has the highest rate of conversion of o-chloronitrobenzene with high selectivity to o-chloroanilines. The concerned Ni/C catalysts are magnetic due to the presence of metallic Ni particles, which makes their recovery easy after the reaction by an external magnetic field. The recovered Ni/C catalysts can be recycled at least ten times without obvious loss both in Ni loading and the catalytic performance. This kind of catalyst is also active for the selective hydrogenation of other nitroarenes to the corresponding anilines.
Introduction
Anilines are important intermediates in the production of organic chemical products such as dyes, drugs, herbicides and pesticides, usually made by hydrogenating nitroarenes. The pathways and the mechanism involved in the reaction are very complicated, as shown in Scheme 1, in which many undesired by-products may be formed.1–3 To tackle this problem, catalysts with high activity and selectivity are highly required. Up to now, many noble metal catalysts such as Pt,4–7 Pd,8 Au,9–11 Ag,12 and Ru13 have been developed, which show a high conversion of substrates, and can effectively suppress the formation of by-products. However, the high cost of noble metals has limited their practical use. It has been found that the supported metallic Ni catalysts14–18 are some of the ideal candidates for the selective hydrogenation of nitroarenes due to their high catalytic activity and low cost. Moreover, the unique magnetic properties of metallic Ni particles make them attractive because the catalysts can be recovered after the reaction by an external magnetic field, and this would greatly reduce the cost in industrial practice.19Carbon materials are excellent catalyst supports for the selective hydrogenation of nitroarenes, owing to their intrinsic properties such as high surface area, unique electronic properties and chemical inertness as well as thermal stability and high mechanical strength.20–25 However, for the carbon-based catalysts available now, the production procedure is time-consuming and complex.21,22 As such, it is highly desirable to develop a simple, efficient and sustainable route to synthesize active Ni/C catalysts for the selective hydrogenation of nitroarenes, and this remains a challenge.
Of various carbon materials, biomass-based hydrothermal carbon (HTC) materials have received an increasing interest due to their good sustainability, tunable surface chemistry and porous structure.26,27 More importantly, they can be easily incorporated into metals or metallic oxides to make functional composites that have been successfully used in various fields such as energy,28–30 catalysis,31,32 and environmental protection.33 Here, we report a facile one-step hydrothermal method to prepare sheet-like Ni/C catalyst precursors with furfural, a biomass derivative, as the carbon source. After heating the precursor at different temperatures, a series of Ni/C catalysts with a unique hierarchical structure were prepared, which show high catalytic activities and selectivities for the hydrogenation of o-chloronitrobenzene (o-CNB) to o-chloroanilines (o-CAN). Compared with other samples, the catalyst made at 300 °C shows the best catalytic performance and a good stability, evidenced by a high conversion of o-CNB and selectivity to o-CAN even after 10 cycles. The Ni/C catalyst is also active for the selective hydrogenation of other nitroarenes to the corresponding anilines.
Experimental
Preparation of the catalysts
All of the reagents involved in the present study were of analytical grade, and used without further purification. The low-temperature hydrothermal method was used to make the precursor for Ni/C catalysts. For a typical run, 0.6 g of nickel acetate, 32 mL of polyethylene glycol-200 (PEG-200) and 1 mL of furfural were added into 32 mL of deionized water under magnetic stirring at room temperature, yielding a clear solution in which all gradients were completely dissolved. The clear solution was transferred into a 100 mL Teflon-lined autoclave, sealed, and maintained at 180 °C for 12 h. When the reaction finished, the autoclave was naturally cooled back to room temperature, yielding powder-like products that were collected by filtering, and washed with deionized water and absolute ethanol for three times. After being dried at 80 °C for 3 h under vacuum, the hydrothermal product finally was obtained and used as the precursor of Ni/C catalysts, which was named Ni/C-p. The as-obtained Ni/C-p was put in a tube furnace, ramped at 3 °C min−1 in flowing H2 to different temperatures, and kept at the final temperature for 3 h, yielding the Ni/C catalysts named Ni/C-T, where T refers to the calcination temperature.Characterization of the catalysts
The morphology and structure of the as-made catalysts were examined by scanning electron microscopy (SEM, Quanta 450), transmission electron microscopy (TEM, JEM-2000EX), high resolution transmission electron microscopy (HRTEM, FEI Tecnai TF30), and energy-dispersive X-ray spectrometry (EDX, Oxford X-Max). Fourier transform infrared (FTIR) spectra were obtained using a Thermo Nicolet 6700 Flex with KBr as the reference. The X-ray diffraction patterns (XRD) were recorded on a Rigaku D/Max-2400X apparatus with Cu Kα irradiation, operated at 40 kV and 100 mA. The average sizes of Ni particles in catalysts were calculated using the Scherrer equation. N2 adsorption–desorption isotherms of the Ni/C catalysts were measured using a Micromeritics ASAP 2020 at −196 °C, and their pore size distributions were calculated using the DFT model. Before the measurements, the samples were degassed at 200 °C for 5 h under vacuum.Hydrogenation of nitroarenes
The selective hydrogenation of nitroarenes was carried out in a 100 mL Parr 4843 autoclave. Typically, the autoclave was loaded with 0.05 g of catalyst, 0.50 g of substrates and 50 mL of ethanol, then was completely sealed and flushed three times with pure hydrogen to remove air. After introducing H2 at an initial pressure of 2.0 MPa, the reactor was heated to 140 °C under constant stirring, and held at 140 °C for 1–2 h. After the reaction, the autoclave was cooled back to room temperature, and the reaction mixture was separated using a magnet, and the solution was analysed by gas chromatography (GC, Techcomp 7890F, equipped with an SE-54 capillary column and a flame ionization detector). The Ni/C-300 catalyst was collected, washed with absolute ethanol three times, and dried at 80 °C before reuse.Results and discussion
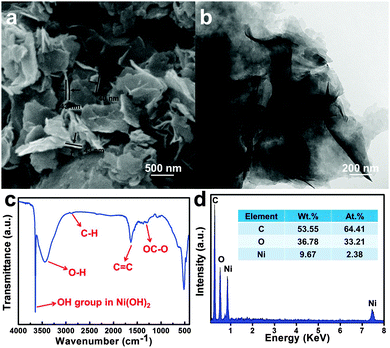
The typical SEM image of the as-made Ni/C-p is shown in Fig. 1a, showing a nanosheet-shaped structure with a thickness of 40–50 nm. Further TEM examination of Ni/C-p (Fig. 1b) reveals that the nanosheets are interconnected with each other, and exist as a bundle of agglomerated sheets. The FTIR spectrum (Fig. 1c) shows a sharp peak at 3600 cm−1, corresponding the geminal OH groups in the brucite-like structure,34,35 and peaks at ca. 3500, 1700 and 1000–1300 cm−1 that can be assigned to the stretching vibration of –OH, COO− and other oxygen-containing groups.36,37 The FTIR analysis suggests that the surface chemistry of the Ni/C-p is similar to the HTC. The EDX results (Fig. 4d) also confirm the presence of C, O, and Ni species in Ni/C-p, while the Ni species are present in the form of Ni(OH)2, confirmed by the XRD data discussed below.The catalysts made at different calcination temperatures were analyzed by XRD, of which the results are shown in Fig. 2. It can be clearly seen that the components of the catalysts vary with the calcination temperature. For Ni/C-p and Ni/C-200, Ni species are mainly Ni(OH)2, and no peaks related to metallic Ni can be seen. When the calcination temperature increases to 300 °C or higher, the characteristic peaks of Ni(OH)2 disappear, and new peaks of metallic Ni species can be observed,22 indicating that Ni(OH)2 species in the Ni/C-p are reduced to metallic Ni particles at 300 °C or above in H2. Obviously, except for the Ni/C-p and Ni/C-200, all of the catalysts obtained in the present study contain metallic Ni species.
The catalytic performance of different Ni/C catalysts for the selective hydrogenation of o-CNB to o-CAN was evaluated, of which the results are shown in Fig. 3 and Table S1 (ESI†). It can be seen that all of the Ni/C catalysts used in the present work show catalytic activities to some degree, with a selectivity to o-CAN over 90%. In particular, Ni/C-300 shows a much better activity than other catalysts under the same conditions (140 °C, 2 MPa H2, 1 h) in terms of both o-CNB conversion of 88.4% and o-CAN selectivity of 90.4%, and reaches nearly 100% in conversion of o-CNB in 2 h. Compared with Ni/C-p and Ni/C-200, the enhanced catalytic performance of Ni/C-300 can be attributed to the formation of metallic Ni species, evidenced by XRD results shown in Fig. 2. For the Ni/C catalysts obtained at temperatures over 300 °C, the catalytic activity drops greatly in terms of the o-CNB conversion as the calcination temperature increases. This is mainly due to the aggregation of the Ni particles, the decrease of the surface area and the destruction of the porous structure of Ni/C catalysts, which will be further discussed below.
 | ||
| Fig. 3 The catalytic performance of Ni/C catalysts for selective hydrogenation of o-CNB. Reaction conditions: 0.50 g o-CNB, 0.05 g catalyst, 50 mL ethanol, 140 °C, 2.0 MPa H2, time 1 h. | ||
Fig. 4 shows the typical SEM and TEM images of Ni/C-300, showing that the nanosheets in the Ni/C-p are transformed into irregular spherical assemblies (Fig. 4a) after calcination at 300 °C, mainly due to the shrinkage of hydrothermal carbon nanosheets37,38 and the aggregation of metallic Ni particles.39 The magnified SEM image (Fig. 4b) shows that Ni/C-300 has a unique hierarchical structure made of sheet-like subunits. Fig. 4c shows the TEM image of Ni/C-300, in which many pores made of disorderly assembled sheet-like subunits can be seen, which would provide channels for the transportation of reactants and products. A higher resolution TEM image (Fig. 4d) shows that metallic Ni particles are abundant, evenly embedded in the nanosheet-like carbon matrix, and have a narrow size distribution of 5–15 nm (Fig. 4f) with an average size of ca. 9 nm. Besides, the HRTEM image of Ni/C-300 (Fig. 4e) reveals that the Ni particle has a perfect single-crystal structure. The interplanar distance is measured to be about 2.04 Å, assigning to the lattice plane of Ni(111). It is because of the relatively uniform and small-sized Ni particles that the Ni/C-300 catalyst shows superior catalytic performance for the selective hydrogenation of o-CNB to o-CAN.
 | ||
| Fig. 4 (a) SEM image, (b) high-magnification SEM image, (c) typical TEM image, and (d) magnified TEM image of Ni/C-300, (e) HRTEM image and (f) size distribution of Ni particles in Ni/C-300. | ||
Fig. S1–S4 (ESI†) show the SEM and TEM images of the Ni/C catalysts calcined at temperatures over 300 °C, showing an obvious difference from Ni/C-300. For the Ni/C-400 catalyst, the sheet-like subunits disappear, instead, the irregular particle subunits are formed (Fig. S1a, ESI†), and meanwhile, the size of metallic Ni particles increases dramatically (Fig. S1b and c, ESI†). As the calcination temperature increases further, the subunits in the irregular spherical catalyst gradually collapse (Fig. S2 and S3, ESI†). In the case of Ni/C-700, its hierarchical structure is completely destroyed, evidenced from its SEM (Fig. S4a, ESI†) and TEM images (Fig. S4b, ESI†) in which a compact instead of a porous structure is observed. The magnified TEM image (Fig. S4c, ESI†) shows that it is hard to see a monodispersed Ni particle in the carbon matrix, which may be due to the agglomeration of the metallic Ni particles in Ni/C-700. According to the Scherrer equation, the average sizes of Ni particles in Ni/C-400, Ni/C-500, Ni/C-600, and Ni/C-700 are 35, 46, 56, and 65 nm, respectively. Nevertheless, the crystal structure characteristics of the Ni particles remain unchanged, which are confirmed by HRTEM images of Ni/C-400 and Ni/C-700 (Fig. S1d and S4d, ESI†).
In addition, the destruction of the hierarchical structure in Ni/C catalysts resulted in a smaller surface area. Fig. S5 (ESI†) shows the N2 adsorption–desorption isotherms, showing that the N2 adsorption amount of Ni/C-300 is much higher than that of Ni/C-400, and their Brunauer–Emmett–Teller (BET) specific surface areas are 20 and 8 m2 g−1, respectively. This is further confirmed by the disappeared micropores and mesopores in Ni/C-400, and the pore size distributions of Ni/C-300 and Ni/C-400 shown in Fig. S6 (ESI†). Obviously, higher calcination temperature would result in the collapse of hierarchical structure and the agglomeration of Ni particles, thus leading to poorer catalytic performance of the Ni/C catalyst for the selective hydrogenation of o-CNB, as shown in Fig. 3.
For high performance catalysts, in addition to the catalytic activity and selectivity, their stability is another important issue. The catalytic stability of the Ni/C-300 catalyst was also evaluated by the hydrogenation of o-CNB to o-CAN in the present work, of which the results are shown in Fig. 5 and Table S2 (ESI†). It can be seen that no obvious drop in both the o-CNB conversion and the o-CAN selectivity is observed over the Ni/C-300 catalyst, even after ten cycles. Both the o-CNB conversion and selectivity to o-CAN are over 90% under reaction conditions of 140 °C, 2 MPa H2, and time 2 h. Moreover, the supported metallic Ni particles that are magnetic make Ni/C-300 to be easily separated from the solution by an external magnetic field, as shown in the inset of Fig. 5.19 On an average, about 96.0 wt% of the Ni/C-300 catalyst can be recovered for each magnetic separation. Nevertheless, Ni particles aggregate after 10 cycles to some extent, and their size is in the range of 25–70 nm, as shown in the TEM image in Fig. S7 (ESI†). The results show that the Ni/C catalysts fabricated by the strategy reported in the present study have good reproducibility, and can be used repeatedly.
Moreover, it is interesting to note that the as-made Ni/C-300 also exhibits a high catalytic performance for the selective hydrogenation of other nitroarenes to the corresponding anilines (Table 1). For the m-CNB and p-CNB, Ni/C-300 can obtain the full conversion of substrates under the general reaction conditions, as well as over 84% selectivity to m-CAN and p-CAN. Meanwhile, 100% o-nitrophenol, 100% p-nitroaniline, 65.3% p-nitrotoluene, and 73.1% nitrobenzene have been converted over Ni/C-300, to ca. 86.4% o-aminophenol, 75.1% p-phenylenediamine, 100% p-methylaniline, and 96.9% aniline selectivity, respectively (entries 4–7 of Table 1). This suggests that Ni/C-300 is one of the promising catalysts for the selective hydrogenation of various nitroarenes.
Conclusions
In summary, magnetic Ni/C catalysts with a unique hierarchical structure have been prepared by directly heating sheet-like Ni(OH)2/HTC composites at different temperatures. Among the Ni/C catalysts obtained in the present work, Ni/C-300 has smaller Ni particles with high dispersion and a unique hierarchical structure, and exhibits a much better catalytic performance for selective hydrogenation of o-CNB to o-CAN. After the reaction, Ni/C-300 can be easily recovered using an external magnetic force, and reused for over 10 times without obvious drop in the conversion of o-CNB and selectivity to o-CAN. The Ni/C-300 also exhibits a good catalytic performance in the selective hydrogenation of other various nitroarenes. The present work may provide a facile approach to synthesize Ni/C catalysts that hold promise for the selective hydrogenation of nitroarenes to the corresponding anilines in large scale production.Acknowledgements
This work was partly supported by the NSFC (No. 21336001 and 21361162004) and the Education Ministry of China (No. 20120041110020).Notes and references
- H. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210–221 CrossRef CAS.
- A. Corma, P. Concepcion and P. Serna, Angew. Chem., Int. Ed., 2007, 46, 7266–7269 CrossRef CAS PubMed.
- E. Gelder, D. Jackson and M. Lok, Chem. Commun., 2005, 522–524 RSC.
- M. Liu, J. Zhang, J. Liu and W. W. Yu, J. Catal., 2011, 278, 1–7 CrossRef CAS PubMed.
- C. J. Serpell, J. Cookson, D. Ozkaya and P. D. Beer, Nat. Chem., 2011, 3, 478–483 CAS.
- F. Wang, J. Liu and X. Xu, Chem. Commun., 2008, 2040–2042 RSC.
- J. Zhang, Y. Wang, H. Ji, Y. Wei, N. Wu, B. Zuo and Q. Wang, J. Catal., 2005, 229, 114–118 CrossRef CAS PubMed.
- V. Kratky, M. Kralik, M. Mecarova, M. Stolcova, L. Zalibera and M. Hronec, Appl. Catal., A, 2002, 235, 225–231 CrossRef CAS.
- F. Cárdenas-Lizana, D. Lamey, N. Perret, S. Gómez-Quero, L. Kiwi-Minsker and M. A. Keane, Catal. Commun., 2012, 21, 46–51 CrossRef PubMed.
- Y. Chen, J. Qiu, X. Wang and J. Xiu, J. Catal., 2006, 242, 227–230 CrossRef CAS PubMed.
- D. He, H. Shi, Y. Wu and B.-Q. Xu, Green Chem., 2007, 9, 849–851 RSC.
- Y. Chen, C. Wang, H. Liu, J. Qiu and X. Bao, Chem. Commun., 2005, 5298–5300 RSC.
- B. Zuo, Y. Wang, Q. Wang, J. Zhang, N. Wu, L. Peng, L. Gui, X. Wang, R. Wang and D. Yu, J. Catal., 2004, 222, 493–498 CrossRef CAS PubMed.
- F. Cao, R. Liu, L. Zhou, S. Song, Y. Lei, W. Shi, F. Zhao and H. Zhang, J. Mater. Chem., 2010, 20, 1078–1085 RSC.
- J. Chen, N. Yao, R. Wang and J. Zhang, Chem. Eng. J., 2009, 148, 164–172 CrossRef CAS PubMed.
- X. Meng, H. Cheng, S.-I. Fujita, Y. Hao, Y. Shang, Y. Yu, S. Cai, F. Zhao and A. Masohiko, J. Catal., 2010, 269, 131–139 CrossRef CAS PubMed.
- J. Xiong, J. Chen and J. Zhang, Catal. Commun., 2007, 8, 345–350 CrossRef CAS PubMed.
- Y. Zheng, K. Ma, H. Wang, X. Sun, J. Jiang, C. Wang, R. Li and J. Ma, Catal. Lett., 2008, 124, 268–276 CrossRef CAS.
- Y. Xie, C. Yu, N. Xiao and J. Qiu, Catal. Commun., 2012, 28, 69–72 CrossRef CAS PubMed.
- N. Mahata, A. F. Cunha, J. J. M. Orfao and J. L. Figueiredo, Catal. Commun., 2009, 10, 1203–1206 CrossRef CAS PubMed.
- M. Oubenali, G. Vanucci, B. Machado, M. Kacimi, M. Ziyad, J. Faria, A. Raspolli-Galetti and P. Serp, ChemSusChem, 2011, 4, 950–956 CrossRef CAS PubMed.
- C. Wang, J. Qiu, C. Liang, L. Xing and X. Yang, Catal. Commun., 2008, 9, 1749–1753 CrossRef CAS PubMed.
- X. Xu, X. Li, H. Gu, Z. Huang and X. Yan, Appl. Catal., A, 2012, 429–430, 17–23 CrossRef CAS PubMed.
- C. Antonetti, M. Oubenali, A. M. Raspolli Galletti, P. Serp and G. Vannucci, Appl. Catal., A, 2012, 421–422, 99–107 CrossRef CAS PubMed.
- J. Wang, G. Fan and F. Li, Catal. Sci. Technol., 2013, 3, 982 CAS.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed.
- M. M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC.
- S. Ding, J. S. Chen and X. W. Lou, Adv. Funct. Mater., 2011, 21, 4120–4125 CrossRef CAS.
- S. Ding, J. S. Chen and X. W. Lou, Chem. – Eur. J., 2011, 17, 13142–13145 CrossRef CAS PubMed.
- T. Zhu, J. S. Chen and X. W. Lou, J. Phys. Chem. C, 2011, 115, 9814–9820 CAS.
- P. Makowski, R. D. Cakan, M. Antonietti, F. Goettmann and M. M. Titirici, Chem. Commun., 2008, 999–1001 RSC.
- C. B. Puttaa and S. Ghosh, Adv. Synth. Catal., 2011, 353, 1889–1896 CrossRef.
- L. F. Chen, H. W. Lang, Y. Lu, C. H. Cui and S. H. Yu, Langmuir, 2011, 27, 8998–9004 CrossRef CAS PubMed.
- G. J. d. A. A. Soler-Illia, M. Jobbágy, A. E. Regazzoni and M. A. Blesa, Chem. Mater., 1999, 11, 3140–3146 CrossRef.
- L. Xu, Y.-S. Ding, C.-H. Chen, L. Zhao, C. Rimkus, R. Joesten and S. L. Suib, Chem. Mater., 2008, 20, 308–316 CrossRef CAS.
- X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- L. Yu, C. Falco, J. Weber, R. J. White, J. Y. Howe and M. M. Titirici, Langmuir, 2012, 28, 12373–12383 CrossRef CAS PubMed.
- C. Falco, F. P. Caballero, F. Babonneau, C. Gervais, G. Laurent, M. M. Titirici and N. Baccile, Langmuir, 2011, 27, 14460–14471 CrossRef CAS PubMed.
- L. Xing, J. Qiu, C. Liang, C. Wang and L. Mao, J. Catal., 2007, 250, 369–372 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental results of Ni/C catalysts in the selective hydrogenation of o-CNB; SEM images of Ni/C-400, Ni/C-500, Ni/C-600, and Ni/C-700; TEM and magnified TEM images of Ni/C-400 and Ni/C-700; HRTEM images of Ni/C-400 and Ni/C-700; nitrogen adsorption–desorption isotherms of Ni/C-300 and Ni/C-400; pore size distributions of Ni/C-300 and Ni/C-400; experimental results of Ni/C-300 in the selective hydrogenation of o-CNB for 10 cycles; the TEM image of Ni/C-300 after 10 cycling reactions. See DOI: 10.1039/c4cp03978e |
| This journal is © the Owner Societies 2015 |