Croconaine rotaxane for acid activated photothermal heating and ratiometric photoacoustic imaging of acidic pH†
Samit
Guha
a,
Gillian Karen
Shaw
a,
Trevor M.
Mitcham
b,
Richard R.
Bouchard
b and
Bradley D.
Smith
*a
aDepartment of Chemistry and Biochemistry, 236 Nieuwland Science Hall, University of Notre Dame, IN 46556, USA. E-mail: smith.115@nd.edu
bDepartment of Imaging Physics, University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA
First published on 22nd October 2015
Abstract
Absorption of 808 nm laser light by liposomes containing a pH sensitive, near-infrared croconaine rotaxane dye increases dramatically in weak acid. A stealth liposome composition permits acid activated, photothermal heating and also acts as an effective nanoparticle probe for ratiometric photoacoustic imaging of acidic pH in deep sample locations, including a living mouse.
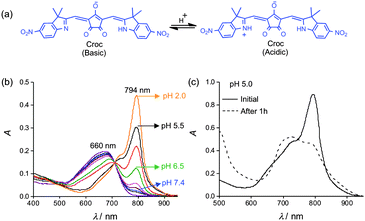
Molecules and materials that can absorb near-infrared (NIR) laser light and create localized heating are potentially valuable for numerous therapeutic applications.1 These photothermal agents can also enhance photoacoustic imaging, an emerging modality in molecular imaging with many performance features that are superior to fluorescence imaging and more amenable to clinical translation.2 To date, most NIR photothermal agents have absorbance profiles that cannot be chemically modulated. However, many photothermal and photoacoustic applications would be improved if the light absorbing properties could be switched on by specific local conditions. A good example is the tissue acidosis associated with pathological states such as cancer, infection, inflammation, and fibrosis.3 There are a few reports of NIR agents that can undergo changes in absorbance cross-section due to triggered self-aggregation but an inherent drawback with this approach is a dependence on local concentration which can be hard to control.4 New NIR absorbing agents are needed with chromophores that can be altered directly by the local chemical environment. A logical strategy is to design appropriate dyes with switchable absorbance, but there are very few NIR chromophores with the correct combination of chemical and photophysical properties.5 Recently, we discovered that croconaine dyes exhibit excellent laser heating properties.6 They strongly absorb NIR light (ε > 105 M−1 cm−1) and have short excited state lifetimes with little fluorescence emission, singlet oxygen generation, or dye photobleaching. We have described a supramolecular encapsulation strategy that modulates a croconaine's NIR absorbance wavelength, but this method is susceptible to the concentration dependence mentioned above.6a Here we report a conceptual advance that is based on the pH dependent croconaine (Croc) dye shown in Fig. 1a.7 The dye's absorption profile can be switched between an anionic basic form (λmax ∼ 660 nm) and a zwitterionic acidic form (λmax ∼ 794 nm). An important spectral feature is the relatively narrow bandwidths which permit large amplitude switching of molar absorptivity at the two wavelengths. To utilize the lipophilic Croc dye for biological applications we incorporated it within liposome membranes and employed supramolecular strategies to achieve two crucial photothermal and photoacoustic performance features: stable ratiometric absorption response that is unaltered by laser irradiation, and fine-tuning of the dye pKa to match the local pH.
A preliminary study using a 808 nm diode laser showed that irradiation of an ethanol solution of Croc in its basic form created very little sample heating, but conversion to the acidic form greatly enhanced laser induced heating (Fig. S1, ESI†). Formulation of the Croc dye for operation in aqueous solution, was achieved by incorporating it into stealth liposomes as an amphiphilic ion-pair with 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) as the counter cation.‡ Stealth liposomes have polyethylene glycol chains protruding from the membrane surface which inhibit liposome aggregation and extend circulation time in the blood stream.8 Initially, stealth liposomes doped with Croc dye (Croc-SL) were prepared as unilamellar vesicles (diameter = 194 nm) with a composition of POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol-PEG600
Chol-PEG600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP
DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Croc 84
Croc 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
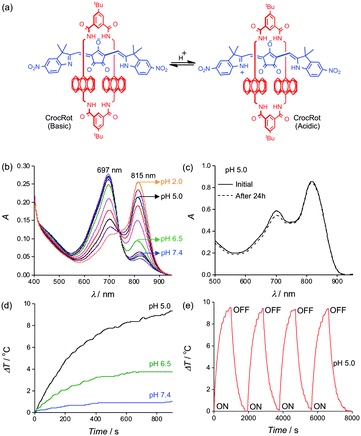
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S3, ESI†).‡ The sample color was blue at pH 7.4 due to the Croc conjugate base absorption at 660 nm. Titration with acid abolished the blue color and an intense new peak appeared at 794 nm with a pKa of 6.5 (Fig. 1b and Fig. S3, ESI†). Irradiation of cuvettes containing Croc-SL with an 808 nm diode laser produced acid activated photothermal heating (Fig. S3b, ESI†). Dynamic light scattering measurements indicated that Croc-SL do not change size in acidic pH, but the absorption spectrum slowly broadened with time (Fig. 1c, Fig. S3 and S4, ESI†). The absorption broadening is consistent with self-aggregation of the zwitterionic Croc conjugate acid within the liposome membrane and is a potential drawback for important applications, such as repetitive photothermal heating and ratiometric photoacoustic imaging of pH (see below). We hypothesized that the problem could be circumvented by trapping the Croc dye within a tetralactam macrocycle and generating an interlocked croconaine rotaxane (CrocRot) (Fig. 2a). Even under self-aggregation conditions, we expected the surrounding macrocycle in CrocRot to prevent electronic coupling of the encapsulated dye.6b Initial attempts to prepare CrocRot by a direct slippage process in organic solvent or within liposome membranes were unsuccessful, presumably due to the steric bulk of the indolenine dimethyl substituents. Thus, a Leigh-type templated clipping reaction was used to convert Croc dye to CrocRot and the rotaxane structure was elucidated using a battery of spectroscopic methods (Fig. S5–S11, ESI†).9 As expected, rotaxane formation produced a 22 nm red-shift of the dye absorption maxima.6 The interlocked CrocRot molecule does not unthread in competitive organic solvents or when it is located within a liposome membrane (Fig. S9, ESI†). We prepared CrocRot doped stealth liposomes (CrocRot-SL) composed of POPC
3 (Fig. S3, ESI†).‡ The sample color was blue at pH 7.4 due to the Croc conjugate base absorption at 660 nm. Titration with acid abolished the blue color and an intense new peak appeared at 794 nm with a pKa of 6.5 (Fig. 1b and Fig. S3, ESI†). Irradiation of cuvettes containing Croc-SL with an 808 nm diode laser produced acid activated photothermal heating (Fig. S3b, ESI†). Dynamic light scattering measurements indicated that Croc-SL do not change size in acidic pH, but the absorption spectrum slowly broadened with time (Fig. 1c, Fig. S3 and S4, ESI†). The absorption broadening is consistent with self-aggregation of the zwitterionic Croc conjugate acid within the liposome membrane and is a potential drawback for important applications, such as repetitive photothermal heating and ratiometric photoacoustic imaging of pH (see below). We hypothesized that the problem could be circumvented by trapping the Croc dye within a tetralactam macrocycle and generating an interlocked croconaine rotaxane (CrocRot) (Fig. 2a). Even under self-aggregation conditions, we expected the surrounding macrocycle in CrocRot to prevent electronic coupling of the encapsulated dye.6b Initial attempts to prepare CrocRot by a direct slippage process in organic solvent or within liposome membranes were unsuccessful, presumably due to the steric bulk of the indolenine dimethyl substituents. Thus, a Leigh-type templated clipping reaction was used to convert Croc dye to CrocRot and the rotaxane structure was elucidated using a battery of spectroscopic methods (Fig. S5–S11, ESI†).9 As expected, rotaxane formation produced a 22 nm red-shift of the dye absorption maxima.6 The interlocked CrocRot molecule does not unthread in competitive organic solvents or when it is located within a liposome membrane (Fig. S9, ESI†). We prepared CrocRot doped stealth liposomes (CrocRot-SL) composed of POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol-PEG600
Chol-PEG600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP
DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CrocRot 84
CrocRot 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S12, ESI†) and found that they exhibited very similar pH dependent changes in absorption spectra as above, with a slightly decreased dye pKa of ∼6.0 (Fig. 2b and Fig. S13, ESI†). We were pleased to find that the spectral stability of CrocRot-SL was greatly improved; for example, there was very little spectral change after sitting for 24 hours in acidic pH (Fig. 2c and Fig. S14, ESI†). As shown in Fig. 2d, pH dependent photothermal heating was observed using an 808 diode laser (3.5 W cm−2), and the high spectral stability of the CrocRot-SL enabled multiple laser heating cycles using the same sample with no measurable change in the absorption profile and photothermal heating response (Fig. 2e and Fig. S15, ESI†). CrocRot-SL are highly stable in the presence of serum, biological salts (Fig. S16a, ESI†), and small biomolecules such as glutathione, cysteine, and H2O2 (Fig. S16b, ESI†). Furthermore, CrocRot-SL were not toxic to cultured mammalian cells (Fig. S16c, ESI†). These results demonstrate that CrocRot-SL are robust biocompatible nanoparticles that can be switched reversibly between acid and base forms and that they survive strong and repeated laser heating. They likely can be used for photothermal therapy and controlled release in disease states that have tissue acidosis.10 In addition, they can also be utilized for another important application, namely, ratiometric photoacoustic imaging of acidic pH.
3 (Fig. S12, ESI†) and found that they exhibited very similar pH dependent changes in absorption spectra as above, with a slightly decreased dye pKa of ∼6.0 (Fig. 2b and Fig. S13, ESI†). We were pleased to find that the spectral stability of CrocRot-SL was greatly improved; for example, there was very little spectral change after sitting for 24 hours in acidic pH (Fig. 2c and Fig. S14, ESI†). As shown in Fig. 2d, pH dependent photothermal heating was observed using an 808 diode laser (3.5 W cm−2), and the high spectral stability of the CrocRot-SL enabled multiple laser heating cycles using the same sample with no measurable change in the absorption profile and photothermal heating response (Fig. 2e and Fig. S15, ESI†). CrocRot-SL are highly stable in the presence of serum, biological salts (Fig. S16a, ESI†), and small biomolecules such as glutathione, cysteine, and H2O2 (Fig. S16b, ESI†). Furthermore, CrocRot-SL were not toxic to cultured mammalian cells (Fig. S16c, ESI†). These results demonstrate that CrocRot-SL are robust biocompatible nanoparticles that can be switched reversibly between acid and base forms and that they survive strong and repeated laser heating. They likely can be used for photothermal therapy and controlled release in disease states that have tissue acidosis.10 In addition, they can also be utilized for another important application, namely, ratiometric photoacoustic imaging of acidic pH.
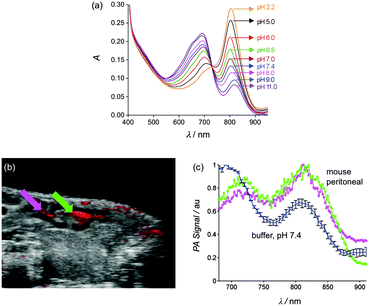
Photothermal agents facilitate photoacoustic imaging by absorbing short-duration, low power density laser pulses (∼0.06 W cm−2) and emitting ultrasound waves which are scattered less than light waves and thus penetrate further through biological media to produce higher resolution images.2 There is a need for new types of exogenous probes for enhanced photoacoustic imaging. The current literature contains several reports of probes that can be activated by cleavage enzymes to produce changes in signal intensity, but to the best of our knowledge, there is no NIR photoacoustic probe with a rapid ratiometric pH response.11 A preliminary evaluation of phantoms (narrow tubes oriented parallel to the face of the transducer, see Fig. S17, ESI†) containing ethanol solutions of Croc or CrocRot showed that they generated about the same photoacoustic signal intensities as the commonly used NIR dye indocyanine green (ICG) (Fig. S18, ESI†).12 We then examined two phantoms containing CrocRot-SL at pH 7.4 or 5.0 and observed that the photoacoustic and absorption spectra at each pH value were closely matched (Fig. 3a and Fig. S19, ESI†). Additional photoacoustic imaging studies examined the two phantoms at different depths in a light scattering medium (0.7% milk fat solution) that is opaque to normal fluorescence imaging methods. We found that the difference in phantom pH could easily be distinguished by comparing the ratio of photoacoustic signal intensities at 812 and 690 nm. The image in Fig. 3b shows a large difference in ratiometric response (more than 5-fold) for the two phantoms, each located at a depth of 11–13 mm from the transducer face. Additional imaging experiments at phantom depths of 9 and 26 mm confirmed that the photoacoustic signal-to-noise decreased with depth, but the pH dependent 812/690 signal ratio hardly changed.
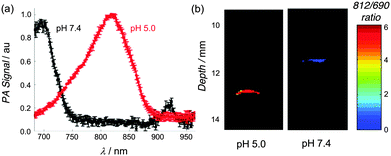 | ||
| Fig. 3 (a) Photoacoustic scans of CrocRot-SL at pH 7.4 (black) and pH 5.0 (red), mean (N = 3) and SD shown. (b) Ratiometric photoacuostic images of two phantoms containing CrocRot-SL in buffer at pH 7.4 or 5.0, proximally located at 11–13 mm depth in light scattering media. See Fig. S17 (ESI†) for a diagram of the transverse orientation of the phantoms. | ||
With these proof-of-concept imaging results in hand we moved to a more demanding biological system to assess the potential of acid sensitive CrocRot-doped liposomes. In some diseases, the tissue pH can be as low as 5.5 (e.g., intracellular endosomes, inflamed tissue) but in others it is closer to 6.5 (e.g., extracellular matrix of solid tumors).3 This means it will be necessary to optimize the pKa of the CrocRot for each case, to ensure a significant degree of absorbance switching. After examining the literature,13 we reasoned that the pKa can be fine-tuned in a rational way by simply adding controlled amounts of appropriately charged amphiphiles to the liposome formulation. To demonstrate the utility of this concept we purposely raised the CrocRot pKa by substituting the neutral Ch-PEG600 in the CrocRot-SL membrane with anionic DSPE-PEG2000.‡ After a short sequence of trials, we found that a modified liposome formulation composed of POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPE-PEG2000
DSPE-PEG2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP
DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CrocRot 79
CrocRot 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and given the descriptor name, CrocRot-IVSL, exhibited a pKa of ∼6.5 (Fig. 4a and Fig. S20, ESI†).§
3 and given the descriptor name, CrocRot-IVSL, exhibited a pKa of ∼6.5 (Fig. 4a and Fig. S20, ESI†).§
To demonstrate the capability of CrocRot-IVSL for in vivo photoacoustic imaging we chose to image the pH of peritoneal fluid in a living mouse which is known to be in the range of 6.1–6.3.14 Following a protocol that was approved by the appropriate animal care and use committee, a single dose of CrocRot-IVSL was injected into the peritoneal cavity of a living mouse (N = 2), and the sagittal plane of the mouse abdomen was imaged using co-registered B-mode ultrasound and multi-wavelength photoacoustic imaging. The image in Fig. 4b is comprised of a B-mode ultrasound image (grayscale) clearly showing the peritoneal cavity and an overlay (red) depicting the corresponding photoacoustic response when the excitation wavelength was 740 nm. There are three photoacoustic spectra in Fig. 4c. One spectrum corresponds to the sample of CrocRot-IVSL in buffer at pH 7.4 before injection into the mouse, and the other two spectra correspond to the different regions-of-interest (ROI) in the mouse peritoneal indicated by the arrows in Fig. 4b. A comparison of the two in vivo ratiometric photoacoustic scans with the UV/absorption plots indicates a peritoneal pH of 6.0–6.5.¶ Thus, the in vivo imaging correctly identified the weakly acidic pH of the mouse peritoneal. With further development, this photoacoustic method may become a new in vivo technique for measuring the pH of peritoneal fluid, which is known to decrease with pathological conditions such as bacterial peritonitis, a frequent complication in patients on peritoneal dialysis.15 It should also be effective at identifying local regions of weakly acidic tissue associated with other types of disease.3 In addition, the liposome architecture can be further customized by incorporating drugs or additional imaging reporter groups to make a wide array of novel laser responsive therapeutic and diagnostic agents.16
We are grateful for funding support from the Walther Cancer Foundation Advancing Basic Cancer Research Grant (2013/14) administered by the Harper Cancer Research Institute (USA) and the NIH (GM059078 to B. D. S., and P30 CA016672, S10 OD010403 to R. R. B.).
Notes and references
- (a) S. Lal, S. E. Clare and N. J. Halas, Acc. Chem. Res., 2008, 41, 1842–1851 CrossRef CAS PubMed; (b) V. Shanmugam, S. Selvakumar and C.-S. Yeh, Chem. Soc. Rev., 2014, 43, 6254–6287 RSC; (c) L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- (a) S. Zackrisson, S. M. W. Y. van de Ven and S. S. Gambhir, Cancer Res., 2014, 74, 979–1004 CrossRef CAS PubMed; (b) S. Mallidi, G. P. Luke and S. Emelianov, Trends Biotechnol., 2011, 29, 213–221 CrossRef CAS PubMed; (c) L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed.
- (a) B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed; (b) A. I. Hashim, X. Zhang, J. W. Wojtkowiakk and R. J. Gillies, NMR Biomed., 2011, 24, 582–591 Search PubMed; (c) O. A. Andreev, A. D. Dupuy, M. Segala, S. Sandugu, D. A. Serra, C. O. Chichester, D. M. Engelman and Y. K. Reshetnyak, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7893–7898 CrossRef CAS PubMed; (d) Y.-T. Tsai, J. Zhou, H. Weng, J. Shen, L. Tang and W.-J. Hu, Adv. Healthcare Mater., 2014, 3, 221–229 CrossRef CAS PubMed; (e) F. Okajima, Cell. Signalling, 2013, 25, 2263–2271 CrossRef CAS PubMed; (f) A. Honasoge and H. Sontheimer, Front. Physiol., 2013, 4, 316 Search PubMed; (g) K. M. Jones, E. A. Randtke, C. M. Howison, J. Cárdenas-Rodríguez, P. J. Sime, M. R. Kottmann and M. D. Pagel, Mol. Imaging Biol., 2015, 17, 177–184 CrossRef PubMed.
- (a) J. Nam, N. Won, H. Jin, H. Chung and S. Kim, J. Am. Chem. Soc., 2009, 131, 13639–13645 CrossRef CAS PubMed; (b) J. Nam, W.-G. La, S. Hwang, Y. S. Ha, N. Park, N. Won, S. Jung, S. H. Bhang, Y.-J. Ma, Y.-M. Cho, M. Jin, J. Han, J.-Y. Shin, E. K. Wang, S. G. Kim, S.-H. Cho, J. Yoo, B.-S. Kim and S. Kim, ACS Nano, 2013, 7, 3388–3402 CrossRef CAS PubMed; (c) A. Dragulescu-Andrasi, S. Kothapalli, G. A. Tikhomirov, J. Rao and S. S. Gambhir, J. Am. Chem. Soc., 2013, 135, 11015–11022 CrossRef CAS PubMed; (d) H. Li, X. Liu, N. Huang, K. Ren, Q. Jin and J. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 18930–18937 CrossRef CAS PubMed; (e) J. F. Hainfeld, M. J. O'Connor, P. Lin, L. Qian, D. N. Slatkin and H. M. Smilowitz, PLoS One, 2014, 9, e88414 Search PubMed.
- W. Shi and H. Ma, Chem. Commun., 2012, 48, 8732–8744 RSC.
- (a) G. T. Spence, G. V. Hartland and B. D. Smith, Chem. Sci., 2013, 4, 4240–4244 RSC; (b) G. T. Spence, S. S. Lo, C. Ke, H. Destecroix, A. P. Davis, G. V. Hartland and B. D. Smith, Chem. – Eur. J., 2014, 20, 12628–12635 CrossRef CAS PubMed . For a summary of absorption properties for nanoparticles containing croconaine dyes, see: ; (c) M. S. Devadas, T. Devkota, S. Guha, S. K. Shaw, B. D. Smith and G. V. Hartland, Nanoscale, 2015, 7, 9779–9785 RSCS. Guha, S. K. Shaw, G. T. Spence, F. M. Roland and B. D. Smith, Langmuir, 2015, 31, 7826–7834 CrossRef CAS PubMed.
- C. Encinas, E. Otazo, L. Rivera, S. Miltsov and J. Alonso, Tetrahedron Lett., 2002, 43, 8391–8393 CrossRef CAS.
- M. L. Immordino, F. Dosio and L. Cattel, Int. J. Nanomed., 2006, 1, 297–315 CrossRef CAS PubMed.
- J. J. Gassensmith, L. Barr, J. M. Baumes, A. Paek, A. Nguyen and B. D. Smith, Org. Lett., 2008, 10, 3343–3346 CrossRef CAS PubMed.
- (a) E. Ju, K. Dong, Z. Liu, F. Pu, J. Ren and X. Qu, Adv. Funct. Mater., 2015, 25, 1574–1580 CrossRef CAS; (b) M. Guo, J. Huang, Y. Deng, H. Shen, Y. Ma, M. Zhang, A. Zhu, Y. Li, H. Hui, Y. Wang, X. Yang, Z. Zhang and H. Chen, Adv. Funct. Mater., 2015, 25, 59–67 CrossRef CAS; (c) X. Wu, M. Yu, B. Lin, H. Xing, J. Han and S. Han, Chem. Sci., 2015, 6, 798–803 RSC.
- For examples of photoacoustic probes with chemically or biochemically activated response, see: (a) K. Pu, A. J. Shuhendler, J. V. Jokerst, J. Mei, S. S. Gambhir, Z. Bao and J. Rao, Nat. Nanotechnol., 2014, 9, 233–239 CrossRef CAS PubMed; (b) A. Ray, H. K. Yoon, Y. E. K. Lee, R. Kopelman and X. Wang, Analyst, 2013, 138, 3126–3130 RSC; (c) K. Yang, L. Zhu, L. Nie, X. Sun, L. Cheng, C. Wu, G. Niu, X. Chen and Z. Liu, Theranostics, 2014, 4, 134–141 CrossRef CAS PubMed; (d) J. Levi, S. R. Kothapalli, T.-J. Ma, K. Hartman, B. T. Khuri-Yakub and S. S. Gambhir, J. Am. Chem. Soc., 2010, 132, 11264–11269 CrossRef CAS PubMed; (e) D. Razansky, N. J. Harlaar, J. L. Hillebrands, A. Taruttis, E. Herzog, C. J. Zeebregts, G. M. van Dam and V. Ntziachristos, Mol. Imaging Biol., 2012, 14, 277–285 CrossRef PubMed; (f) K. J. Cash, C. Li, J. Xia, L. V. Wang and H. A. Clark, ACS Nano, 2015, 9, 1692–1698 CrossRef CAS PubMed; (g) G. Huang, Z. Si, S. Yang, C. Li and D. Xing, J. Mater. Chem., 2012, 22, 22575–22581 RSC; (h) J. Zhang, Z. Qiao, P. Yang, J. Pan, L. Wang and H. Wang, Chin. J. Chem., 2015, 33, 35–52 CrossRef CAS.
- N. Beziere, N. Lozano, A. Nunes, J. Salichs, D. Queiros, K. Kostarelos and V. Ntziachristos, Biomaterials, 2015, 37, 415–424 CrossRef CAS PubMed . For recent insight into the photophysical factors that control photoacoustic signal intensity using dyes, see: M. Frenette, M. Hatamimoslehabadi, S. Bellinger-Buckley, S. Laoui, J. La, S. Bag, S. Mallidi, T. Hasan, B. Bouma, C. Yelleswarapu and J. Rochford, J. Am. Chem. Soc., 2014, 136, 15853–15856 CrossRef PubMed.
- (a) I. M. Hafez, S. Ansell and P. R. Cullis, Biophys. J., 2000, 79, 1438–1446 CrossRef CAS PubMed; (b) G. Shi, W. Guo, S. M. Stephenson and R. J. Lee, J. Controlled Release, 2002, 80, 309–319 CrossRef CAS.
- A. Beck, S. Bergner-Rabinowitz and I. Ofek, J. Bacteriol., 1969, 100, 1204–1207 CAS.
- H. Johno, R. Ogata, S. Nakajima, N. Hiramatsu, T. Kobayashi, H. Hara and M. Kitamura, Nephrol., Dial., Transplant., 2012, 27, 4053–4060 CrossRef CAS PubMed.
- (a) R. D. Corato, G. Béalle, J. Kolosnjaj-Tabi, A. Espinosa, O. Clément, A. K. A. Silva, C. Ménager and C. Wilhelm, ACS Nano, 2015, 9, 2904–2916 CrossRef PubMed; (b) M. S. Muthu, D. T. Leong, L. Mei and S.-S. Feng, Theranostics, 2014, 4, 660–677 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemical structures, synthesis and characterization; liposome data; photoacoustic imaging data. See DOI: 10.1039/c5cc08317f |
| ‡ Abbreviations: Cholesterol-PEG600 n = 10–11 (Ch-PEG600), croconaine (Croc), croconaine rotaxane (CrocRot), 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP), N-(carbonyl-methoxypolyethyleneglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanol-amine (DSPE-PEG2000), Indocyanine Green (ICG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). See Fig. S2 (ESI†) for chemical structures. |
| § This result highlights an important technical advantage of pH sensor production by nanoparticle self-assembly versus covalent synthesis of a discrete molecular sensor. Fine-tuning the pKa of a molecular sensor requires a much longer and more labour intensive cycle of structure redesign and chemical synthesis. |
| ¶ Inspection of Fig. 4c shows that the two major peaks within the in vivo photoacoustic spectra are slightly red-shifted when compared to the spectrum in buffer before injection. This is attributed to in vivo attenuation of the lower photoacoustic excitation wavelengths by the blood-perfused skin and tissue. This anatomical-dependent variation may explain why the two in vivo photoacoustic spectra from nearby peritoneal locations are slightly different. A goal for future imaging studies is to develop calibration methods that correct these tissue-dependent changes in the in vivo photoacoustic spectrum. |
| This journal is © The Royal Society of Chemistry 2016 |