A two-photon fluorescent probe for lysosomal zinc ions†
Hyo-Jun
Lee‡
a,
Chang-Woo
Cho
*a,
Hyewon
Seo‡
b,
Subhankar
Singha‡
b,
Yong Woong
Jun
b,
Kyung-Ha
Lee
c,
Youngseob
Jung
c,
Kyong-Tai
Kim
c,
Seongjun
Park
d,
Sung Chul
Bae
d and
Kyo Han
Ahn
*b
aDepartment of Chemistry, Kyungpook National University, Daegu 702-701, Republic of Korea. E-mail: cwcho@knu.ac.kr
bDepartment of Chemistry, POSTECH, Pohang 790-784, Republic of Korea. E-mail: ahn@postech.ac.kr
cDivision of Integrative Biosciences and Biotechnology, POSTECH, Pohang 790-784, Republic of Korea
dSchool of Life Sciences, UNIST, Ulsan 689-798, Republic of Korea
First published on 21st October 2015
Abstract
The selective detection of zinc ions in lysosomes over that in cytosol is achieved with a fluorescent probe, which enabled the fluorescence imaging of endogenous zinc ions in lysosomes of NIH 3T3 cells as well as mouse hippocampal tissues by two-photon microscopy under excitation at 900 nm.
Being the second most abundant transition metal ion in our body, Zn(II) is of paramount importance for maintaining biological functions including the modulation of biological redox systems, enzymatic functions, and cellular signalling.1 Imbalance in the intracellular Zn(II) level is associated with various diseases such as Alzheimer's disease, Parkinson's disease, diabetes, prostate cancer and immune dysfunction.2 The influence of Zn(II) on human health has motivated scientists to investigate “Zn(II) biology” by fluorescence methods. Accordingly, much efforts have been made to develop fluorescent Zn(II) probes for biological applications. A fluorescence assay based on the chelation of Zn(II) with N-(6-methoxy-8-quinolyl)-p-toluenesulfonamide was one of the earliest developments.3 Since then, significant progress has been made, and at present several fluorescent Zn(II) probes with high affinity, fast response, good biocompatibility, and preferably with longer excitation and emission wavelengths are available.4 Recently, the subcellular detection of Zn(II) by targeting a specific organelle has become a subject of great interest in this research field. For example, fluorescence imaging of zinc ions in a subcellular compartment such as mitochondria or lysosome has received considerable interests.5
In the biological systems, Zn(II)-bound metallothioneins are the main cytoplasmic proteins that maintain the intracellular Zn(II) homeostasis. During the oxidative stress, a rapid influx of hydrogen peroxide causes oxidation of the cysteine residues in Zn(II)-metallothioneins to disulfides and subsequent release of Zn(II) ions.6 The released Zn(II) ions are rapidly accumulated into the lysosomes, causing the lysosomal membrane permeabilization (LMP), a potentially lethal event. In this process, the additional Zn(II) ions in lysosomes induce lysosomal membrane disintegration and release several hydrolytic enzymes (including cathepsins) from the lysosomal lumen to the cytosol. The presence of lysosomal hydrolases in the cytosol causes the digestion of vital cytosolic proteins and the activation of additional hydrolases to initiate a cell death pathway.5d,7,8 Therefore, the development of fluorescent probes that selectively detect lysosomal Zn(II) ions, which act as a downstream marker for the LMP processes, is necessary to study the oxidative stress level.8
Among the many fluorescent Zn(II) probes developed so far, only a few are claimed to detect lysosomal Zn(II).9 Those probes have a lysosomal targeting moiety and thus accumulate in the lysosome to report Zn(II) ions there. Such probes thus rely on the “concentration gradient” of the probes between the cytosol and lysosomes; they hardly discriminate the Zn(II) ions in lysosomes from those in cytosol, as they show fluorescence response upon binding with Zn(II) in a broad pH range covering that of lysosomal pH (4.5–5.5) and cytosolic pH (7.2–7.4). Such concentration gradient-dependent probes report the probe's concentration gradient from the cytosol to lysosomes. Such probes also may present interference from non-specific imaging in the case of slow accumulation of the probe into lysosomes. On the contrary, a fluorescent probe that shows “quenched” fluorescence at cytosolic pH (7.4) even after binding with Zn(II) but strong fluorescence only at lysosomal pH (pH 4.5–5.5) could provide highly specific lysosomal Zn(II) detection irrespective of the probe accumulation process.
Most of the existing Zn(II) probes are also one-photon excitable at short wavelengths outside the biological optical window. For tissue imaging applications, additional issues such as photobleaching of the probe and autofluorescence from tissues become serious concerns under one-photon excitation at the shorter wavelengths. The use of the low-energy near-infrared (NIR) excitation light under two-photon excitation conditions enables deeper tissue penetration and also alleviates the photobleaching and autofluorescence issues.10
Two-photon probes excitable at NIR wavelengths furthermore allow 3D imaging of tissues with very high spatial resolution. Accordingly, several two-photon Zn(II) probes have been developed, which showed turn-on11 or ratiometric fluorescence response.12 A few of them are also equipped with an organelle targeting function, which enabled the detection of the Zn(II) ions in the cellular membrane or mitochondria; but Zn(II) detection in the lysosomal site using two-photon microscopy (TPM) is rarely known. Recently a two-photon probe was reported that could detect endogenous Zn(II) ions in the Zn(II)-enriched acid vesicles such as insulin granules only; however, the imaging of the low level of endogenous lysosomal Zn(II) was not possible.13 Moreover, this two-photon probe also showed a similar level of response towards Zn(II) as well as Cd(II). Herein, we report a two-photon probe that allows selective detection of the endogenous Zn(II) ions in lysosomes, which also has an improved selectivity over Cd(II).
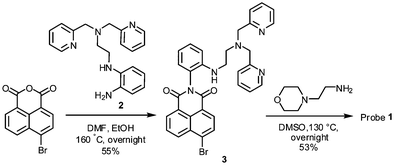
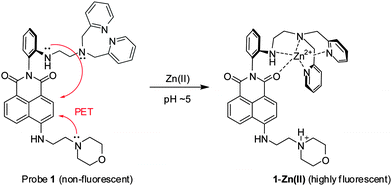
We designed probe 1 (Scheme 1) where a morpholine group and a N,N-di-(2-picolyl)ethylenediamine (DPEN) ligand are introduced into a naphthalimide dye. The naphthalimide dye is dipolar and has the maximum absorbance of around 450 nm; hence, it would allow tissue imaging by TPM under excitation at 900 nm, a wavelength at the longer side of the first biological optical window (650–950), which is beneficial in reducing autofluorescence observed in tissue imaging.14 Recently, we have disclosed a novel approach to make such a dipolar dye emit strongly in aqueous media, in particular under two-photon excitation conditions.15 Based on our findings and a previous example,16 we have chosen the naphthalimide dye for the development of the two-photon probe. It is known that a morpholine moiety is useful for the sensing purpose of lysosomal pH, as a protonated morpholine has a pKa value of ∼5, a lysosomal pH. DPEN is known to bind metal ions such as Zn(II), Cd(II), and Cu(II).17 In this work, we have introduced the DPEN group to the naphthalimide dye in such a way that one of the imide carbonyl oxygen atoms could coordinate Zn(II) together with the DPEN nitrogen atoms, with a hope that this additional coordination would improve the metal ion selectivity as well as the binding affinity. Both are realized indeed.
 | ||
| Scheme 1 Detection of zinc ions under acidic conditions with probe 1, through the blocking of two PET processes. | ||
Probe 1 thus designed can be readily synthesized from 4-bromo-1,8-naphthalic anhydride (Scheme 2). Thus, the imide formation with DPEN containing amine precursor 2 and then electrophilic amine substitution at 4-position with 4-(2-aminoethyl)morpholine afforded probe 1 in a good yield (details are described in the ESI†).
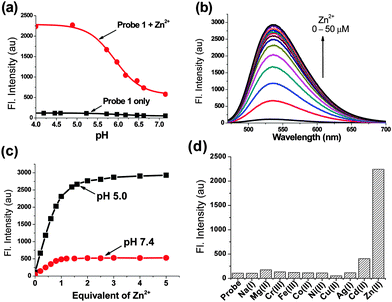
First we examined whether probe 1 could detect Zn(II) in the lysosomal pH range (pH = 4.5–5.5). The fluorescence measurement at various pH values shows that probe 1 emits almost negligible fluorescence, whereas its Zn(II) complex emits strong fluorescence in the lysosomal pH range (pH = 4.5–5.5) (ΦF of probe 1 = 0.03; ΦF of 1-Zn(II) = 0.23 in pH 5 buffer, Table S1 in the ESI†); this is plausibly owing to the suppressed PET processes both from the Zn(II)-coordinated DPEN site as well as from the protonated morpholine unit (Fig. 1a). The pKa values of probe 1 and its Zn(II) complex were 6.05 and 5.91, respectively, as determined from the fluorescence intensity changes within the pH range of 4.0–8.0 (Fig. S2 in the ESI†).
The probe's emission intensity gradually increased with increasing Zn(II) concentration and finally saturated with an equivalent of Zn(II) ions at pH = 5.0 (Fig. 1b and Fig. S3 in the ESI†), indicative of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between the probe and Zn(II) in the acidic environment. The 1
1 binding between the probe and Zn(II) in the acidic environment. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was also observed at physiological pH of 7.4 (Fig. S4 in the ESI†), albeit the final fluorescence intensity was much weaker (∼1/6 times) than that at pH = 5.0 (Fig. 1c). The lower fluorescence intensity of probe 1 in the presence of Zn(II) ions at neutral pH can be ascribed to the PET process from the morpholine to the naphthalimide dye. In fact, the behaviour of weak emission at cytosolic pH (7.4) and strong emission at lysosomal pH (4.5–5.5) is highly desirable for lysosomal Zn(II) imaging.
1 binding stoichiometry was also observed at physiological pH of 7.4 (Fig. S4 in the ESI†), albeit the final fluorescence intensity was much weaker (∼1/6 times) than that at pH = 5.0 (Fig. 1c). The lower fluorescence intensity of probe 1 in the presence of Zn(II) ions at neutral pH can be ascribed to the PET process from the morpholine to the naphthalimide dye. In fact, the behaviour of weak emission at cytosolic pH (7.4) and strong emission at lysosomal pH (4.5–5.5) is highly desirable for lysosomal Zn(II) imaging.
The previously reported lysosomal Zn(II) probes,9 after binding with Zn(II), showed strong fluorescence even at the cytosolic pH (pH = 7.2–7.4); however, the accumulation of the probes into lysosomes provided enhanced fluorescence intensity in lysosomes compared to that in cytosol as noted above. On the contrary, our probe shows a low fluorescence intensity at cytosolic pH (7.4) even after binding with Zn(II) ions. A strong fluorescence intensity can be only observable when the probe binds Zn(II) ions inside the lysosomes (pH 4.5–5.5).
Probe 1 showed a good linear response to [Zn(II)] even in the lower concentration region (0–1.0 μM) at pH = 5.0 (Fig. S5 in the ESI†), offering a very high sensitivity toward Zn(II). The limit of detection was determined to be 0.18 μM on the basis of the signal-to-noise ratio of three (Fig. S6 in the ESI†). The association constant (Ka) for probe 1 with Zn(II) was found to be 1.17 × 105 M−1 (error < 10%), as determined from the Benesi–Hildebrand plot18 based on the fluorescence titration data. The high sensitivity and strong binding affinity of probe 1 towards Zn(II) indeed allowed us to detect the intracellular Zn(II) ions present in lysosomes where the free Zn(II) concentration is reached up to a micromolar level during certain stimulations, such as inflammation and oxidative stress.19
The high selectivity of Zn(II) over other common transition metals is also a key issue to be addressed in the development of a fluorescent probe. In comparison with the frequently used di(2-picolyl)amine (DPA) receptors, the additional coordination site (one of the imide carbonyl groups) is found to not only improve the binding affinity to Zn(II) but also improve the selectivity over other competing metal ions.4b,9b Probe 1 thus showed high selectivity towards Zn(II) among various metal ions, in particular with a small interference from Cd(II), which is most interfering (Fig. 1d). Note that a related system with a para-substituted DPEN moiety showed a similar binding response towards Zn(II) as well as Cd(II).20 The competition assay revealed that probe 1 was also able to sense Zn(II) in presence of other metal ions, except Cu(II) and Co(II) that caused quenching due to their paramagnetic nature (Fig. S7 in the ESI†). As the concentrations of these two metal ions are negligible in the human body (intracellular free copper is undetectable) compared to zinc ion,21 their interference is not a problematic issue during the detection and bioimaging of Zn(II).
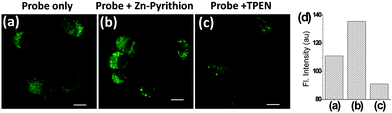
Next we examined whether probe 1 could be used to detect intracellular Zn(II) ions in lysosomes by TPM. Prior to the bioimaging studies, a low level of cytotoxicity of probe 1 towards NIH 3T3 cells was confirmed by the MTT assay (Fig. S9 in the ESI†). First, we evaluated the sensing capability of probe 1 in live NIH 3T3 cells in the absence and presence of an exogenous source of Zn(II) or a chelator that suppresses the intracellular Zn(II) level. The NIH 3T3 cells incubated with probe 1 for 30 min showed strong fluorescence when observed by TPM, apparently from the intracellular Zn(II) ions (Fig. 2a). When the probe-treated cells were again incubated for 10 min with an exogenous Zn(II) source (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Zn(ClO4)2 and pyrithion, which in situ forms the Zn–pyrithion complex that enhances the cell-membrane permeability22), there was a substantial increase in the fluorescence intensity in the cells (Fig. 2b). In contrast, the fluorescence signal decreased significantly when the probe-treated cells were incubated with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), for 10 min (Fig. 2c).
1 mixture of Zn(ClO4)2 and pyrithion, which in situ forms the Zn–pyrithion complex that enhances the cell-membrane permeability22), there was a substantial increase in the fluorescence intensity in the cells (Fig. 2b). In contrast, the fluorescence signal decreased significantly when the probe-treated cells were incubated with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), for 10 min (Fig. 2c).
TPEN is a strong chelator for Zn(II) ions, which inhibits the binding of zinc ions by the probe. The relative fluorescence intensity data of the respective images show the fluorescence changes dependent on the Zn(II) ions (Fig. 2d). From the above set of experiments, it is evident that probe 1 allows us to detect and image the lysosomal Zn(II) ions by using TPM. Similar results were also obtained when the cells were observed by using a one-photon confocal microscope (Fig. S10 in the ESI†).
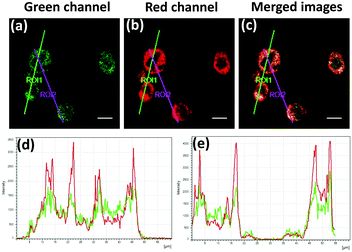
To ensure that probe 1 can sense the Zn(II) ions in lysosomes, we performed a co-localization experiment with a commercial marker for lysosomes (LysoTracker® Deep Red) in live NIH 3T3 cells. The TPM images obtained through different optical windows clearly show well merging (Fig. 3a–c). Additionally, the intensity profiles of the two linear regions of interest (ROI 1 and ROI 2) across the cells also vary in close synchronicity (Fig. 3d and e). The Pearson's co-localization coefficient, which describes the correlation of the intensity distributions to characterize the degree of overlap between images, was calculated to be 0.87 by using the LAS AF software (Table S2 in the ESI†). Also, the co-localization experiments performed using commercial dyes showed that the probe poorly localizes in the mitochondria and the endoplasmic reticulum (Fig. S11 and S12 in the ESI†). Hence, all the above data support that probe 1 detects intracellular lysosomal Zn(II) ions with a high fidelity.
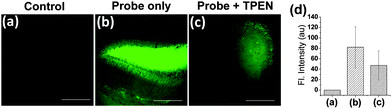
With the two-photon excitable probe 1, we also imaged Zn(II) ions in deep tissues of a mouse brain (hippocampal slice). The TPM images of the tissue slice incubated with probe 1 show strong fluorescence apparently due to endogenous free Zn(II) in the mouse brain,1b,2a whereas the tissue without probe treatment (used as the control) does not show any fluorescence (Fig. 4). Again, the addition of a metal chelator TPEN to the probe-treated tissue reduces the fluorescence intensity. These imaging experiments altogether demonstrate the potential applicability of probe 1 for the imaging of endogenous Zn(II) ions in tissues by TPM.
In conclusion, we have developed a novel two-photon probe that selectively detects the Zn(II) ions in lysosomes over those in cytosol. The probe, a naphthalimide dye composed of an N,N-di-(2-picolyl)ethylenediamine (DPEN) ligand and a morpholine unit, only fluoresces in the presence of Zn(II) ions at the lysosomal pH, with high sensitivity as well as improved selectivity over the most competing Cd(II) ions. The probe enabled the fluorescence imaging of a lower level of intracellular Zn(II) ions present in lysosomes as well as in mouse brain tissues under two-photon excitation conditions at 900 nm. The probe thus provides a useful tool for investigating various biological processes associated with lysosomal Zn(II) ions by two-photon microscopy.
K. H. Ahn thanks the financial supports from the Ministry of Health & Welfare (HI13C1378) and Global Research Laboratory Program (2014K1A1A2064569) through the National Research Foundation (NRF) funded by Ministry of Science, ICT & Future Planning. C. W. Cho thanks the financial support from the Kyungpook National University Research Fund, 2014.
Notes and references
-
(a) C. J. Frederickson, Int. Rev. Neurobiol., 1989, 31, 145 CrossRef CAS PubMed
; (b) A. S. Nakashima and R. H. Dyck, Brain Res. Rev., 2009, 59, 347 CrossRef CAS PubMed
.
-
(a) C. J. Frederickson, J. Y. Koh and A. I. Bush, Nat. Rev. Neurosci., 2005, 6, 449 CrossRef CAS PubMed
; (b) S. A. Parasad, Annu. Rev. Nutr., 1985, 5, 341 CrossRef PubMed
; (c) S. K. Ghosh, P. Kim, X. A. Zhang, S. H. Yun, A. Moore, S. J. Lippard and Z. Medarova, Cancer Res., 2010, 70, 6119 CrossRef CAS PubMed
.
- C. J. Frederickson, E. J. Kasarskis, D. Ringo and R. E. Frederickson, J. Neurosci. Methods, 1987, 20, 91 CrossRef CAS PubMed
.
-
(a) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed
; (b) Z. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996 RSC
; (c) E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193 CrossRef CAS PubMed
; (d) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS PubMed
; (e) Y. Chen, Y. Bai, Z. Han, W. He and Z. Guo, Chem. Soc. Rev., 2015, 44, 4517 RSC
.
-
(a) S. L. Sensi, H. Z. Yin and J. H. Weiss, Eur. J. Neurosci., 2000, 12, 3813 CrossRef CAS PubMed
; (b) S. L. Sensi, D. Ton-That, P. G. Sullivan, E. A. Jonas, K. R. Gee, L. K. Kaczmarek and J. H. Weiss, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6157 CrossRef CAS PubMed
; (c) J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622 CrossRef CAS PubMed
; (d) J. J. Hwang, S.-J. Lee, T.-Y. Kim, J.-H. Cho and J.-Y. Koh, J. Neurosci., 2008, 28, 3114 CrossRef CAS PubMed
.
-
(a) A. Krężel and W. Maret, JBIC, J. Biol. Inorg. Chem., 2008, 13, 401 CrossRef PubMed
; (b) R. A. Colvin, W. R. Holmes, C. P. Fontaine and W. Maret, Metallomics, 2010, 2, 306 RSC
; (c) S. G. Bell and B. L. Vallee, ChemBioChem, 2009, 10, 55 CrossRef CAS PubMed
.
-
(a) S.-J. Lee and J.-Y. Koh, Mol. Brain, 2010, 3, 30 CrossRef PubMed
; (b) S. L. Sensi, P. Paoletti, J.-Y. Koh, E. Aizenman, A. I. Bush and M. Hershfinkel, J. Neurosci., 2011, 31, 16076 CrossRef CAS PubMed
; (c) P. Boya and G. Kroemer, Oncogene, 2008, 27, 6434 CrossRef CAS PubMed
.
- H. C. Roh, S. Collier, J. Guthrie, J. D. Robertson and K. Kornfeld, Cell Metab., 2012, 15, 88 CrossRef CAS PubMed
.
-
(a) L. Xue, G. Li, D. Zhu, Q. Liu and H. Jiang, Inorg. Chem., 2012, 51, 10842 CrossRef CAS PubMed
; (b) H. Zhu, J. Fan, S. Zhang, J. Cao, K. Song, D. Ge, H. Dong, J. Wang and X. Peng, Biomater. Sci., 2014, 2, 89 RSC
; (c) K. Sreenath, Z. Yuan, J. R. Allen, M. W. Davidson and L. Zhu, Chem. – Eur. J., 2014, 20, 867 Search PubMed
.
- W. R. Zipfel, R. M. Williams and W. W. Webb, Nat. Biotechnol., 2003, 2, 1369 CrossRef PubMed
.
-
(a) H. M. Kim, M. S. Seo, M. J. An, J. H. Hong, Y. S. Tian, J. H. Choi, O. Kwon, K. J. Lee and B. R. Cho, Angew. Chem., Int. Ed., 2008, 47, 5167 CrossRef CAS PubMed
; (b) G. Masanta, C. S. Lim, H. J. Kim, J. H. Han, H. M. Kim and B. R. Cho, J. Am. Chem. Soc., 2011, 133, 5698 CrossRef CAS PubMed
; (c) Z. Mao, L. Hu, X. Dong, C. Zhong, B.-F. Liu and Z. Liu, Anal. Chem., 2014, 86, 6548 CrossRef CAS PubMed
; (d) C. Huang, J. Qu, J. Qi, M. Yan and G. Xu, Org. Lett., 2011, 13, 1462 CrossRef CAS PubMed
; (e) X.-Y. Chen, J. Shi, Y.-M. Li, F.-L. Wang, X. Wu, Q.-X. Guo and L. Liu, Org. Lett., 2009, 11, 4426 CrossRef CAS PubMed
; (f) M. Khan, C. R. Goldsmith, Z. Huang, J. Georgiou, T. T. Luyben, J. C. Roder, S. J. Lippard and K. Okamoto, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 6786 CrossRef CAS PubMed
.
-
(a) M. Taki, J. L. Wolford and T. V. O'Halloran, J. Am. Chem. Soc., 2004, 126, 712 CrossRef CAS PubMed
; (b) X. Meng, S. Wang, Y. Li, M. Zhu and Q. Guo, Chem. Commun., 2012, 48, 4196 RSC
; (c) K. P. Divya, S. Sreejith, P. Ashokkumar, K. Yuzhan, Q. Peng, S. K. Maji, Y. Tong, H. Yu, Y. Zhao, P. Ramamurthy and A. Ajayaghosh, Chem. Sci., 2014, 5, 3469 RSC
.
- P. Rivera-Fuentes, A. T. Wrobel, M. L. Zastrow, M. Khan, J. Georgiou, T. T. Luyben, J. C. Roder, K. Okamoto and S. J. Lippard, Chem. Sci., 2015, 6, 1944 RSC
.
- D. Kim, H. Moon, S. H. Baik, S. Singha, Y. W. Jun, T. Wang, K. H. Kim, B. S. Park, J. Jung, I. Mook-Jung and K. H. Ahn, J. Am. Chem. Soc., 2015, 137, 6781 CrossRef CAS PubMed
.
- S. Singha, D. Kim, B. Roy, S. Sambasivan, H. Moon, A. S. Rao, J. Y. Kim, T. Joo, J. W. Park, Y. M. Rhee, T. Wang, K. H. Kim, Y. H. Shin, J. Jung and K. H. Ahn, Chem. Sci., 2015, 6, 4335 RSC
.
- H. Yu, Y. Xiao and L. Jin, J. Am. Chem. Soc., 2012, 134, 17486 CrossRef CAS PubMed
.
- E. Kawabata, K. Kikuchi, Y. Urano, H. Kojima, A. Odani and T. Nagano, J. Am. Chem. Soc., 2005, 127, 818 CrossRef CAS PubMed
.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS
.
-
(a) Y. Li, C. J. Hough, S. W. Suh, J. M. Sarvey and C. J. Frederickson, J. Neurophysiol., 2001, 86, 2597 CAS
; (b) T. E. Kehl-Fie and E. P. Skaar, Curr. Opin. Chem. Biol., 2010, 14, 218 CrossRef CAS PubMed
.
- J. Wang, Y. Xiao, Z. Zhang, X. Qian, Y. Yanga and Q. Xu, J. Mater. Chem., 2005, 15, 2836 RSC
.
-
(a) T. D. Rae, P. J. Schmidt, R. A. Pufahl, V. C. Culotta and T. V. O'Halloran, Science, 1999, 284, 805 CrossRef CAS PubMed
; (b) P. Kajič, I. Milošev, B. Pihlar and V. Pišot, J. Trace Elem. Med. Biol., 2003, 17, 153 CrossRef
.
- C. J. Chang, J. Jaworski, E. M. Nolan, M. Sheng and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1129 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and procedures regarding the synthesis of the probe, one-photon and two-photon spectroscopic analysis, cell and tissue imaging experiments. See DOI: 10.1039/c5cc06976a |
| ‡ Equally contributed. |
| This journal is © The Royal Society of Chemistry 2016 |