Open Access Article
Open Access ArticleOne-dimensional coordination polymers of [Co3(dpa)4]2+ and [MF6]2− (M = ReIV, ZrIV and SnIV)†
Vladimir
Bulicanu
ab,
Kasper S.
Pedersen
abc,
Mathieu
Rouzières
ab,
Jesper
Bendix
d,
Pierre
Dechambenoit
ab,
Rodolphe
Clérac
ab and
Elizabeth A.
Hillard
*ab
aCNRS, CRPP, UPR 8641, 33600 Pessac, France. E-mail: hillard@crpp-bordeaux.cnrs.fr
bUniv. Bordeaux, CRPP, UPR 8641, 33600 Pessac, France
cCNRS, ICMCB, UPR 9014, F-33600 Pessac, France
dDepartment of Chemistry, University of Copenhagen, Universitetsparken 5, 2100 Copenhagen, Denmark
First published on 16th October 2015
Abstract
One-dimensional coordination polymers of alternating metal–metal bonded trinuclear [Co3(dpa)4]2+ (dpa = the anion of 2,2′-dipyridylamine) building blocks and [ReF6]2− (1), [ZrF6]2− (2) or [SnF6]2− (3) linkers have been self-assembled and crystallographically characterized. Magnetic measurements reveal a significant ferromagnetic coupling (J/kB = +9.9 K) between S = 1/2 {Co36+} and S = 3/2 ReIV magnetic sites through a single, unsupported fluoride bridge in 1.
One-dimensional metal–ligand coordination polymers are of particular interest in the molecular magnetism community for their potential as single-chain magnets (SCMs).1 Because the stereochemistry around the metal ion determines the topology of coordination-driven self-assembled systems, a simple approach to magnetic linear polymers entails the use of paramagnetic metal complexes possessing two empty, or at least kinetically labile, coordination sites. These acceptors can then be associated into chains using paramagnetic linkers with two donor sites, the most common of which have been trans-cyanidometallates.2
Possessing rich magnetic and electronic properties, in addition to axial geometry, paddlewheel complexes are attractive building blocks for the construction of linear one-dimensional systems. Indeed, many dinuclear metal ion tetracarboxylates, e.g. {Cr24+}, {Cu24+}, {Rh24+} and {Ru24+}, have strongly Lewis acidic axial sites and form extended chain structures in the absence of exogenous ligands by coordination of a carboxylate oxygen atom to the axial position of an adjacent molecule.3 On the other hand, copper and zinc paddlewheels have been extensively used in the formation of three-dimensional metal–organic frameworks,4 while one-dimensional coordination polymers using exogenous spacers and {Rh24+},5 {Cu24+},6 {Re26+},7 {Zn24+}8 units are also well represented. Of particular note are magnetic systems, some quite sophisticated, constructed of {Ru25+} (S = 3/2)9 and {Ru24+} (S = 1)10 building blocks and which display remarkable properties.
We here turn our attention to Extended Metal Atom Chains (EMACs), which contain three or more metal ions typically supported by oligopyridylamines.11 While dinuclear divalent paddlewheel complexes are often diamagnetic, EMACs tend to have an odd number of metal centers and to be paramagnetic. This is due to a variety of factors, including an odd number of electrons, accidental degeneracy of frontier orbitals, or dissymmetry in the linear complex giving rise to an isolated high-spin metal ion. However, with the exception of a few coordination polymers based on [Ni3(dpa)4]2+,12 EMACs have rarely been used to build extended systems.
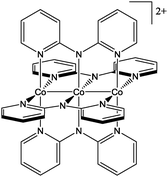
The [Co3(dpa)4]2+ unit (Chart 1) is expected to be a useful building block, based on the interesting physical properties of the [Co3(dpa)4Cl2] analogue. Its core consists of three aligned cobalt metal ions possessing a delocalized 3-electron 3-center bond, and depending on crystal packing effects, the spacing of the Co ions can be equal (as in the orthorhombic [Co3(dpa)4Cl2]·CH2Cl2 phase), or unequal, (as in the tetragonal [Co3(dpa)4Cl2]·2CH2Cl2 phase).13 Both forms show a spin-crossover (SCO) process from S = 1/2 to S = 3/2 or 5/2. Remarkably, the one electron oxidized compound, [Co3(dpa)4Cl2](BF4) undergoes a two-step SCO, from S = 0 to S = 1 to S = 2.14 Finally, the axial chloride ligands can be cleanly removed from [Co3(dpa)4Cl2] using silver salts, generating MeCN,15 BF4−,16 CN−, N(CN)2− or NCS−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 adducts. Nonetheless, [Co3(dpa)4]2+ has not been widely used in extended structures, even if organization into rigid multi-dimensional systems is an appealing strategy to introduce cooperativity in the SCO process and thus to generate possible spin transition phenomena. Recent work by Shatruk and coworkers on putative 2D grids formed by four ditopic [Co3(dpa)4]2+ units coordinated to [Co(CN)6]3− or [Fe(CN)6]3− is the only example of the assembly of [Co3(dpa)4]2+ units into extended networks.18 Unfortunately, these materials could not be crystallographically characterized.
17 adducts. Nonetheless, [Co3(dpa)4]2+ has not been widely used in extended structures, even if organization into rigid multi-dimensional systems is an appealing strategy to introduce cooperativity in the SCO process and thus to generate possible spin transition phenomena. Recent work by Shatruk and coworkers on putative 2D grids formed by four ditopic [Co3(dpa)4]2+ units coordinated to [Co(CN)6]3− or [Fe(CN)6]3− is the only example of the assembly of [Co3(dpa)4]2+ units into extended networks.18 Unfortunately, these materials could not be crystallographically characterized.
In order to obtain 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 neutral chains, we have selected the dianionic metalloligands [ReF6]2−, [ZrF6]2− and [SnF6]2− to link the [Co3(dpa)4]2+ building block. (PPh4)2[ReF6]·2H2O was recently reported to exhibit a pronounced magnetic anisotropy,19 a property that was retained in 1D coordination polymers featuring {MII–F–ReIV} linkages. Furthermore, the [Ni(viz)4(ReF6)] (viz = 1-vinylimidazole) chain additionally exhibits relatively strong fluoride-mediated ferromagnetic coupling between the ReIV and NiII magnetic sites (J/kB = +17 K; H = −2JS1S2 Hamiltonian definition).
1 neutral chains, we have selected the dianionic metalloligands [ReF6]2−, [ZrF6]2− and [SnF6]2− to link the [Co3(dpa)4]2+ building block. (PPh4)2[ReF6]·2H2O was recently reported to exhibit a pronounced magnetic anisotropy,19 a property that was retained in 1D coordination polymers featuring {MII–F–ReIV} linkages. Furthermore, the [Ni(viz)4(ReF6)] (viz = 1-vinylimidazole) chain additionally exhibits relatively strong fluoride-mediated ferromagnetic coupling between the ReIV and NiII magnetic sites (J/kB = +17 K; H = −2JS1S2 Hamiltonian definition).
Notably, (PPh4)2[ReF6]·2H2O can be dehydrated without decomposition and subsequently recrystallized to afford (PPh4)2[ReF6]·MeCN, (ESI†) which is a convenient starting material for assembly reactions with moisture sensitive building blocks. The combination of equimolar solutions of in situ formed [Co3(dpa)4(BF4)2] and (PPh4)2[MF6] (M = ReIV, ZrIV and SnIV) immediately gave a dark precipitate, insoluble in all common solvents. In order to obtain crystals, a dilute solution of (PPh4)2[MF6] in MeCN was layered on a DMF solution of [Co3(dpa)4(BF4)2] in a thin tube and left to slowly diffuse over several weeks.‡ Green blocks of [Co3(dpa)4(ReF6)]·2DMF (1), [Co3(dpa)4(ZrF6)]·2DMF (2) and [Co3(dpa)4(SnF6)]·2DMF (3) were obtained in moderate yield. Powder X-ray diffraction revealed the presence of only one phase and thermal gravimetric analysis was consistent with the presence of two DMF molecules (ESI†).
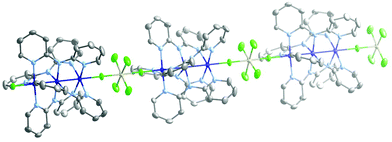
Compounds 1–3 are isostructural. They crystallize in the space group P4/ncc (Table S1, ESI†) with the four-fold axis coincident with the rigorously linear Co–F–M axis. The chains are racemic, being made up of alternating Δ and Λ [Co3(dpa)4]2+ helicoidal moieties (Fig. 1). A small disorder is found with respect to the wrapping of the dpa− ligands, and each position is occupied by ca. 80% of one enantiomer and 20% of the other. The {Co36+} core is slightly asymmetrical with differences in Co–Co distances of 0.008(1), 0.013(1) and 0.013(1) Å in 1, 2 and 3, respectively (at 200 K; Table 1). Surprisingly, the Co–F distances are quite unequal, with differences of 0.066(4) Å (1), 0.059(4) Å (2) and 0.069(4) Å (3), with the longer distance associated with the terminal cobalt engaged in the longer Co–Co bonding interaction. This asymmetry is however not reflected in the two individual M–Fax distances, which are distinctly and equally elongated due to their coordination to the Co centers (Table 1).
| 1 | 2 | 3 | |
|---|---|---|---|
| Co1–Co3 | 2.270(1) | 2.275(1) | 2.275(1) |
| Co2–Co3 | 2.278(1) | 2.288(1) | 2.288(1) |
| Co1–F1 | 2.115(4) | 2.097(4) | 2.104(4) |
| Co2–F2 | 2.181(4) | 2.156(4) | 2.173(4) |
| M–F1 | 1.962(4) | 2.037(4) | 2.002(4) |
| M–F2 | 1.970(4) | 2.044(4) | 2.009(4) |
| M–Feq | 1.931(3) | 1.973(3) | 1.928(3) |
Magnetic susceptibility measurements were performed on polycrystalline samples of 1–3 between 1.85 and 300 K. At room temperature, the χT product of 1 amounts to 2.4 cm3 K mol−1, slightly higher than the theoretical value of 1.97 cm3 K mol−1 for isolated S = 1/2 ([Co3(dpa)4]2+, g = 2.35, C = 0.52 cm3 K mol−1) and S = 3/2 ([ReF6]2−, g = 1.76, C = 1.45 cm3 K mol−1) spins (Fig. 2). On lowering the temperature, the χT product increases steadily, with a more abrupt increase below ca. 30 K eventually reaching 9.6 cm3 K mol−1 at 1.85 K, suggestive of a significant ferromagnetic coupling between {Co36+} and ReIV magnetic sites. The data were fit to a Seiden model20 derived from the exchange-coupling Hamiltonian 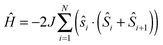 , where ŝi and Ŝi represent spin-operators of {Co36+} and ReIV, yielding g = 2.05(5) and J/kB = +9.9(1) K. As expected, this average g value falls in between the previously reported values for [Co3(dpa)4Cl2]·CH2Cl2 (g = 2.35)13 and (PPh4)2[ReF6]·2H2O (g = 1.76).19 Compounds 2 and 3, possessing diamagnetic [ZrF6]2− and [SnF6]2− linkers, show an almost temperature independent χT product between about 50 and 300 K. The low temperature decrease of the χT product is likely due to weak antiferromagnetic coupling between the {Co36+} centers through the diamagnetic linkers, and fitting the data to a regular quantum s = 1/2 spin chain model21 derived from the Hamiltonian
, where ŝi and Ŝi represent spin-operators of {Co36+} and ReIV, yielding g = 2.05(5) and J/kB = +9.9(1) K. As expected, this average g value falls in between the previously reported values for [Co3(dpa)4Cl2]·CH2Cl2 (g = 2.35)13 and (PPh4)2[ReF6]·2H2O (g = 1.76).19 Compounds 2 and 3, possessing diamagnetic [ZrF6]2− and [SnF6]2− linkers, show an almost temperature independent χT product between about 50 and 300 K. The low temperature decrease of the χT product is likely due to weak antiferromagnetic coupling between the {Co36+} centers through the diamagnetic linkers, and fitting the data to a regular quantum s = 1/2 spin chain model21 derived from the Hamiltonian 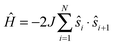 yields J/kB = −1.0 K for 2 while magnetic interactions are virtually undetectable above 1.85 K for 3 (with g = 2.36 for both). The difference in the magnetic coupling between 2 and 3 may be attributed to the lack of empty d orbitals in SnIV to mediate a superexchange interaction, in contrast with ZrIV in 2.
yields J/kB = −1.0 K for 2 while magnetic interactions are virtually undetectable above 1.85 K for 3 (with g = 2.36 for both). The difference in the magnetic coupling between 2 and 3 may be attributed to the lack of empty d orbitals in SnIV to mediate a superexchange interaction, in contrast with ZrIV in 2.
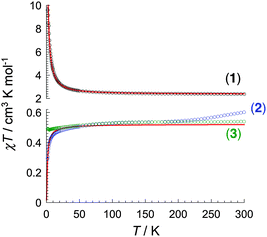 | ||
Fig. 2 Temperature dependence of the χT product for 1 (○), 2 ( ) and 3 ( ) and 3 ( ) at 1000 Oe (χ is defined as molar magnetic susceptibility equal to M/H). The red lines are the fits discussed in the text. ) at 1000 Oe (χ is defined as molar magnetic susceptibility equal to M/H). The red lines are the fits discussed in the text. | ||
The magnetic behaviour of these coordination polymers is significantly different to that of their building units. For example, the parent compound [Co3(dpa)4Cl2]·CH2Cl2 demonstrates a SCO event above 200 K.13 No such thermal behaviour is observed in 1 and 3 up to 300 K, while for 2, an onset of what may be a SCO event appears reversibly above 250 K. However, above 300 K, a rapid and irreversible rise in the χT product of these compounds was observed in concert with DMF loss. Therefore, the assembly of ostensibly SCO [Co3(dpa)4]2+ units with [MF6]2− linkers into chains increased the SCO temperature to inaccessible temperatures without evidence of cooperativity increase. As previously reported, the [ReF6]2− anion in (PPh4)2[ReF6]·2H2O displays single-molecule magnet (SMM) properties, a behavior which is retained upon assembly of [ReF6]2− units by diamagnetic linkers.19 In 1, slow dynamics of the magnetization were not detected by ac susceptibility (in zero-dc or applied dc field, up 10 kHz and down to 1.8 K). As we previously discussed,19,22 an electronic elongation of the [ReF6]2− octahedron, while keeping the tetragonal symmetry implies the axial zero-field splitting parameter, D, to be positive. Therefore, despite relatively strong intra-chain ferromagnetic interactions, magnetic bistability, e.g. single-chain magnet behavior,1 is not expected nor experimentally observed in the present system.
In summary, the metal–metal bonded [Co3(dpa)4]2+ moiety has been used for the first time as a building block in a structurally characterized coordination network leading to one-dimensional extended architectures. These unique systems pave the way toward the synthesis of new bistable coordination networks incorporating metal–metal bonding clusters with spin-crossover or spin-transition properties.
This work was supported by the CNRS, the University of Bordeaux, the Conseil Régional d'Aquitaine, the ANR and the Erasmus Mundus European program for a PhD fellowship of VB. JB acknowledges support from The Danish Research Council under grant 12-125226. KSP thanks The Danish Research Council for a DFF | Sapere Aude: Research Talent award (grant 4090-00201). The authors thank L. Favello for crystallographic assistance, P. Dagault for TGA analysis, E. Lebraud for PXRD and C. Mathionère and D. Samohvalov for technical assistance.
Notes and references
- C. Coulon, C. Pianet, M. Urdampilleta and R. Clérac, Struct. Bonding, 2015, 164, 143 CrossRef.
- M. Ferbinteanu, H. Miyasaka, W. Wernsdorfer, K. Nakata, K.-I. Sugiura, M. Yamashita, C. Coulon and R. Clérac, J. Am. Chem. Soc., 2005, 127, 3090 CrossRef CAS PubMed; J.-F. Guo, W.-F. Yeung, P.-H. Lau, X.-T. Wang, S. Gao, W.-T. Wong, S. S.-Y. Chui, C.-M. Che, W.-Y. Wong and T.-C. Lau, Inorg. Chem., 2010, 49, 1607 CrossRef PubMed; D. Zhang, L.-F. Zhang, Y. Chen, H. Wang, Z.-H. Ni, W. Wernsdorfer and J. Jiang, Chem. Commun., 2010, 46, 3550 RSC; T. D. Harris, C. Coulon, R. Clérac and J. R. Long, J. Am. Chem. Soc., 2011, 133, 123 CrossRef PubMed; I. Bhowmick, E. A. Hillard, P. Dechambenoit, C. Coulon, T. D. Harris and R. Clérac, Chem. Commun., 2012, 48, 9717 RSC; S. Wang, X. H. Ding, Y. H. Li and W. Huang, Coord. Chem. Rev., 2012, 256, 439 CrossRef PubMed; Q. Zhang, H. Zhou, X. Shen, H. Zhou and Y. Yang, New J. Chem., 2013, 37, 941 RSC; J. Xiang, L.-H. Jia, B.-W. Wang, S.-M. Yiu, S.-M. Peng, W.-Y. Wong, S. Gao and T.-C. Lau, Dalton Trans., 2013, 42, 3876 RSC.
- F. A. Cotton, C. A. Murillo, J. L. Eglin, M. H. Chisholm, C. B. Hollandsworth, A. P. Sattelberger, R. A. Walton, P. Angaridis, T. Ren, H. T. Chifotides, K. R. Dunbar, D. J. Timmons, M. P. Doyle and J. F. Berry, Multiple Bonds between Metal Atoms, Springer, USA, 2005 CrossRef CAS PubMed; E. V. Dikarev, A. S. Filatov, R. Clérac and M. A. Petrukhina, Inorg. Chem., 2006, 45, 744 CrossRef CAS PubMed; B. Li, H. Zhang, L. Huynh, M. Shatruk and E. V. Dikarev, Inorg. Chem., 2007, 46, 9155 CrossRef PubMed; M. V. Peskov, X.-H. Miao, D. Heryadi, J. Eppinger and U. Schwingenschlogl, J. Phys. Chem. C, 2013, 117, 5462 Search PubMed.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561 RSC.
- S. Takamizawa, E. Nakata, H. Yokoyama, K. Mochizuki and W. Mori, Angew. Chem., Int. Ed., 2003, 42, 4331 CrossRef CAS PubMed; Y. Fuma and M. Ebihara, Chem. Lett., 2006, 35, 1298 CrossRef; K. Uemura and M. Ebihara, Inorg. Chem., 2011, 50, 7919 CrossRef PubMed; K. Uemura and M. Ebihara, Inorg. Chem., 2013, 52, 5535 CrossRef PubMed; W. Kosaka, K. Yamagishi, A. Hori, H. Sato, R. Matsuda, S. Kitagawa, M. Takata and H. Miyasaka, J. Am. Chem. Soc., 2013, 135, 18469 CrossRef PubMed.
- J. S. Valentine, A. J. Silverstein and Z. G. Soos, J. Am. Chem. Soc., 1974, 96, 97 CrossRef CAS; G. Smith, C. H. L. Kennard and K. A. Byriel, Polyhedron, 1991, 10, 873 CrossRef; F. P. W. Agterberg, H. A. J. Provó Kluit, W. L. Driessen, H. Oevering, W. Buijs, M. T. Lakin, A. L. Spek and J. Reedijk, Inorg. Chem., 1997, 36, 4321 CrossRef PubMed; X.-Y. Li, L.-Q. Liu, M.-L. Ma, X.-L. Zhao and K. Wen, Dalton Trans., 2010, 39, 8646 RSC; D. Pogozhev, S. A. Baudron, G. Rogez and M. W. Hosseini, Polyhedron, 2013, 52, 1329 CrossRef PubMed.
- M. Q. Dequeant, R. McGuire, Jr., D. R. McMillin and T. Ren, Inorg. Chem., 2005, 44, 6521 CrossRef CAS PubMed.
- S. Hazra, B. Sarkar, S. Naiya, M. G. B. Drew and A. Ghosh, Polyhedron, 2012, 46, 8 CrossRef CAS PubMed.
- G. Arribas, M. C. Barral, R. González-Prieto, R. Jiménez-Aparicio, J. L. Priego, M. R. Torres and F. A. Urbanos, Inorg. Chem., 2005, 44, 5770 CrossRef CAS PubMed; M. C. Barral, R. González-Prieto, R. Jiménez-Aparicio, J. L. Priego, M. R. Torres and F. A. Urbanos, Eur. J. Inorg. Chem., 2006, 4229 CrossRef PubMed; M. Handa, H. Ishida, K. Ito, T. Adachi, T. Ikeue, I. Hiromitsu, M. Mikuriya and K. Kasuga, Chem. Pap., 2008, 62, 410 Search PubMed; R. Kuwahara, S. Fujikawa, K. Kuroiwa and N. Kimizuka, J. Am. Chem. Soc., 2012, 134, 1192 CrossRef PubMed; H. Miyasaka, Y. Asai, N. Motokawa, K. Kubo and M. Yamashita, Inorg. Chem., 2010, 49, 9116 CrossRef PubMed; H. Miyasaka, Acc. Chem. Res., 2013, 46, 248 CrossRef PubMed; P. Delgado-Martínez, R. González-Prieto, C. J. Gómez-García, R. Jiménez-Aparicio, J. L. Priego and M. R. Torres, Dalton Trans., 2014, 43, 3227 RSC.
- S. Furukawa, M. Ohba and S. Kitagawa, Chem. Commun., 2005, 865 RSC; H. Miyasaka, T. Izawa, N. Takahashi, M. Yamashita and K. R. Dunbar, J. Am. Chem. Soc., 2006, 128, 11358 CrossRef CAS PubMed; N. Motokawa, T. Oyama, S. Matsunaga, H. Miyasaka, K. Sugimoto, M. Yamashita, N. Lopez and K. R. Dunbar, Dalton Trans., 2008, 4099 RSC; H. Miyasaka, N. Motokawa, S. Matsunaga, M. Yamashita, K. Sugimoto, T. Mori, N. Toyota and K. R. Dunbar, J. Am. Chem. Soc., 2010, 132, 1532 CrossRef PubMed; H. Miyasaka, N. Motokawa, T. Chiyo, M. Takemura, M. Yamashita, H. Sagayama and T. Arima, J. Am. Chem. Soc., 2011, 133, 5338 CrossRef PubMed; M. Nishio, N. Hoshino, W. Kosaka, T. Akutagawa and H. Miyasaka, J. Am. Chem. Soc., 2013, 135, 17715 CrossRef PubMed.
- J. F. Berry, in Multiple Bonds between Metal Atoms, ed. F. A. Cotton, C. A. Murillo and R. A. Walton, Springer, USA, 2005, pp. 669–706 Search PubMed.
- C.-H. Peng, C.-C. Wang, H.-C. Lee, W.-C. Lo, G.-H. Lee and S.-M. Peng, J. Chin. Chem. Soc., 2001, 48, 987 CrossRef CAS PubMed; J. Zhang and L.-G. Zhu, CrystEngComm, 2011, 13, 553 RSC.
- R. Clérac, F. A. Cotton, L. M. Daniels, K. R. Dunbar, K. Kirschbaum, C. A. Murillo, A. A. Pinkerton, A. J. Schultz and X. Wang, J. Am. Chem. Soc., 2000, 122, 6226 CrossRef.
- R. Clérac, F. A. Cotton, K. R. Dunbar, T. Lu, C. A. Murillo and X. Wang, J. Am. Chem. Soc., 2000, 122, 2272 CrossRef.
- R. Clérac, F. A. Cotton, K. R. Dunbar, T. Lu, C. A. Murillo and X. Wang, Inorg. Chem., 2000, 39, 3065 CrossRef.
- F. A. Cotton, L. M. Daniels, G. T. Jordan IV and C. A. Murillo, J. Am. Chem. Soc., 1997, 119, 10377 CrossRef CAS.
- R. Clérac, F. A. Cotton, S. P. Jeffery, C. A. Murillo and X. Wang, Inorg. Chem., 2001, 40, 1265 CrossRef PubMed.
- J. Wang, A. Ozarowski, K. Kovnir, C. M. Thompson, A. A. Yaroslavtsev, R. V. Chernikov, N. S. Dalal and M. Shatruk, Eur. J. Inorg. Chem., 2012, 4652 CrossRef CAS PubMed.
- K. S. Pedersen, M. Sigrist, M. A. Sørensen, A.-L. Barra, T. Weyhermüller, S. Piligkos, C. Thuesen, M. G. Vinum, H. Mutka, H. Weihe, R. Clérac and J. Bendix, Angew. Chem., Int. Ed., 2014, 53, 1351 CrossRef CAS PubMed.
- J. Seiden, J. Phys. Lett., 1983, 44, L-947 CrossRef.
- J. C. Bonner and M. E. Fisher, Phys. Rev., 1964, 135, A640 CrossRef.
- K. S. Pedersen, M. A. Sørensen and J. Bendix, Coord. Chem. Rev., 2015, 299, 1 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Physical methods, IR, TGA, PXRD, magnetization data, crystal structure data for 1–3 and (PPh4)2[ReF6]·MeCN. CCDC 1415823–1415826. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc06704a |
‡ General synthesis of [Co3(dpa)4(MF6)]·2DMF, 1–3: [Co3(dpa)4Cl2] (50 mg, 0.05 mmol) and AgBF4 (20 mg, 0.10 mmol) were combined in 10 mL of DMF in a glovebox. The mixture was stirred overnight and filtered. Anhydrous (PPh4)2[MF6] (1 eq.) was dissolved in 10 mL of MeCN. The MeCN solution was layered upon the DMF solution, separated by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DMF 1 mixture of DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN in glass tubes (200 mm, ∅ 10 mm). Dark green blocks were collected from the walls of the tubes after 4 weeks. [Co3(dpa)4(ReF6)]·2DMF, 1. Yield 30.2 mg (46%). FT-IR ( MeCN in glass tubes (200 mm, ∅ 10 mm). Dark green blocks were collected from the walls of the tubes after 4 weeks. [Co3(dpa)4(ReF6)]·2DMF, 1. Yield 30.2 mg (46%). FT-IR (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) , cm−1): 657, 740, 761, 885, 1024, 1089, 1153, 1283, 1314, 1366, 1421, 1457, 1466, 1550, 1595, 1606, 1668. Elemental anal. for C40H32Co3F6N12Re·2(C3H7NO), % calc. C 42.37, H 3.56, N 15.04; % found C 42.48, H 3.52, N 15.26. [Co3(dpa)4(ZrF6)]·2DMF, 2: yield 35.1 mg (58%). FT-IR ( , cm−1): 657, 740, 761, 885, 1024, 1089, 1153, 1283, 1314, 1366, 1421, 1457, 1466, 1550, 1595, 1606, 1668. Elemental anal. for C40H32Co3F6N12Re·2(C3H7NO), % calc. C 42.37, H 3.56, N 15.04; % found C 42.48, H 3.52, N 15.26. [Co3(dpa)4(ZrF6)]·2DMF, 2: yield 35.1 mg (58%). FT-IR (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) , cm−1): 656, 741, 763, 885, 1025, 1084, 1152, 1284, 1316, 1363, 1421, 1459, 1471, 1550, 1596, 1606, 1671. Elemental anal. for C40H32Co3F6N12Zr·2(H2O) % calc. C 43.72, H 3.30, N 15.30; % found C 43.86, H 3.52, N 15.32. [Co3(dpa)4(SnF6)]·2DMF, 3. Yield 32.2 mg (53%). FT-IR ( , cm−1): 656, 741, 763, 885, 1025, 1084, 1152, 1284, 1316, 1363, 1421, 1459, 1471, 1550, 1596, 1606, 1671. Elemental anal. for C40H32Co3F6N12Zr·2(H2O) % calc. C 43.72, H 3.30, N 15.30; % found C 43.86, H 3.52, N 15.32. [Co3(dpa)4(SnF6)]·2DMF, 3. Yield 32.2 mg (53%). FT-IR (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) , cm−1): 656, 740, 761, 884, 1024, 1084, 1150, 1280, 1315, 1363, 1421, 1457, 1470, 1551, 1596, 1606, 1671. Elemental anal. for C40H32Co3F6N12Sn·(C3H7NO)(H2O) % calc. C 43.72, H 3.50, N 15.41; % found C 44.30, H 3.76, N 15.15. , cm−1): 656, 740, 761, 884, 1024, 1084, 1150, 1280, 1315, 1363, 1421, 1457, 1470, 1551, 1596, 1606, 1671. Elemental anal. for C40H32Co3F6N12Sn·(C3H7NO)(H2O) % calc. C 43.72, H 3.50, N 15.41; % found C 44.30, H 3.76, N 15.15. |
| This journal is © The Royal Society of Chemistry 2015 |