Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical generation and modification of peptides containing multiple dehydroalanines†
Philip M.
Morrison
,
Patrick J.
Foley
,
Stuart L.
Warriner
and
Michael E.
Webb
*
School of Chemistry and Astbury Centre for Structural Molecular Biology, University of Leeds, Leeds, LS2 9JT, UK. E-mail: m.e.webb@leeds.ac.uk
First published on 23rd July 2015
Abstract
Chemical formation of dehydroalanine has been widely used for the post-translational modification of proteins and peptides, however methods to incorporate multiple dehydroalanine residues into a single peptide have not been defined. We report the use of methyl 2,5-dibromovalerate which can be used to cleanly carry out this transformation.
Incorporation of dehydroalanine (Dha) into proteins and peptides can be used to generate a diverse range of chemical modifications. The electrophilic nature of the Dha residue means that it is readily modified in a site-selective fashion using thiols; this has been utilised to label proteins with dyes1 and sugars,2,3 incorporate unnatural side chains,4–6 and install post-translational modifications.7,8 A particular advantage of using Dha is that a single modified protein can be reacted with an array of thiols to generate a range of protein conjugates.5,6 Metal-catalysed chemistry has also been used to create unnatural amino acids from Dha,9 such as those with boron- and silicon-containing side chains10 and fluorescent alanine derivatives.11 Dha itself is an integral constituent of modified ribosomal peptides such as the lanthipeptides and thiopeptides, being used to generate lanthionine and pyridine cross-links as well as remaining unmodified in many lantibiotic structures. Finally, incorporation of Dha has been used as a chemical biology tool for example as a electrophilic probe in the investigation of diubiquitinase activity and selectivity.12,13
Both its application for site-selective protein modification and the involvement of Dha in lanthipeptide biosynthesis have driven the development of methods to chemically generate Dha in peptide structures. Dehydroamino-acids cannot be incorporated using standard peptide synthesis strategies and a masked amino acid is commonly incorporated and subsequently converted to Dha.14–16 Biosynthetic methods rely on incorporation of selenocysteine derivatives which are then unmasked using peroxide oxidation2,17 whereas enzymatic transformation of serine residues can be achieved utilising dehydratase enzymes from lanthipeptide pathways.18–23 All of the chemical methods of Dha generation were reviewed extensively by Davis and coworkers,24 who have pioneered the double-alkylation elimination reagents to convert cysteine to Dha. This approach is selective for Cys residues, utilises mild reaction conditions and avoids the incorporation of non-canonical amino acids however it had not been applied to the simultaneous generation of multiple dehydroalanine residues in a single peptide, as has previously only been achieved using unnatural amino acids.16,25
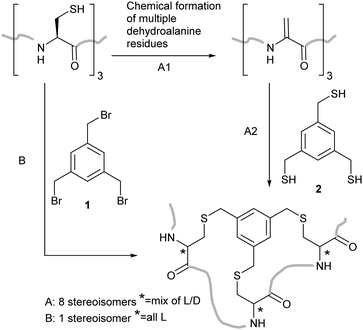
Our initial aim was to create constrained peptides in a two-step strategy via the creation of multiple Dha residues and their subsequent cyclisation with a small molecule polythiol core (Scheme 1). Similar molecules have been shown to bind to proteins with nanomolar affinities and large surface areas – making them potential modulators of protein–protein interactions.26–28 The addition of a thiol to Dha scrambles the stereochemistry at the alpha carbon, often limiting the yield due to formation of the D-Cys derivatives. We aimed to take advantage of the stereochemical scrambling and increase the diversity of peptide loop structures accessible upon cyclisation while incorporating D-amino acids which have previously been shown to improve protease resistance in the bicyclic peptide structures.29 Successful application of this strategy was however dependent upon developing a strategy to incorporate the multiple dehydroalanine residues.
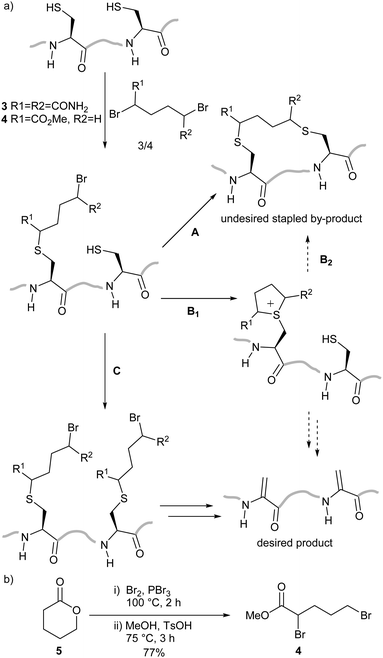
We planned to utilise cysteine double alkylation for Dha conversion due to the mild reaction conditions and wide tolerance of other amino acids. A cyclic sulfonium intermediate, formed through double cysteine alkylation, is eliminated to generate the dehydroalanine residue.7,24 In preliminary experiments with a model peptide containing two cysteines (H2N-ACGDDACG-CO2H) the use of the most common reagent, dibromoadipamide 3, led to formation of an undesired stapled by-product (Scheme 2a). While the proportion of the stapled by-product could be decreased by increasing the equivalents of 3 used in the reaction, even at high ratios of reagent to cysteine (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) residual stapled peptide was still observed. Corresponding experiments with peptides containing three cysteines gave complex mixtures which included a yet higher proportion of stapled peptides.
1) residual stapled peptide was still observed. Corresponding experiments with peptides containing three cysteines gave complex mixtures which included a yet higher proportion of stapled peptides.
We hypothesised that the stapled by-product forms either by double alkylation of the dibromoadipamide across two cysteine residues (Scheme 2a, Route A), or cysteine interception of the cyclic sulfonium ion (Route B) in a manner similar to elongated thiol modification previously observed by Nathani et al.30 An unsymmetrical reagent containing both an α-bromoacyl group and an alkyl bromide would mitigate the observed stapling; the first alkylation step at the α-position would be rapid, whereas a second alkylation at the ω-alkyl bromide would be slow. By changing the relative rates of the two steps we anticipated that all cysteine residues would be monoalkylated before the intramolecular alkylation or sulfonium ion formation, preventing either potential competing reaction pathway (Scheme 2a, Route C).
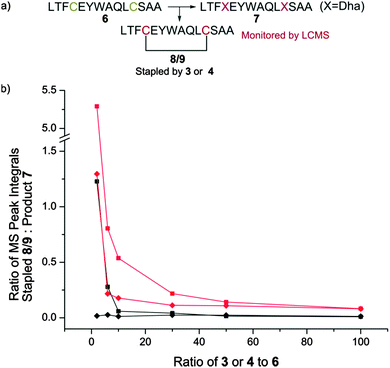
We therefore generated methyl dibromovalerate 4via PBr3-mediated bromination and ring-opening of valerolactone 5 (Scheme 2b). We used 4 to promote alkene formation in the same set of model peptides and observed clean conversion to dehydroalanine residues. Similar conversion was observed for peptides containing three cysteine residues and we were readily-able to purify these peptides by HPLC. To investigate the scope of the reagent, we investigated the reaction of the peptide H2N-LTFCEYWAQLCSAA-CO2H 6 using both reagents 3 and 4 (Fig. 1a) in a variety of solvent conditions. We monitored by LCMS the formation of both the product peptide 7 containing two Dha residues and the undesired stapled by-products 8 and 9 wherein the two cysteine residues have reacted with a single molecule of 3 and 4 respectively. At low ratios of 4 to 6, we still observed some formation of the stapled by-product 9 however this was significantly reduced relative to formation of 8 when 3 was used. At higher ratios of 4 to 6 (greater than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), little to no stapling could be observed. In DMSO/H2O mixtures, no stapling was observed even at low ratios of reagent 4 to peptide 6. Additionally, the reaction could be conducted in acetonitrile (ESI,† Fig. S1), which is not compatible with 3 since it is not soluble in this solvent. All subsequent conversions were carried out in DMSO/H2O followed by HPLC purification.
1), little to no stapling could be observed. In DMSO/H2O mixtures, no stapling was observed even at low ratios of reagent 4 to peptide 6. Additionally, the reaction could be conducted in acetonitrile (ESI,† Fig. S1), which is not compatible with 3 since it is not soluble in this solvent. All subsequent conversions were carried out in DMSO/H2O followed by HPLC purification.
Having defined robust chemistry to generate multiple dehydroalanine residues, we turned to the kallikrein inhibitor PK1528 as a model system to exemplify the increased diversity generated in our two-step strategy for bicyclic peptide formation. The linear PK15-derived peptide 10 (H2N-ACSDRFRNCPADEALCG-CO2H) was converted to its dehydroalanine-containing analogue 12 in good yield. To replicate the mesitylene core of PK15 11 we synthesised the trithiol trismercaptomethylbenzene 2 from tris(bromomethyl)benzene (TBMB) 1. Addition of 2 to 12 gave a cyclised product as a mixture of stereoisomers (ESI,† Fig. S2).
We were conscious that the bicyclic product has the same mass as monocyclic and uncyclised single-addition products. To confirm the presence of only bicyclic peptide structures, the purified product mixture was individually reacted with either excess N-methyl maleimide or β-mercaptoethanol. No reaction of β-mercaptoethanol was observed, and a single addition of N-methylmaleimide to the free amino-terminus of the peptide was observed. The same addition was observed on the bicyclic peptide PK15 11 which was synthesised independently via direct cyclisation of peptide 10 with TBMB 1 (Scheme 3).28
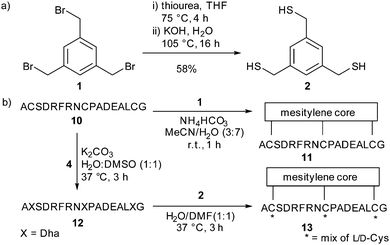 | ||
| Scheme 3 (a) The synthesis of 2 to be used as the central core. (b) The two-step conversion of peptide 10 to the stereochemically-scrambled mixture 13via dehydroalanine-containing peptide 12. | ||
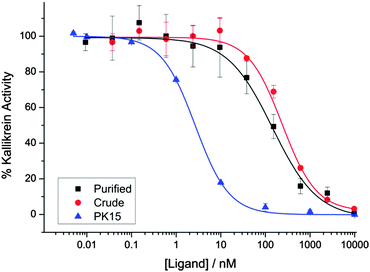
We evaluated the biological activity of the mixture of stereoisomers 13 obtained via cyclisation using a kallikrein inhibition assay. The mixture retained high potency (IC50 144 ± 31 nM), even as a crude mixture before purification (IC50 238 ± 21 nM) (Fig. 2). To further investigate the effect of this stereochemical scrambling on the PK15 sequence we resynthesized the individual stereoisomers using solid phase peptide synthesis and cyclisation with TBMB 1. Following purification by HPLC, they were then tested in the kallikrein inhibition assay (Table 1 and ESI,† Fig. S3). The range of observed activity spans more than 4 orders of magnitude, albeit with none of the stereoisomers being more potent inhibitors of kallikrein than the literature PK15 LLL-sequence. This differential activity demonstrates that the peptide loops adopt significantly different conformations in response to changes in stereochemistry at a single centre, validating our initial hypothesis that stereochemical scrambling of a peptide library will effectively increase its diversity. Intriguingly, analysis of inhibition by the stereochemically-scrambled peptides revealed an unexpected interaction between the two peptide loops; while the LLD-stereoisomer was inactive in the concentration range tested, a further stereocentre inversion to generate a DLD-variant rescues activity slightly, suggesting that the two loops must interact with each other. The wide range of activities means that we envisage that a deconvolution-by-resynthesis approach could be readily used to identify the most active stereoisomer, if the chemistry were to be adapted to a screening context.
In summary, we have reported an effective strategy to generate multiple dehydroalanine residues in peptides via mild chemical conversion of cysteines with methyl 2,5-dibromovalerate 4. This strategy avoids the formation of stapled by-products observed with other reagents. Additionally, 4 is more readily soluble and has a wider solvent tolerance. This reagent has multiple potential applications, including in the synthesis of modified peptides and modified peptide precursors for lanthipeptides. In this report, we have utilised the reagent in a two-step approach to generate stereochemically diverse bicyclic peptide structures from a single peptide precursor. Generation of such mixtures in a peptide or phage-display context and their subsequent deconvolution has the potential to increase the diversity of peptide libraries while simultaneous incorporating in-built resistance to cellular and plasma L-amino- and L-carboxypeptidases as well as increasing the potential range of core molecules which can be used in bicyclic peptide library generation.
We acknowledge support from EPSRC (EP/K03135X/1) for equipment. Personal funding to PMM was provided by the Wellcome Trust (grant 096687/Z/11/Z).
References
- E. Branigan, C. Pliotas, G. Hagelueken and J. H. Naismith, Nat. Protoc., 2013, 8, 2090–2097 CrossRef CAS PubMed.
- J. Wang, S. M. Schiller and P. G. Schultz, Angew. Chem., 2007, 119, 6973–6975 CrossRef PubMed.
- C. Aydillo, I. Compañón, A. Avenoza, J. H. Busto, F. Corzana, J. M. Peregrina and M. M. Zurbano, J. Am. Chem. Soc., 2014, 136, 789–800 CrossRef CAS PubMed.
- Y. A. Lin, O. Boutureira, L. Lercher, B. Bhushan, R. S. Paton and B. G. Davis, J. Am. Chem. Soc., 2013, 135, 12156–12159 CrossRef CAS PubMed.
- N. Timms, C. L. Windle, A. Polyakova, J. R. Ault, C. H. Trinh, A. R. Pearson, A. Nelson and A. Berry, ChemBioChem, 2013, 14, 474–481 CrossRef CAS PubMed.
- F. Rowan, M. Richards, M. Widya, R. Bayliss and J. Blagg, PLoS One, 2014, 9, e103935 Search PubMed.
- J. M. Chalker, L. Lercher, N. R. Rose, C. J. Schofield and B. G. Davis, Angew. Chem., Int. Ed., 2012, 51, 1835–1839 CrossRef CAS PubMed.
- K. P. Chooi, S. R. G. Galan, R. Raj, J. McCullagh, S. Mohammed, L. H. Jones and B. G. Davis, J. Am. Chem. Soc., 2014, 136, 1698–1701 CrossRef CAS PubMed.
- C. J. Chapman, A. Matsuno, C. G. Frost and M. C. Willis, Chem. Commun., 2007, 3903–3905 RSC.
- F. Bartoccini, S. Bartolucci, S. Lucarini and G. Piersanti, Eur. J. Org. Chem., 2015, 3352–3360 CrossRef CAS PubMed.
- P. M. T. Ferreira, L. S. Monteiro, G. Pereira, E. M. S. Castanheira and C. G. Frost, Eur. J. Org. Chem., 2013, 550–556 CrossRef CAS PubMed.
- N. Haj-Yahya, H. P. Hemantha, R. Meledin, S. Bondalapati, M. Seenaiah and A. Brik, Org. Lett., 2014, 16, 540–543 CrossRef CAS PubMed.
- M. P. C. Mulder, F. El Oualid, J. ter Beek and H. Ovaa, ChemBioChem, 2014, 15, 946–949 CrossRef CAS PubMed.
- N. M. Okeley, Y. Zhu and W. A. van Der Donk, Org. Lett., 2000, 2, 3603–3606 CrossRef CAS PubMed.
- Y. Zhu and W. A. van der Donk, Org. Lett., 2001, 3, 1189–1192 CrossRef CAS PubMed.
- S. Burrage, T. Raynham, G. Williams, J. W. Essex, C. Allen, M. Cardno, V. Swali and M. Bradley, Chem. – Eur. J., 2000, 6, 1455–1466 CrossRef CAS.
- F. P. Seebeck and J. W. Szostak, J. Am. Chem. Soc., 2006, 128, 7150–7151 CrossRef CAS PubMed.
- C. Chatterjee, G. C. Patton, L. Cooper, M. Paul and W. A. van der Donk, Chem. Biol., 2006, 13, 1109–1117 CrossRef CAS PubMed.
- N. Garg, L. M. Salazar-Ocampo and W. A. van der Donk, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7258–7263 CrossRef CAS PubMed.
- J. A. Majchrzykiewicz, J. Lubelski, G. N. Moll, A. Kuipers, J. J. Bijlsma, O. P. Kuipers and R. Rink, Antimicrob. Agents Chemother., 2010, 54, 1498–1505 CrossRef CAS PubMed.
- C. Chatterjee, M. Paul, L. Xie and W. A. van der Donk, Chem. Rev., 2005, 105, 633–684 CrossRef CAS PubMed.
- P. J. Knerr and W. A. van der Donk, Annu. Rev. Biochem., 2012, 81, 479–505 CrossRef CAS PubMed.
- Y. O. You, M. R. Levengood, L. A. Ihnken, A. K. Knowlton and W. A. van der Donk, ACS Chem. Biol., 2009, 4, 379–385 CrossRef CAS PubMed.
- J. M. Chalker, S. B. Gunnoo, O. Boutureira, S. C. Gerstberger, M. Fernández-González, G. J. L. Bernardes, L. Griffin, H. Hailu, C. J. Schofield and B. G. Davis, Chem. Sci., 2011, 2, 1666 RSC.
- Y. Zhu, M. D. Gieselman, H. Zhou, O. Averin and W. A. van der Donk, Org. Biomol. Chem., 2003, 1, 3304–3315 CAS.
- A. Angelini, L. Cendron, S. Chen, J. Touati, G. Winter, G. Zanotti and C. Heinis, ACS Chem. Biol., 2012, 7, 817–821 CrossRef CAS PubMed.
- S. Chen, D. Bertoldo, A. Angelini, F. Pojer and C. Heinis, Angew. Chem., Int. Ed., 2014, 53, 1602–1606 CrossRef CAS PubMed.
- C. Heinis, T. Rutherford, S. Freund and G. Winter, Nat. Chem. Biol., 2009, 5, 502–507 CrossRef CAS PubMed.
- S. Chen, D. Gfeller, S. A. Buth, O. Michielin, P. G. Leiman and C. Heinis, ChemBioChem, 2013, 14, 1316–1322 CrossRef CAS PubMed.
- R. Nathani, P. Moody, M. E. Smith, R. J. Fitzmaurice and S. Caddick, ChemBioChem, 2012, 13, 1283–1285 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc05469a |
| This journal is © The Royal Society of Chemistry 2015 |