Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An integrin-targeted photoactivatable Pt(IV) complex as a selective anticancer pro-drug: synthesis and photoactivation studies†
Albert
Gandioso
a,
Evyenia
Shaili
b,
Anna
Massaguer
c,
Gerard
Artigas
a,
Alejandro
González-Cantó
a,
Julie A.
Woods
d,
Peter J.
Sadler
b and
Vicente
Marchán
*a
aDepartament de Química Orgànica and IBUB, Universitat de Barcelona, Barcelona, E-08028, Spain. E-mail: vmarchan@ub.edu
bDepartment of Chemistry, University of Warwick, Warwick, CV4 7AL, Coventry, UK
cDepartament de Biologia, Universitat de Girona, Campus Montilivi, Girona, E-17071, Spain
dPhotobiology Unit, Department of Dermatology, Ninewells Hospital, Dundee, DD1 9SY, UK
First published on 28th April 2015
Abstract
A new anticancer agent based on the conjugation of a photoactivatable Pt(IV) pro-drug to a cyclic RGD-containing peptide is described. Upon visible light irradiation, phototoxicity was induced preferentially in SK-MEL-28 melanoma cancer cells overexpressing αVβ3 integrin compared to control DU-145 human prostate carcinoma cells.
The use of visible light has enormous potential in chemotherapy for controlling, at a desired time, the place and dose, and release of cytotoxic species from inert anticancer pro-drugs. For this reason, much effort has been dedicated to the development of photoactivatable metal-based anticancer complexes for improving drug efficacy and reducing toxic side-effects associated with platinum-based chemotherapeutic drugs currently used in clinics.1 In addition, photoactivation offers the possibility for new mechanisms of action as well as the formation of novel adducts with the final biological target (not only DNA, but also RNA or proteins), which are important variables to overcome inherent or acquired resistance to cisplatin. Among photoactivated metallodrugs, Pt(IV) diazidodihydroxido complexes are particularly promising since they are inert and nontoxic in the dark, but become highly active against a range of cancer cell lines upon irradiation with visible light, including cisplatin-resistant cells (A2780cis).2 Such Pt(IV) pro-drugs accumulate in tumour cells and bind strongly to DNA by generating adducts distinct from those of cisplatin.3 Ru(II) arene complexes such as [(η6-p-cym)Ru(bpm)(py)]2+ or its peptide derivatives can also be activated by visible light to induce the dissociation of the Ru–pyridine bond and the generation of an active species with capacity to react with DNA.4 A similar pro-drug approach has been described with some Ru polypyridyl complexes masked with thioether groups that can be removed selectively upon visible light irradiation.5 Very recently, caging groups have also been applied to control the activity of Ru(II) and Re(I) complexes.6
Despite these promising examples, it is desirable to improve some of the pharmacological properties of photoactivatable metallodrugs, such as aqueous solubility and cell uptake, as well as higher selectivity against cancer cells. In this context, targeted approaches based on peptide vectors whose receptors are overexpressed on cancer cells in combination with light activation can be used to develop anticancer agents with a dual mechanism of selectivity, such as the conjugates between a photoactivatable Ru(II) arene complex and receptor-binding peptides recently described by us4b or a conjugate between a caged Re(I) organometallic complex and bombesin.6b
Herein we report the conjugation of a photoactivatable Pt(IV) pro-drug, trans,trans,trans-[Pt(N3)2(OH)2(py)2] (1)2 (Fig. 1), to a cyclic peptide containing the RGD sequence (–Arg–Gly–Asp–), which is selectively recognized by αVβ3 and αVβ5 integrins. The overexpression of these transmembrane heterodimeric glycoproteins in different tumor cells together with their known relationship with tumor angiogenesis, which is an essential process for tumor growth and metastasis, makes them relevant targets in medicinal chemistry.7 In fact, RGD-containing peptides have been exploited extensively for tumour imaging and for targeted drug delivery of cytotoxic compounds,8 including some metal-based anticancer drugs.4b,9 As recently found by us for conjugates between a Pt(IV) derivative of picoplatin and RGD-containing peptides,9d we hypothesize that the peptide vector will confer complex 1 with selectivity for cancer cells overexpressing pro-angiogenic integrins such as αVβ3 and αVβ5. The novelty of this approach resides in the use of a photoactivatable Pt(IV) pro-drug since irradiation with visible light directly within the tumour will trigger the release of cytotoxic Pt(II) species from the internalized conjugate (3 in Fig. 1), thus providing a Pt(IV)-based anticancer agent with dual control over selectivity.
 | ||
| Fig. 1 Structure of trans,trans,trans-[Pt(N3)2(OH)2(py)2] (1), trans,trans,trans-[Pt(N3)2(OH)(succ)(py)2] (2) and the schematic representation of the Pt–c(RGDfK) conjugate (3). | ||
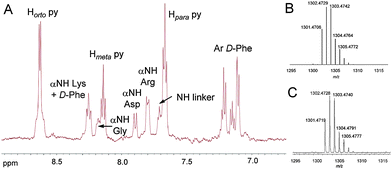
First, one of the axial hydroxyl groups of complex 1 was esterified with succinic anhydride to generate trans,trans,trans-[Pt(N3)2(OH)(succ)(py)2] (2) (Fig. 1),10 which contains a carboxylic acid function suitable for attaching the peptide moiety. As shown in Fig. 2, we selected as a carrier the cyclic pentapeptide c(RGDfK),11 which is a conjugatable version of Cilengitide, c(RGDf[N-Me]V), an antagonist of pro-angiogenic integrins, and currently in clinical phase III trials for the treatment of patients with brain tumors.12 The incorporation of non-natural D-Phe and [N-Me]-Val in the cyclic structure is responsible for increasing both the stability in biological fluids and the higher selectivity for αVβ3 integrin over αVβ5 and α5β1.12b In our case, replacement of N-methyl Val by Lys allowed further derivatization of the RGD-containing peptide with a polyethyleneglycol spacer at the ε-NH2 function. Then, complex 2 was attached to peptide intermediate 49d by using HATU in the presence of DIPEA in anhydrous DMF for 2 h at RT in the dark. The expected Pt–c(RGDfK) conjugate (3) was obtained as a pale yellow solid (54% yield) after purification by reversed-phase HPLC and lyophilisation (Fig. S1, ESI†). Conjugate 3 was unambiguously characterized by high-resolution ESI mass spectrometry and 1H NMR spectroscopy. As shown in Fig. 3 and Fig. S2 (ESI†), an m/z value consistent with the calculated value of the charged species ([M + H]+) and with the expected isotopic mass distribution pattern of Pt was obtained. In addition, diagnostic signals from the platinum complex (pyridine ligands) and from the peptide moiety (amide NH protons and aromatic protons of D-Phe) in the aromatic region of the 1H NMR spectra confirmed the covalent attachment of the Pt complex to the peptide vector (Fig. 3 and Fig. S3, ESI†).
 | ||
| Fig. 2 Schematic representation of the synthesis of the Pt–c(RGDfK) conjugate (3) and of the photo-reaction with 5′-GMP. | ||
Next, the efficiency of the photoactivation of Pt–c(RGDfK) conjugate (3) in the presence of 5′-GMP (2 mol equiv.) was investigated by HPLC-MS. As shown in Fig. S4 (ESI†), irradiation (λirr = 420 nm, 11 mW cm−2, 45 min, 37 °C) led to the complete disappearance of 3 and to the formation of a major species that was characterized by HR-ESI-MS as the Pt(II)–GMP adduct, trans-[Pt(N3)(5′-GMP)(py)2]+ (6 in Fig. 2) (GMP is considered neutral in all the formulae). In addition, two minor GMP adducts were identified, trans-[Pt(py)2(5′-GMP)2]2+ and [Pt2(N3)(py)4(5′-GMP)]+. The photodissociation of conjugate 3 to form the Pt(II)–GMP adduct as a major product, parallels the behaviour observed for the parent complexes 12a and 2,10 indicating that the attached peptide does not alter the photochemical properties or the type of photoadducts with a model nucleobase. Furthermore, the release of the intact succinate–c(RGDfK) moiety (5 in Fig. 2) implies that the carrier ligand neither competes with 5′-GMP for binding to the photoreleased Pt(II) species, nor does it form any secondary reactions, being a simple targeting vector of the Pt(IV) pro-drug.
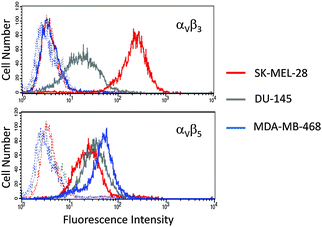
Having established the photoactivation properties of the Pt–c(RGDfK) conjugate, our next objective was to investigate its toxicity towards different cancer cell lines in the presence of visible light to assess the capacity of the peptide vector to deliver the photoactivatable Pt(IV) pro-drug into cancer cells overexpressing integrin receptors. On the basis of flow cytometry studies (Fig. 4), we selected SK-MEL-28 human malignant melanoma cell line as a model to evaluate the phototoxicity of 3 since it expresses high levels of αVβ3 integrin compared with αVβ5 integrin (mean cell fluorescence intensity of 217.4 and 23.3 for αVβ3 and αVβ5 integrins, respectively).9d As negative control, the DU-145 human prostate carcinoma cell line was selected since the expression of αVβ3 integrin was considerably lower, whereas that of αVβ5 integrin was similar (mean cell fluorescence intensity of 16.6 and 31.8 for αVβ3 and αVβ5 integrins, respectively). As expected, the internalization of the fluorescein-labelled RGD-containing peptide, Fluo-c(RGDfK) (7),9d was slightly higher in the αVβ3 integrin overexpressing SK-MEL-28 cells than in DU-145 (by 1.6-fold when incubated at 10 μM; see Fig. S5, ESI†), which points to the active participation of this integrin receptor in the uptake of the peptide.
The photocytotoxicity of the Pt–c(RGDfK) conjugate (3) and of the parent Pt complexes (1 and 2) was determined upon irradiation with visible light (λirr = 420 nm, 5 J cm−2) in both cell lines. The photoactivated dose-dependent inhibition of cell viability for compounds 1–3 towards SK-MEL-28 and DU-145 cells and their phototoxic indices are summarised in Table 1 and the cytotoxicity plots are shown in Fig. S6 (ESI†). First, it is worth noting that the IC50 value for complex 1 in SK-MEL-28 was similar to those previously found in other cancer cell lines (IC50 = 6.8 μM in HaCaT, 8.3 μM in A2780 or 8.4 μM in OE19, under blue light irradiation),2b although the cytotoxicity in DU-145 cells was about 4-fold lower than in the melanoma cancer cell line. Hence, these results confirm the high antitumour efficiency of the Pt(IV) pro-drug against cancer cells of different origin when photoactivated with visible light. Second, the cytotoxic effect of 1 was slightly reduced in SK-MEL-28 (about 1.5-fold) upon derivatization with a succinate group (2). This tendency was not reproduced in DU-145, since the cytotoxic effect was increased upon succinylation (IC50 = 20 μM for 2vs. IC50 = 43 μM for 1).
| SK-MEL-28 | DU-145 | ||||||
|---|---|---|---|---|---|---|---|
| IC50a (μM) | PIb | IC50a (μM) | PIb | ||||
| a IC50 is defined as the concentration of compound that inhibits dye uptake by 50%. The lowest value indicates the highest toxicity to cells. b PI: phototoxic index. | |||||||
| 1 | 420 nm | 10.2 | (7.9–13.0) | 20.8 | 43.2 | (33.0–56.6) | 4.9 |
| 2 | 420 nm | 15.5 | (10.2–23.6) | 11.3 | 20.0 | (14.7–27.3) | 8.8 |
| 3 | 420 nm | 19.5 | (13.4–28.2) | 3.6 | 53.9 | Wide | 1.3 |
| 3 | 365 nm | 9.9 | (9.5–10.1) | 8 | 56.4 | (50.9–61.9) | 2.7 |
As shown in Table 1, the phototoxicity of conjugate 3 in SK-MEL-28 (IC50 = 19.5 μM) was similar to that of the parent succinylated complex 2 (IC50 = 15.5 μM), whereas the differences in the irradiated mean IC50 values were higher in DU-145 cells (IC50 = 20 μM for 2 and 54 μM for 3). This result is in good agreement with the levels of expression of the αVβ3 integrin and with the cellular uptake experiments with the fluorescein-labelled peptide, and is consistent with the participation of the receptor in the biological activity of the conjugate. Hence, conjugation to the RGD-containing peptide vector seems to confer selectivity to complex 2 since the anticancer activity is higher in the melanoma cancer cell line that overexpresses αVβ3 integrin receptor whereas a lower phototoxicity was found in the human prostate cancer cell line. This difference was increased when conjugate 3 was irradiated with UVA (λirr = 365 nm, 5 J cm−2) in both cell lines. Interestingly, DU-145 cells treated with aminolevulinic acid (ALA) using the same blue light source were twice more susceptible than SK-MEL-28 cells (irradiated mean IC50 values = 0.12 mM and 0.23 mM, respectively). Thus, the melanoma cancer cells were more resistant to the porphyrin-based therapy, but more sensitive to the Pt-based therapy compared to the prostate cancer cells.
Since the cellular uptake of the Pt–peptide conjugate depends both on the level of expression of the pro-angiogenic integrins and on the binding affinity of the RGD-containing peptide towards these receptors, the determination of the intracellular accumulation is of high importance to assess the effect of the peptide conjugation on the biological activity of the Pt(IV) pro-drug as well as to investigate the contribution of each integrin subtype. For this purpose, in addition to SK-MEL-28 and DU-145 cells, we selected the MBA-MD-468 breast adenocarcinoma cell line as positive control for αVβ5 integrin. As shown in Fig. 4, the expression of αVβ5 integrin was considerably higher than that of αVβ3 integrin (mean cell fluorescence intensity of 3.8 and 42.4 for αVβ3 and αVβ5 integrins, respectively). Then, the three cancer cell lines were exposed to 10 μM solutions of 1–3 in the dark for 1 h, and the intracellular level of platinum was quantified by inductively-coupled plasma mass spectrometry (ICP-MS).
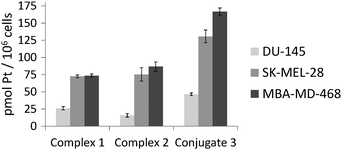
As shown in Fig. 5, the accumulation of platinum after exposure of the three cell lines to Pt–c(RGDfK) conjugate (3) (46.6 ± 2 pmol Pt per 106 cells in DU-145, 130.7 ± 9 pmol Pt per 106 cells in SK-MEL-28 and 166.5 ± 6 pmol Pt per 106 cells in MBA-MD-468) was higher than that of complex 1 (26.2 ± 2.4 pmol Pt per 106 cells in DU-145, 72.6 ± 1.9 pmol Pt per 106 cells in SK-MEL-28 and 73.6 ± 2 pmol Pt per 106 cells in MBA-MD-468) or 2 (15.9 ± 2.7 pmol Pt per 106 cells in DU-145, 75.4 ± 9.6 pmol Pt per 106 cells in SK-MEL-28 and 86.8 ± 7 pmol Pt per 106 cells in MBA-MD-468). This clearly indicates that peptide conjugation has a positive effect on the intracellular accumulation of the photoactivatable Pt(IV) pro-drug. Notably, platinum accumulation in SK-MEL-28 cells after exposure to conjugate 3 was higher (about 2.8-fold) than in DU-145 cells, which agree with the higher expression of αVβ3 integrin in the human malignant melanoma cell line compared with the prostate carcinoma cell line, as well as with the internalization studies with the fluorescein-labelled peptide.
To our surprise, the intracellular accumulation of 3 in MBA-MD-468 was also higher than in DU-145 cells (about 3.6-fold) despite the very low expression of αVβ3 integrin in the breast carcinoma cell line. These results and the fact that accumulation of 3 in MBA-MD-468 was about 1.3-fold higher than in SK-MEL-28 points out to the internalization of the Pt–c(RGDfK) conjugate mediated by αVβ5 integrin as well. These results are in agreement with the known selectivity of RGD-containing peptides, particularly the cyclic version c(RGDfK), for cancer cells overexpressing αVβ3 and αVβ5 integrins and suggest in all cases the participation of the peptide in the internalization of the conjugate. The reduced selectivity of conjugate 3 for αVβ3 integrin compared with Cilengitide can be attributed to the replacement of [N-Me]-Val by the Lys residue where the photoactivatable Pt(IV) complex is attached. Hence, on the basis of the overall results, we can envisage the integrin-mediated internalization and accumulation of the intact Pt–peptide conjugate in cancer cells overexpressing αVβ3 and/or αVβ5 integrins, where it will be photoactivated to generate cytotoxic Pt(II) species with a capacity to react with nucleic acids, as inferred by the adduct generated with 5′-GMP. Otherwise, a premature activation of the Pt(IV) pro-drug or hydrolysis of the conjugate would lead to similar or even lower Pt accumulation ratios than those obtained with control complexes. Interestingly, a correlation was found between intracellular accumulation of conjugate 3 and phototoxicity (see Table 1): a lower IC50 value upon visible light irradiation and a higher phototoxic index was found in the melanoma cancer cells that accumulated a higher amount of the compound compared with prostate carcinoma cells. Notably, the accumulation of 1 and 2 in SK-MEL-28 and MBA-MD-468 cells was also higher than in DU-145, thereby revealing a preference for the melanoma and breast cancer cells. It is also interesting that despite the higher accumulation of conjugate 3 compared with the parent complexes, the phototoxicity was slightly reduced, particularly when compared with 1 in SK-MEL-28. This might be attributable to differences in the quantum yield of the compounds and to the accumulation of the conjugate in intracellular vesicles that might interfere with the interaction of the released Pt(II) species with the target.
In conclusion, our results demonstrate the potential of conjugating photoactivatable metal complexes, such as Pt(IV) pro-drugs, to peptides with the aim of generating receptor-targeted metal-based anticancer drugs with reduced toxic side effects based on dual control over selectivity. The fact that the Pt–c(RGDfK) conjugate can also be internalized by αVβ5 integrin opens up the door to delivering such promising anticancer metallodrugs to tumours overexpressing αVβ5 integrin13 or to tumours coexpressing both αVβ3 and αVβ5 integrins.7b,14 Such a multi-integrin targeting approach would provide new metal-based anticancer strategies and so benefit a wider range of patients by increasing the number of tumours which can be targeted.15
This work was supported by funds from the Spanish Ministerio de Ciencia e Innovación (grants CTQ2010-21567-C02-01-02, CTQ2014-52658-R and the RNAREG project, grant CSD2009-00080), the Generalitat de Catalunya (2009SGR-208 and XRB), the ERC (grant 247450), EPSRC (EP/F034210/1) and EPSRC (MOAC Doctoral Training Centre, EP/F500378/1). The authors acknowledge helpful assistance of Dr Irene Fernández and Laura Ortiz (MS), Dr Maite Romero (ICP-MS) and Dr M. Antònia Molins (NMR) from CCiTUB.
Notes and references
- (a) C. Moucheron, New J. Chem., 2009, 33, 235 RSC; (b) D. Crespy, K. Landfester, U. S. Schubert and A. Schiller, Chem. Commun., 2010, 46, 6651 RSC; (c) N. A. Smith and P. J. Sadler, Philos. Trans. R. Soc., A, 2013, 371, 20120519, DOI:10.1098/rsta.2012.0519.
- (a) N. J. Farrer, J. A. Woods, L. Salassa, Y. Zhao, K. S. Robinson, G. Clarkson, F. S. Mackay and P. J. Sadler, Angew. Chem., Int. Ed., 2010, 49, 8905 CrossRef CAS PubMed; (b) Y. Zhao, J. A. Woods, N. J. Farrer, K. S. Robinson, J. Pracharova, J. Kasparkova, O. Novakova, H. Li, L. Salassa, A. M. Pizarro, G. J. Clarkson, L. Song, V. Brabec and P. J. Sadler, Chem. – Eur. J., 2013, 19, 9578 CrossRef CAS PubMed; (c) A. M. Pizarro, R. J. McQuitty, F. S. Mackay, Y. Zhao, J. A. Woods and P. J. Sadler, ChemMedChem, 2014, 9, 1169 CrossRef CAS PubMed.
- (a) J. Pracharova, L. Zerzankova, J. Stepankova, O. Novakova, N. J. Farrer, P. J. Sadler, V. Brabec and J. Kasparkova, Chem. Res. Toxicol., 2012, 25, 1099 CrossRef CAS PubMed; (b) H.-C. Tai, R. Brodbeck, J. Kasparkova, N. J. Farrer, V. Brabec, P. J. Sadler and R. J. Deeth, Inorg. Chem., 2012, 51, 6830 CrossRef CAS PubMed.
- (a) S. Betanzos-Lara, L. Salassa, A. Habtemanriam and P. J. Sadler, Chem. Commun., 2009, 6622 RSC; (b) F. Barragán, P. López-Senín, L. Salassa, S. Betanzos-Lara, A. Habtemariam, V. Moreno, P. J. Sadler and V. Marchán, J. Am. Chem. Soc., 2011, 133, 14098 CrossRef PubMed.
- (a) R. E. Goldbach, I. Rodriguez-Garcia, J. H. van Lenthe, M. A. Siegler and S. Bonnet, Chem. – Eur. J., 2011, 17, 9924 CrossRef CAS PubMed; (b) A. Bahreman, B. Limburg, M. A. Siegler, E. Bouwman and S. Bonnet, Inorg. Chem., 2013, 52, 9456 CrossRef CAS PubMed; (c) S. H. C. Askes, A. Bahreman and S. Bonnet, Angew. Chem., Int. Ed., 2014, 53, 1029 CrossRef CAS PubMed.
- (a) T. Joshi, V. Pierroz, C. Mari, L. Gemperle, S. Ferrari and G. Gasser, Angew. Chem., Int. Ed., 2014, 53, 2960 CrossRef CAS PubMed; (b) A. Leonidova, V. Pierroz, R. Rubbiani, Y. Lan, A. G. Schmitz, A. Kaech, R. K. O. Sigel, S. Ferrari and G. Gasser, Chem. Sci., 2014, 5, 4044 RSC.
- (a) M. Friedlander, P. C. Brooks, R. W. Shaffer, C. M. Kincaid, J. A. Varner and D. A. Cheresh, Science, 1995, 270, 1500 CAS; (b) J. S. Desgrosellier and D. A. Cheresh, Nat. Rev. Cancer, 2010, 10, 9 CrossRef CAS PubMed; (c) L. Auzzas, F. Zanardi, L. Battistini, P. Burreddu, P. Carta, G. Rassu, C. Curti and G. Casiraghi, Curr. Med. Chem., 2010, 17, 1255 CrossRef CAS; (d) D. G. Stupack and D. A. Cheresh, Curr. Top. Dev. Biol., 2004, 64, 207 CrossRef CAS.
- (a) K. Temming, R. M. Schiffelers, G. Molema and R. J. Kok, Drug Resist. Updates, 2005, 8, 381 CrossRef CAS PubMed; (b) S. Liu, Mol. Pharmaceutics, 2006, 3, 472 CrossRef CAS PubMed; (c) F. Danhier, A. Le Breton and V. Préat, Mol. Pharmaceutics, 2012, 9, 2961 CrossRef CAS PubMed.
- (a) S. Mukhopadhyay, C. M. Barnés, A. Haskel, S. M. Short, K. R. Barnes and S. J. Lippard, Bioconjugate Chem., 2008, 19, 39 CrossRef CAS PubMed; (b) N. Graf, D. R. Bielenberg, N. Kolishetti, C. Muus, J. Banyard, O. C. Farokhzad and S. J. Lippard, ACS Nano, 2012, 6, 4530 CrossRef CAS PubMed; (c) Y. Yuan, R. T. K. Kwok, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2014, 136, 2546 CrossRef CAS PubMed; (d) A. Massaguer, A. González-Cantó, E. Escribano, S. Barrabés, G. Artigas, V. Moreno and V. Marchán, Dalton Trans., 2015, 44, 202 RSC.
- E. Shaili, PhD thesis, University of Warwick, 2013.
- (a) K.-E. Gottschalk and H. Kessler, Angew. Chem., Int. Ed., 2002, 41, 3767 CrossRef CAS; (b) F. Gaertner, H. Kessler, H. Wester, M. Schwaiger and A. Beer, Eur. J. Nucl. Med. Mol. Imaging, 2012, 39, S126 CrossRef PubMed.
- (a) M. A. Dechantsreiter, E. Planker, B. Mathä, E. Lohof, G. Hölzemann, A. Jonczyk, S. L. Goodman and H. Kessler, J. Med. Chem., 1999, 42, 3033 CrossRef CAS PubMed; (b) C. Mas-Moruno, F. Rechenmacher and H. Kessler, Anti-Cancer Agents Med. Chem., 2010, 10, 753 CrossRef CAS.
- S. L. Goodman, H. J. Grote and C. Wilm, Biol. Open, 2012, 1, 329 CrossRef CAS PubMed.
- A. Erdreich-Epstein, H. Shimada, S. Groshen, M. Liu, L. S. Metelitsa, K. S. Kim, M. F. Stins, R. C. Seeger and D. L. Durden, Cancer Res., 2000, 60, 712 CAS.
- H. M. Sheldrake and L. H. Patterson, J. Med. Chem., 2014, 57, 6301 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data for conjugate 3, and results from photoactivation studies. See DOI: 10.1039/c5cc03180j |
| This journal is © The Royal Society of Chemistry 2015 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: