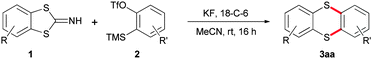Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploiting amphiphilicity: facile metal free access to thianthrenes and related sulphur heterocycles†
Martin
Pawliczek
ab,
Lennart K. B.
Garve
a and
Daniel B.
Werz
*a
aTechnische Universität Braunschweig, Institut für Organische Chemie, Hagenring 30, 38106 Braunschweig, Germany. E-mail: d.werz@tu-braunschweig.de
bGeorg-August-Universität Göttingen, Institut für Organische und Biomolekulare Chemie, Tammannstraße 2, 37077 Göttingen, Germany
First published on 28th April 2015
Abstract
Benzodithioloimines are reacted with arynes or alkynes substituted with electron-withdrawing groups to afford the corresponding thianthrene or benzo[b][1,4]dithiine derivatives. The transformation takes place under mild reaction conditions without any transition metal. Furthermore, the reaction mode could be expanded to 2-thiocyanatopyrroles yielding pyrrolothiazoles.
In the last decades sulphur-containing molecules have received great attention because of their interesting biological properties1 and their unique application in material science.2 For that purpose, the development of new methodologies dealing with the formation of carbon–sulphur bonds has become a major topic in modern organic synthesis.3 Traditional methods which take advantage of the high nucleophilicity of sulphur require harsh reaction conditions to form arene–sulphur bonds. Therefore, elevated temperatures, strong bases or a specific substitution pattern at the arene are needed making them unattractive for numerous target molecules.4 These drawbacks could be bypassed utilising metal catalysed cross-coupling reactions for C–S bond formation.5 Nevertheless, applications in material science require highly pure compounds without any extraneous metal impurities. Thus, transition metal free protocols for the formation of C–S bonds under mild reaction conditions are of considerable interest.6
Regarding the reaction properties of thiophenols, sulphur reacts as a nucleophile. However, its philicity can be reversed by converting thiophenol into the corresponding sulfenyl chloride or thiocyanate. In this shape, sulphur acts as electrophile suitable for substitution by nucleophiles (e.g. lithium or Grignard reagents).7 For the facile synthesis of heterocycles containing two sulphur atoms, 1,2-thiophenol derivatives equipped with both abilities are of utmost importance (Scheme 1a). This means one sulphur atom should act as nucleophile, the other one as electrophile. 2-Thiocyanatobenzenethiol (1a′) which exists in its closed form 1a was chosen as target substrate.8 Providing the required abilities easily visualised by its open form 1a′′, compound 1a should be able to react with other easily polarisable or polarised substrates such as arynes9 or alkynes substituted with electron-withdrawing (EWG) groups.10 As products, the corresponding dithiaheterocycles 3aa and 5, respectively, should be formed (Scheme 1b).
 | ||
| Scheme 1 (a) Reaction mode of mononitrile-substituted 1,2-dithiophenol. (b) Possible reaction partners and desired products. | ||
At the beginning of our investigations, we intended to establish a fast and efficient access to key compound 1a since the methods described in the literature proved to be tedious.8 We discovered, when treating aromatic 1,2-dithiocyanatobenzene 6a with PPh3 in the presence of base in acetonitrile, 1a is formed in up to 91% yield within 1 h at room temperature (Scheme 2a). With compound 1a in hand, we proved the amphiphilic behaviour of the two sulphur atoms. Based on our previous considerations, we chose a common aryne precursor for the desired metal free transformation which would provide a novel entry to the thianthrene scaffold.11 To our delight, common conditions using KF and 18-crown-6 in acetonitrile at ambient temperature yielded the desired thianthrene 3aa in excellent 95% yield (Scheme 2b).
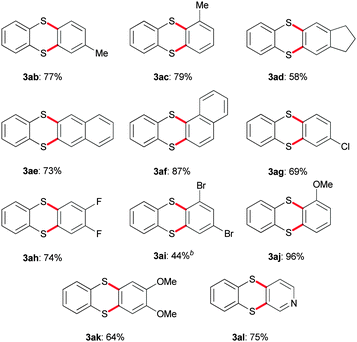
Exposition of 1a to various aryne precursors under the above-mentioned reaction conditions yielded the corresponding thianthrenes (Table 1). Alkyl-substituted arynes and such with extended π-systems were successfully converted to their thianthrene counterparts 3ab–3af in 58–87% yield. Chloro-, fluoro-, and bromo-substituted thianthrenes 3ag, 3ah, and 3ai were obtained in yields ranging from 44 to 74%. In the case of the dibromo derivative 3ai higher loadings of aryne precursor were required. Thianthrenes equipped with one or two methoxy substituents 3aj and 3ak were accessed in 64% and 96% yield, respectively. Furthermore, also a pyridine-based aryne precursor was able to undergo the transformation to azathianthrene 3al in 75% yield.
Similarly, variations with respect to the dithioloimine bearing core are possible (Table 2). Methyl-substituted 1b in combination with the unfunctionalised aryne precursor was exposed to the reaction conditions yielding 3ba in 71%. Naphthyl-based 3be, difluoro- 3bh and dimethyoxy-substituted systems 3bk were obtained in yields ranging from 55 to 70%. Because of their high electronegativity the latter two substituents render the important in-plane π orbital of the aryne highly electron-deficient. Further, benzodithioloimines containing pyrazine or thiadiazole heterocyclic cores were also applied. Pyrazine derivative 3ca could be obtained in 69% yield, whereas the formation of 3ca stayed out.
| a Reaction conditions: 1 (50 μmol), 2 (75 μmol), KF (150 μmol), 18-C-6 (150 μmol), MeCN (2 mL), rt, 16 h. |
|---|

|
Finally, 1b was exposed to unsymmetrical aryne precursors substituted with a methyl or a methoxy group. As result, inseparable mixtures of 3bc/3bc′ (64%, ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 3bj/3bj′ (79%, ratio of 2
1) and 3bj/3bj′ (79%, ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were obtained, respectively. This observation demonstrates that even the methoxy-substituted aryne, known for its high distortion and hence its high regioselectivity in aryne chemistry, does not achieve a satisfying ratio of regioisomers.12
1) were obtained, respectively. This observation demonstrates that even the methoxy-substituted aryne, known for its high distortion and hence its high regioselectivity in aryne chemistry, does not achieve a satisfying ratio of regioisomers.12
In addition, we also tried a one-step procedure applying 1,2-dithiocyanatobenzene 6a by addition of PPh3 to the aryne reaction. Unfortunately, the resulting yields were lower with 65% as the best result. A further drawback was the separation of PPh3 from the product by column chromatography wherefore the two-step procedure proved to be the method of choice.
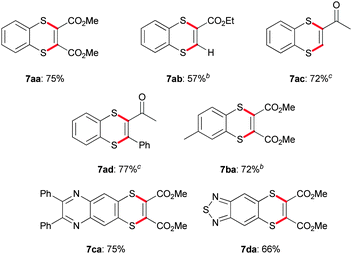
With this procedure for thianthrenes in hand, we tried to expand the scope to other heterocyclic scaffolds. Therefore, alkynes 4 bearing an electron-withdrawing group were utilised resulting in benzo[b][1,4]dithiines.13 Slight adjustments of the reaction conditions were necessary to obtain the desired products, but in all cases much faster transformations were observed using these polarised alkynes.14
Consequently, dimethylester 7aa and mono-methylester 7ab were reached in 75% and 57% yields, respectively (Table 3). Michael acceptors based on ketones could be converted in yields of 72% for 7ac and 77% for 7ad. Modifications regarding the dithioloimine core were performed as well. Methyl-substituted dimethylester derivative 7ba could be achieved in 72% yield. Also, other heterocyclic systems could be introduced resulting in pyrazine 7ca and thiadiazol 7da and in 75% and 66% yield. In addition, the application of Michael acceptors based on double bonds was unsuccessful. We assume that in such a case the retro-Michael reaction in contrast to the ring closure is the preferred mode of action.15
Encouraged by these results, we investigated whether also nitrogen might play the nucleophilic role. Changing the aromatic core to 2-thiocyanatopyrrole the endocyclic nitrogen should be prone to act as nucleophile. As result, the analogous transformation under modified reaction conditions yielded the desired benzo[d]pyrrolo[2,1-b]thiazole16 (9) in 45% yield (Scheme 3).
Further, also Michael acceptors were shown to be suitable substrates to afford the corresponding pyrrolo[2,1-b]thiazole17 derivatives 10ad, 10ba and 10bb in 50–63% yield (Table 4). Because of the high reactivity and amphiphilic character of 8, it rather tends to undergo a homocoupling than a 1,4-addition. This problem was partially counteracted by applying higher loadings of Michael acceptor.
| a Reaction conditions: 8 (200 μmol), 4 (4.00 mmol), Cs2CO3 (300 μmol), MeCN (3 mL), rt, 5 min. |
|---|

|
We propose the following plausible reaction mechanisms being responsible for product formation (Scheme 4). Precursor 2a is transformed into the reactive aryne. Deprotonation of benzodithioloimine favours the nucleophilic attack on the aryne leading to intermediate A (Scheme 4a). This species undergoes a concomitant nucleophilic substitution on sulphur, whereby nitrile acts as leaving group to form product 3aa. The pivotal reaction steps for alkynes substituted with electron-withdrawing groups are similar (Scheme 4b). Nevertheless, in this case the formation of the reactive triple bond is not necessary resulting in shorter reaction times in comparison with the previous transformation. The proposed reaction mechanisms are supported by side products 12 and 13 which are formed if ring-closure stays out because of protonation of intermediate A or B (Scheme 4c).
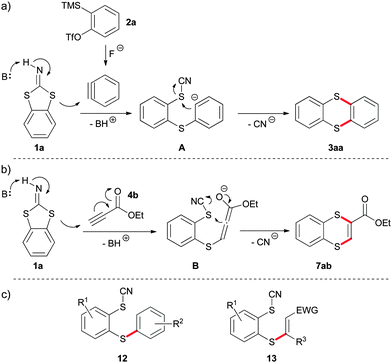 | ||
| Scheme 4 Proposed mechanistic scenario: (a) for the reaction with arynes; (b) for the reaction with Michael acceptors; (c) observed side products. | ||
In conclusion, we developed a broadly applicable transition metal free method for the synthesis of thianthrenes and benzodithiines. For this purpose, amphiphilic dithioloimines containing negatively and positively polarised sulphur were reacted with arynes and alkynes substituted with electron-withdrawing groups. Additionally, the concept of two differently polarised heteroatoms was transferred to a nitrogen–sulphur combination. The use of 2-thiocyanatopyrroles paves the way for a novel, fast and efficient entry to benzo[d]pyrrolo[2,1-b]thiazoles and pyrrolo[2,1-b]thiazoles, respectively. Further studies to expand this methodology to other heterocyclic scaffolds are ongoing.
This research was supported by the German Research Foundation (DFG, Emmy Noether and Heisenberg Fellowships to D.B.W.) and by the Fonds der Chemischen Industrie (PhD Fellowship to M.P. and Dozentenstipendium to D.B.W.). L.K.B.G. thanks the Studienstiftung des deutschen Volkes for a PhD Fellowship.
Notes and references
- (a) B. Stumpf, C. Eberle, M. Kaiser, R. Brun, R. L. Krauth-Siegel and F. Diederich, Org. Biomol. Chem., 2008, 6, 3935 RSC; (b) A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 50, 3046 CrossRef CAS PubMed; (c) S. F. Nielsen, E. Ø. Nielsen, G. M. Olsen, T. Liljefors and D. Peters, J. Med. Chem., 2000, 43, 2217 CrossRef CAS PubMed.
- (a) P. Choto, K. Rasmussen and G. Grampp, Phys. Chem. Chem. Phys., 2015, 17, 3415 RSC; (b) C. C. Ferrón, M. Capdevila-Cortada, R. Balster, F. Hartl, W. Niu, M. He, J. J. Novoa, J. N. L. Navarrete, V. Hernández and M. C. R. Delgado, Chem. – Eur. J., 2014, 20, 10351 CrossRef PubMed; (c) A. M. Khenkin, G. Leitus and R. Neumann, J. Am. Chem. Soc., 2010, 132, 11446 CrossRef CAS PubMed; (d) C. Marti, J. Irurre, A. Alvarez-Larena, J. F. Piniella, E. Brillas, L. Fajari, C. Aleman and L. Julia, J. Org. Chem., 1994, 59, 6200 CrossRef CAS; (e) T. Sarukawa and N. Oyama, J. Electrochem. Soc., 2010, 157, F23 CrossRef CAS PubMed; (f) G. Jones, B. Huang and S. F. Griffin, J. Org. Chem., 1993, 58, 2035 CrossRef CAS.
- (a) J. Mao, T. Jia, G. Frensch and P. J. Walsh, Org. Lett., 2014, 16, 5304 CrossRef CAS PubMed; (b) L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599 RSC; (c) R. B. N. Baig and R. S. Varma, Chem. Commun., 2012, 48, 2582 RSC; (d) P. Bichler and J. A. Love, Top. Organomet. Chem., 2010, 31, 39 CrossRef CAS; (e) C.-K. Chen, Y.-W. Chen, C.-H. Lin, H.-P. Lin and C.-F. Lee, Chem. Commun., 2010, 46, 282 RSC.
- (a) J. Ham, I. Yang and H. Kang, J. Org. Chem., 2004, 69, 3236 CrossRef CAS PubMed; (b) A. V. Bierbeek and M. Gingras, Tetrahedron Lett., 1998, 39, 6283 CrossRef; (c) A. B. Pierini, M. T. Baumgartner and R. A. Rossi, J. Org. Chem., 1987, 52, 1089 CrossRef CAS; (d) J. Lindley, Tetrahedron, 1984, 40, 1433 CrossRef CAS.
- (a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1569 CrossRef PubMed; (b) H. Yang, Y. Li, M. Jiang, J. Wang and H. Fu, Chem. – Eur. J., 2011, 17, 5652 CrossRef CAS PubMed; (c) P. Anbarasan, H. Neumann and M. Beller, Chem. Commun., 2011, 47, 3233 RSC; (d) F. Ke, Y. Qu, Z. Jiang, Z. Li, D. Wu and X. Zhou, Org. Lett., 2011, 13, 454 CrossRef CAS PubMed; (e) M. A. Fernández-Rodríguez, Q. Shen and J. F. Hartwig, Chem. – Eur. J., 2006, 12, 7782 CrossRef PubMed.
- (a) Y. Yang, Z. Chen and Y. Rao, Chem. Commun., 2014, 50, 15037 RSC; (b) J.-K. Qui, W.-J. Hao, D.-C. Wang, P. Wei, J. Sun, B. Jiang and S.-J. Tu, Chem. Commun., 2014, 50, 14782 RSC; (c) F.-L. Yang and S.-K. Tian, Angew. Chem., Int. Ed., 2013, 52, 4929 CrossRef CAS PubMed; (d) N. Umiersky and G. Manolikakes, Org. Lett., 2013, 15, 188 CrossRef PubMed; (e) S. Liang, R. Zhang, L. Xi, S. Chen and X. Yu, J. Org. Chem., 2013, 78, 11874 CrossRef CAS PubMed; (f) S. A. Amelichev, L. S. Konstantinova, N. V. Obruchnikova, O. A. Rakitin and C. W. Rees, Org. Lett., 2006, 8, 4529 CrossRef CAS PubMed.
- (a) L. Melzig, C. B. Rauhut, N. Naredi-Rainer and P. Knochel, Chem. – Eur. J., 2011, 17, 5362 CrossRef CAS PubMed; (b) J. R. Fotsing and K. Banert, Synthesis, 2006, 261 CAS; (c) D. B. Werz, R. Gleiter and F. Rominger, J. Org. Chem., 2004, 69, 2945 CrossRef CAS PubMed; (d) D. B. Werz and R. Gleiter, J. Org. Chem., 2003, 68, 9400 CrossRef CAS PubMed; (e) F. D. Toste and I. W. J. Still, Tetrahedron Lett., 1995, 36, 4361 CrossRef CAS; (f) H. Maruyama, M. Shiozaki, S. Oida and T. Hiraoka, Tetrahedron Lett., 1985, 26, 4521 CrossRef CAS.
- (a) P. Stanetty, G. Hattinger, M. D. Mihovilovic and K. J. Mereiter, J. Heterocycl. Chem., 2000, 37, 955 CrossRef CAS PubMed; (b) J. Nakayama, A. Sakai, S. Tokita and M. Hoshino, Heterocycles, 1984, 22, 27 CrossRef CAS.
- (a) J.-A. García-López, M. Çetin and M. F. Greaney, Angew. Chem., Int. Ed., 2015, 54, 2156 CrossRef PubMed; (b) D. Giallombardo, A. C. Nevin, W. Lewis, C. C. Nawrat, R. R. A. Kitson and C. J. Moody, Tetrahedron, 2014, 70, 1283 CrossRef CAS PubMed; (c) J. Zhao and R. C. Larock, J. Org. Chem., 2007, 72, 583 CrossRef CAS PubMed; (d) D. C. Rognessa and R. C. Larock, Tetrahedron Lett., 2009, 50, 4003 CrossRef PubMed; (e) J. Zhao and R. C. Larock, Org. Lett., 2005, 7, 4273 CrossRef CAS PubMed; (f) D. Hong, Z. Chen, X. Lin and Y. Wang, Org. Lett., 2010, 12, 4608 CrossRef CAS PubMed.
- (a) C. Shao, Q. Zhang, G. Cheng, C. Cheng, X. Wang and Y. Hu, Eur. J. Org. Chem., 2013, 6443 CrossRef CAS PubMed; (b) K. Yoshimura, T. Oishi, K. Yamaguchi and N. Mizuno, Chem. – Eur. J., 2011, 17, 3827 CrossRef CAS PubMed; (c) E. B. Castillo-Contreras and G. R. Dake, Org. Lett., 2014, 16, 1642 CrossRef CAS PubMed; (d) M. Yoshida, K. Saito, Y. Fujino and T. Doi, Chem. Commun., 2012, 48, 11796 RSC.
- (a) D. Kundu, T. Chatterjee and B. C. Ranu, Adv. Synth. Catal., 2013, 355, 2285 CrossRef CAS PubMed; (b) K. Radhakrishnan and C.-H. Lin, Synlett, 2005, 2179 CAS; (c) P. Preedasuriyachai, P. Charoonniyomporn, O. Karoonnirun, T. Thongpanchang and Y. Thebtaranonth, Tetrahedron Lett., 2004, 45, 1343 CrossRef CAS PubMed.
- (a) J. M. Medina, J. L. Mackey, N. K. Garg and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 15798 CrossRef CAS PubMed; (b) F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed; (c) S. S. Bhojgude, T. Kaicharla and A. T. Biju, Org. Lett., 2013, 15, 5452 CrossRef CAS PubMed.
- (a) G. L. Morgans, E. L. Ngidi, L. G. Madeley, S. D. Khanye, J. P. Michael, C. B. de Koning and W. A. L. van Otterlo, Tetrahedron, 2009, 65, 10650 CrossRef CAS PubMed; (b) E. M. Engler, V. V. Patel and R. R. Schumaker, Tetrahedron Lett., 1981, 22, 2035 CrossRef CAS; (c) P. Blatcher, S. Warren, S. Ncube, A. Pelter and K. Smith, Tetrahedron Lett., 1978, 26, 2349 CrossRef.
- Different bases were tested (K2CO3, KOH, DABCO, KOAc, CsF, Cs2CO3, KF/18-C-6). Cs2CO3 or KF/18-C-6 gave the best results in the specific cases as stated in Table 3.
- J. Evarts, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2002, 124, 11093 CrossRef CAS PubMed.
- (a) P. Zhao, H. Yin, H. Gao and C. Xi, J. Org. Chem., 2013, 78, 5001 CrossRef CAS PubMed; (b) M. Kucukdisli and T. Opatz, Eur. J. Org. Chem., 2012, 4555 CrossRef CAS PubMed.
- (a) D. C. Rogness, N. A. Markina, J. P. Waldo and R. C. Larock, J. Org. Chem., 2012, 77, 2743 CrossRef CAS PubMed; (b) K. C. Majumdar and S. Nath, Synthesis, 2011, 1413 CrossRef CAS PubMed; (c) Y. S. Song and K.-J. Lee, Synthesis, 2007, 3037 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc01757b |
| This journal is © The Royal Society of Chemistry 2015 |