Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Online monitoring the isomerization of an azobenzene-based dendritic bolaamphiphile using ion mobility-mass spectrometry†
Leonhard H.
Urner
a,
Bala N. S.
Thota
a,
Olaf
Nachtigall
a,
Stephan
Warnke
,
Gert
von Helden
b,
Rainer
Haag
*a and
Kevin
Pagel
*ab
aFreie Universität Berlin, Department of Biology, Chemistry and Pharmacy, Takustrasse 3, Berlin, Germany. E-mail: haag@chemie.fu-berlin.de; kevin.pagel@fu-berlin.de
bFritz Haber Institute of the Max Planck Society, Department of Molecular Physics, Faradayweg 4-6, Berlin, Germany
First published on 20th April 2015
Abstract
Ion mobility-mass spectrometry was used to obtain detailed information about the kinetics of the light-induced cis/trans isomerization process of a new supramolecular azobenzene-based bolaamphiphile. Further experiments revealed that the investigated light-induced structural transition dramatically influences the aggregation behaviour of the molecule.
Self-assembly of amphiphilic molecules is one of the most commonly observed phenomena in nature. Inspired by the high diversity of the underlying driving forces1–3 and the complexity of the resulting materials, several synthetic amphiphilic molecules have been introduced over the last few decades. One of these evolving classes are so-called bolaamphiphiles – amphiphiles which are characterized by two polar head groups connected through an alkyl spacer.4 Their structure is reminiscent to those of membrane lipids found in archaea bacteria whose high assembly stability is one of the foundations for their colonization of tiny ecological niches, which are hostile to life for all other organisms.5,6 As such, purely native systems are of rather limited use when a controlled assembly and disassembly is crucial for the intended application. A common route to circumvent this problem is to introduce moieties which modulate the physicochemical properties of the molecule in response to external stimuli. In this context, especially photo-responsive moieties like azobenzenes, spiropyrans or diarylethenes have recently attracted increased attention.7–9 In the case of azobenzene derivatives, the absorption of photons is typically accompanied by a change in geometry and polarity, which in turn can be used to control aggregation processes.10,11 Due to the complexity of the formed aggregates, it is often challenging to characterize the assembly kinetics of switchable azobenzene-based materials in full detail. A promising method to provide an additional dimension of molecular level information is ion mobility-mass spectrometry (IM-MS). In IM-MS ions are separated according to the time that is required to traverse a cell filled with inert neutral gas when a weak electric field is applied. This drift time not only depends on the mass-to-charge (m/z) ratio of the ions, but also on their size and shape, which effectively allows the separation of isomers.12–14
Here we present for the first time that IM-MS can be used for the direct online-monitoring of the light induced molecular shape transition of a new azobenzene-based dendritic bolaamphiphile. To do so, the molecule was irradiated with light directly at the nanoelectrospray ionization (nESI) interface followed by a time-resolved analysis of resulting cis/trans ratio in the gas phase using IM-MS. Further TEM measurements revealed that the light-induced structural transition dramatically influences the aggregation behaviour.
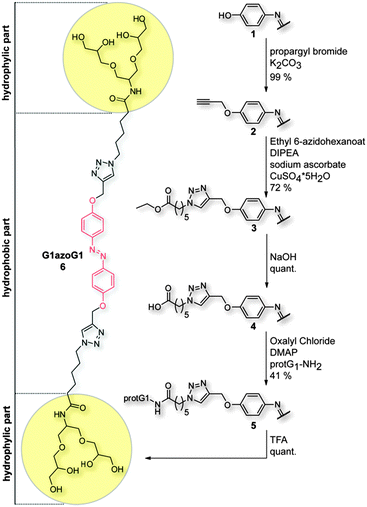
The structure of the symmetric bolaamphiphile G1azoG1 6 focused here is shown in Scheme 1. The synthesis was performed in five steps (for details see ESI†). First, compound 1 was treated with propargyl bromide under basic conditions to generate the symmetric O-propargylated product 2. Coupling of the azido ester with compound 2via “click chemistry” directly lead to the diester 3. Subsequently, the diester was hydrolyzed in ethanol under basic conditions and the resulting acid 4 was converted into the symmetric acid chloride and coupled with protG1-NH2 under basic conditions to give compound 5. Finally, the acetal protecting groups of compound 5 were removed with an excess of trifluoroacetic acid to quantitatively yield compound 6.
 | ||
| Scheme 1 Synthetic paths to G1azoG1 6. The half drawings of 1–5 are symmetric to the rest of the molecule. | ||
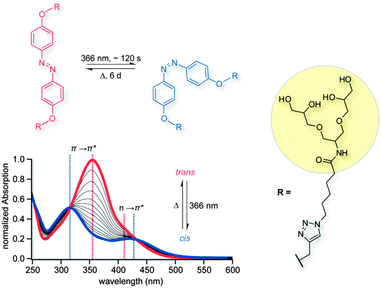
Due to their different absorption properties, cis/trans isomers of G1azoG1 6 can be distinguished by UV/VIS spectroscopy. The absorption spectrum of trans G1azoG1 6 exhibits a strong π → π* absorption band (356 nm) and a weaker n → π* absorption band (410 nm) at longer wavelengths. After conversion into the cis isomer via irradiation with UV light at 366 nm, on the other hand, the π → π* transition is perceptibly blue shifted to 318 nm, while a red shift is observed for the n → π* transition at 431 nm. In order to determine the time that is required to reach the maximum saturation of the cis form, UV/VIS spectra were recorded after stepwise irradiation at 366 nm. To do so, the sample was exposed to light for 5 s and a spectrum was measured. Subsequently, the procedure was repeated until the conversion was complete; a full saturation regarding the cis state was observed after approximately 120 s (blue line, Fig. 1) under certain experimental conditions (see Table ES1, ESI†). Moreover, a fully converted sample thermally reconverts from the cis to the trans form within approximately six days when stored in the dark (Fig. ES1, ESI†). Further attempts to differentiate between both structural isomers in solution using DOSY experiments failed, because here both isoforms exhibit similar diffusion coefficients (Fig. ES2, ESI†).
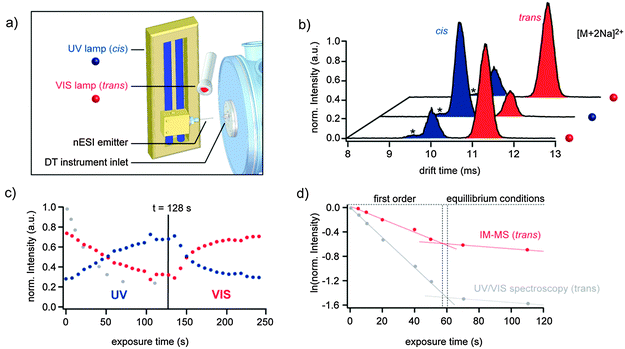
For online-monitoring of the cis/trans isomerization of G1azoG1 6via IM-MS an in-house-built drift tube (DT) IM-MS instrument was used (for details see ESI†).15,16 The source region of this instrument was equipped with two light sources to enable irradiation of the sample prior to ionization (Fig. 2a). Ions are generated via nESI using borosilicate capillaries prepared in-house using a previously described procedure.17 The Pd/Pt coated, but still partially transparent capillary was irradiated with: (a) a mercury vapour UV lamp (λ = 366 nm) to induce trans to cis isomerization or (b) a tungsten filament lamp (T = 3300 K, λmax ≈ 880 nm) to induce cis to trans isomerization (Fig. 2a). As a result, the cis/trans equilibrium within the capillary can be monitored directly in a time-resolved manner via IM-MS analysis.
Regardless of the configuration, mixtures of singly and doubly charged sodiated and/or protonated ions are observed after nESI of G1azoG1 6 (Fig. ES3, ESI†). For singly charged ions only one peak is observed in the IM-MS arrival time distribution (ATD). This indicates, that cis and trans isoforms of singly charged G1azoG1 6 ions adopt very similar structures in the gas phase. Due to the structural flexibility of the molecule, this observation is not surprising since the dendric side chains are likely to collapse in the gas phase to solvate the charge. Coulomb repulsion in doubly charged ions, on the other hand, leads to two distinct conformations, which can be clearly separated by IM-MS as shown exemplarily for doubly sodiated ions in Fig. 2b. After irradiation of the sample inside the nESI capillary at 366 nm, the species at shorter drift time is dominant with only a minor content of the isomer with longer drift time. Subsequent irradiation of the sample with visible light, on the other hand, leads to a complete inversion of the intensities, with the species at longer drift time now being the major component. This leads to the conclusion that the peak at shorter drift time can be assigned to the cis isomer and the peak at larger drift time to the trans isomer. Multiple repetition of the switching process revealed that the isomerization process is completely reversible. Generally, a good separation between the two isoforms was observed for all doubly charged ions, i.e. [M+2H]2+, [M+2Na]2+ and [M+NaH]2+. The relative abundance of the individual doubly charged species, however, was shifted considerably. This is very likely a result of a different ESI ionization efficiency, which is known to strongly depend on the size and accessibility of the molecule. In order to take that into account in our analysis, each observed adduct was considered as a part of an ensemble of cis or trans molecules in solution. The absolute number of all observed cis or trans species was summed up and divided by the total intensity of all doubly charged ions. Subsequently, the relative populations of the cis and trans isomers were normalized and plotted against the exposure time at 366 nm. As shown in Fig. 2c the relative content of cis isomer (blue dots) steadily increases upon irradiation at 366 nm until saturation is reached after approx. 130 s. Subsequent irradiation with visible light lead to a rapid depletion of the cis form. For comparison, also the intensities observed from the trans absorption band via UV/VIS spectroscopy are shown (Fig. 2c, grey dots). The general shape of the curves is surprisingly similar.
In order to also obtain information on the underlying kinetics, the intensities of trans G1azoG1 6 observed by IM-MS and UV/VIS spectroscopy were fitted to a logarithmic first order exponential equation (Fig. 2d). The linear sections observed for the first 60 s clearly indicate a monomolecular reaction with the reaction rate of the trans to cis isomerization exclusively depending on the concentration of molecules in the trans state. The increasing concentration of molecules in the cis state is affecting the cis to trans reconversion. This is reflected in a clear deviation from a monomolecular reaction after 60 s. Surprisingly, both techniques IM-MS as well as UV/VIS analysis perfectly agree with the observable mechanistic change from first order to equilibrium conditions. However, the fact that the observed transition between first order kinetics and equilibrium conditions occurs almost simultaneously in both methods is purely coincidental (for details see Table ES1, ESI†).
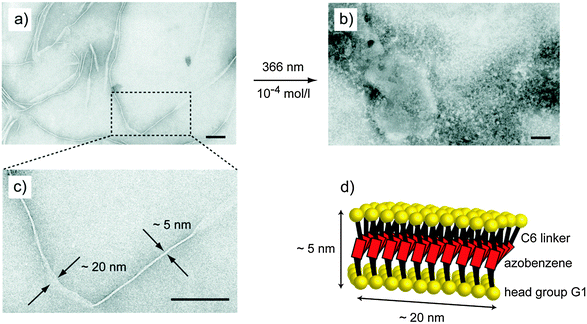
In addition to the analysis of the isolated monomers, the influence of the light induced structural changes on the aggregation behaviour was studied. First, the critical aggregation concentration (cac) was determined before and after irradiation at 366 nm using the pendant drop method (for details see ESI†).18,19 The cac of the non-irradiated trans form (cactrans = 6.3 × 10−5 M) was found to be at least 10 times lower than the cac of the of the irradiated cis form (caccis = 5.7 × 10−4 M). This trend is expected, since the dipole moment of the azobenzene is known to increase considerably during the trans to cis isomerization.10,20 Furthermore, the solubility increases and the amphiphilic nature of G1azoG1 6 is reduced. Secondly, the morphology of the aggregates was studied using TEM. To do so, aliquots of one and the same aqueous solution of G1azoG1 6 with a concentration of 10−4 M (i.e. above cactrans, but below caccis) were studied before and after irradiation at 366 nm. In the non-irradiated trans form, G1azoG1 6 forms regular twisted tapes with lengths up to several micrometers and widths of approximately 20 nm (Fig. 3a and c). Interestingly, the observed aggregates exhibit a constant width of around 5 nm, which agrees well with the length of the monomer in a stretched arrangement. As a result, the overall structure of the twisted tape along the longitudinal axis can be explained using a simple model structure (Fig. 3d). TEM after irradiation at 366 nm for 30 min, on the other hand, showed the complete breakup of the twisted tapes (Fig. 3b). Therefore, it can be concluded that the trans form of G1azoG1 6 forms well-ordered, tape-like structures at 10−4 M while the cis form shows no aggregation at these conditions. As a result, the tendency to form higher order aggregates at 10−4 M can be switched on and off simply by irradiation with light at 366 nm.
In summary, our data show that the photoinduced trans to cis isomerization of a new azobenzene-based dendritic bolaamphiphile can be followed online via direct irradiation of the sample at the nESI interface. The comparison between IM-MS and UV/VIS analysis showed that both methods perfectly agree with the observed mechanistic change from first order to equilibrium conditions. Moreover, TEM measurements revealed that the light-induced structural transition dramatically influences the aggregation behaviour of the investigated bolaamphiphile. Our experiments show that the breakup of the observed tape-like structures can be induced simply by irradiation at 366 nm.
This project was supported by the DFG (SFB 658). Andrea Schulz is gratefully acknowledged for performing the TEM measurements. The authors thank Michael T. Bowers for helpful discussions and continuous support and also Andreas Schäfer is gratefully acknowledged for performing the NMR measurements.
Notes and references
- T. Rehm and C. Schmuck, Chem. Commun., 2008, 801–813 RSC.
- F. Huang, R. O'Reilly and S. C. Zimmerman, Chem. Commun., 2014, 50, 13415–13416 RSC.
- A. Lützen, CHEMKON, 2007, 14, 123–130 CrossRef PubMed.
- J. H. Fuhrhop and T. Wang, Chem. Rev., 2004, 104, 2901–2937 CrossRef CAS PubMed.
- G. D. Sprott, J. Bioenerg. Biomembr., 1992, 24, 555–566 CrossRef CAS.
- A. Gambacorta, A. Gliozzi and M. De Rosa, World J. Microbiol. Biotechnol., 1995, 11, 115–131 CrossRef CAS PubMed.
- M. M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed.
- S. Peng, Q. Guo, P. G. Hartley and T. C. Hughes, J. Mater. Chem., 2014, 2, 8303–8312 RSC.
- T. Sendai, S. Biswas and T. Aida, J. Am. Chem. Soc., 2013, 135, 11509–11512 CrossRef CAS PubMed.
- S. Hecht, Small, 2005, 1, 26–29 CrossRef CAS PubMed.
- C. Stoffelen, J. Voskuhl, P. Jonkheijm and J. Huskens, Angew. Chem., 2014, 126, 3468–3472 CrossRef PubMed.
- J. J. Santos, S. H. Toma, P. M. Lalli, M. F. Riccio, M. N. Eberlin, H. E. Toma and K. Araki, Analyst, 2012, 137, 4045–4051 RSC.
- J. Thiel, D. Yang, M. H. Rosnes, X. Liu, C. Yvon, S. E. Kelly, Y. F. Song, D. L. Long and L. Cronin, Angew. Chem., Int. Ed. Engl., 2011, 50, 8871–8875 CrossRef CAS PubMed.
- Á. Révész, D. Schröder, T. A. Rokob, M. Havlík and B. Dolenský, Angew. Chem., Int. Ed., 2011, 50, 2401–2404 CrossRef PubMed.
- P. R. Kemper, N. F. Dupuis and M. T. Bowers, Int. J. Mass Spectrom., 2009, 287, 46–57 CrossRef CAS PubMed.
- S. Warnke, C. Baldauf, M. T. Bowers, K. Pagel and G. von Helden, J. Am. Chem. Soc., 2014, 136, 10308–10314 CrossRef CAS PubMed.
- H. Hernandez and C. V. Robinson, Nat. Protoc., 2007, 2, 715–726 CrossRef CAS PubMed.
- C. Kordel, C. S. Popeney and R. Haag, Chem. Commun., 2011, 47, 6584–6586 RSC.
- F. M. Menger, A. L. Galloway and M. E. Chlebowski, Langmuir, 2005, 21, 9010–9012 CrossRef CAS PubMed.
- H. Yan, Y. Long, K. Song, C.-H. Tung and L. Zheng, Soft Matter, 2014, 10, 115–121 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and synthetic procedures and characterization data employed in this work are given. See DOI: 10.1039/c5cc01488c |
| This journal is © The Royal Society of Chemistry 2015 |