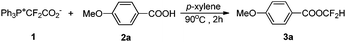Open Access Article
Open Access ArticleDifluoromethylation and gem-difluorocyclopropenation with difluorocarbene generated by decarboxylation†
Xiao-Yun
Deng
,
Jin-Hong
Lin
,
Jian
Zheng
and
Ji-Chang
Xiao
*
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: jchxiao@sioc.ac.cn; Fax: +86-21-6416-6128; Tel: +86-21-5492-5430
First published on 20th April 2015
Abstract
Difluoromethylation of the activated X–H bond (X = N, O and S) and aliphatic thiols, and gem-difluorocyclopropenation of alkynes with difluorocarbene generated in situ from difluoromethylene phosphobetaine (Ph3P+CF2CO2−) by decarboxylation occurred smoothly without the presence of any base or other additives.
As fluorinated moieties usually show profound effects on the physical, chemical, and biological properties of the target molecules, fluorine has been considered as the “second-favorite heteroatom” after nitrogen in drug design. The number of fluorine-containing pharmaceuticals and agrochemicals has been increasing rapidly in the past decades.1 Consequently, determined efforts have been devoted to the exploration of applicable protocols for the incorporation of fluorine-containing groups.2 Difluorocarbene has proved to be a highly valuable intermediate, not only from the perspective of theoretical investigation, but also from its synthetic utilities as the transformation of difluorocarbene can incorporate the difluoromethylene group into various organic molecules.3 The transformation of difluorocarbene include homocoupling to produce tetrafluoroethylene,4 [2+1] cycloaddition with alkenes or alkynes,3a difluoromethylation of the X–H bond (X = N, O, S, etc.),3a [18F]-trifluoromethylation,5 and coordination with transition metals.6 Although a number of difluorocarbene reagents have been developed to realize a variety of reactions due to the increasing research interest in this chemistry, these reactions usually require the addition of a strong base or additive, and some reagents are volatile or highly hygroscopic.7–13 Previously, we have shown that difluoromethylene phosphobetaine (Ph3P+CF2CO2−, PDFA), an efficient phosphonium ylide reagent,14 can readily generate difluorocarbene simply via decarboxylation.15 We have now investigated the use of this difluorocarbene precursor in the difluoromethylation of the activated X–H bond (X = N, O and S), difluoromethylation of aliphatic thiols, and gem-difluorocyclopropenation of alkynes.
Many difluorocarbene precursors can be successfully applied to the difluoromethylation of the activated X–H bond (X = N, O and S), such as ClCF2CO2Na,12d FSO2CF2CO2TMS,16a TMSCF2Br9 and HCF2S(O)(NTs)Ph.17 However, basic conditions are required in these reactions, limiting their wide applicability. The two exceptions are the N-difluoromethylation of imidazoles and benzimidazoles with TMSCF3,7c and N-difluoromethylation of N-(pyridin-2-yl)acetamide with ClCF2CO2Na,12c which can proceed under neutral conditions. But the methods suffer from a high reaction temperature, and/or are applicable only to N-difluoromethylation. In sharp contrast, we found that all of N-, O- and S-difluoromethylation with PDFA can occur smoothly under mild conditions without the presence of a base.
Although S-difluoromethylation with difluorocarbene is a straightforward protocol to incorporate the SCF2H group, which is a valuable moiety in medicinal chemistry and agrochemistry, it has thus far been limited to isolated examples.9,12d,17–19 Especially for the aliphatic S–H difluoromethylation, only two reports have been published. Hu disclosed that both TMSCF2Br9 and HCF2S(O)(NTs)Ph17 can be used to achieve the difluoromethylation of aliphatic thiols. But in both protocols, strong basic conditions are unavoidable.
The difluorocarbene reagents previously used for gem-difluorocyclopropenation to afford gem-difluorocyclopropenes, which have received much attention in synthetic chemistry, include BrCF2CO2Na,13 FSO2CF2CO2TMS,16b and (CF3)2Cd.20 Most of these methods still lack generality due to such disadvantages as harsh reaction conditions, the use of highly toxic reagents, low product yields or inconvenient operations. Although TMSCF3,7a TMSCF2Cl,8 and TMSCF2Br9 are versatile difluorocarbene precursors and effective for gem-difluorocyclopropenation, the reagents are highly volatile and the reaction requires the presence of an initiator for the generation of difluorocarbene.
In this work, PDFA was found to be an efficient difluorocarbene reagent for difluoromethylation and gem-difluorocyclopropenation via decarboxylation under neutral conditions. The attractive decarboxylative protocol is worthy of attention due to its operational convenience and mild reaction conditions.
In our previous study, it was found that low-polarity solvents such as cyclohexane and p-xylene favor the dissociation of PDFA into difluorocarbene.15 For the difluoromethylation of aromatic carboxylic acids with PDFA, p-xylene proved to be a suitable solvent (entries 1–4, Table 1). Elevating the reaction temperature to 90 °C in p-xylene improved the yield to 47% (entry 6). The reaction was quite sensitive to the loading of PDFA. Increasing its amount to 2 equiv. led to a significant increase in the yield (entry 7).
| Entry | Solvent | Temp. (°C) | Molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) 2a) |
Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2a (0.6 mmol) and 1 in solvent (3 mL). b Determined by 19F NMR with trifluoromethylbenzene as the internal standard. | ||||
| 1 | Cyclohexane | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
| 2 | p-Xylene | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18 |
| 3 | DMF | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 |
| 4 | THF | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11 |
| 5 | p-Xylene | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 |
| 6 | p-Xylene | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
47 |
| 7 | p-Xylene | 90 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
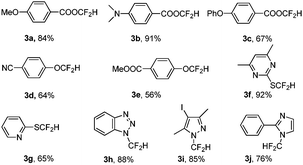
With the optimal reaction conditions in hand (entry 7, Table 1), we then investigated the substrate scope for the difluoromethylation of the activated X–H bond (Table 2). For the difluoromethylation of the O–H bond, the hydroxyl group in both carboxylic acids (3a–3c) and phenols (3d–3e) is reactive, and the carboxylic acids seem more reactive compared with phenols. The aromatic thiols can also be converted smoothly into the desired products (3f–3g). N-heterocycles are key structural units prevalent in biological systems. The incorporation of the difluoromethyl group is of great interest in synthetic and medicinal chemistry. Fortunately, the N-difluoromethylation of heterocycles with PDFA proceeded very well to afford the products in high yields (3h–3j). It is worth noting that no additive or base is required to generate difluorocarbene from PDFA, and the reaction can occur directly without neutralization of the substrates by base (Table 2).
| a Reaction conditions: 2 (0.6 mmol), 1 (1.2 mmol) in p-xylene (3 mL) for 2 h at 90 °C. |
|---|
|
However, the above reaction conditions (entry 7, Table 1) are not effective for the difluoromethylation of alcohols or aliphatic thiols. We have screened many conditions for the difluoromethylation of alcohols, but no condition can afford the desired product over 30% yield. To our delight, the difluoromethylation of aliphatic thiols seems to be very promising. For the reaction of benzyl thiol 4a with PDFA, 1,4-dioxane was found to be a suitable solvent instead of p-xylene. At 60 °C, the reaction furnished the desired product in 44% yield (entry 7, Table 3). Lowering or elevating the reaction temperature cannot increase the yield (entries 8–12). Increasing the loading of PDFA from 1 equiv. to 2 equiv. led to a dramatic improvement in the yield from 44% to 66% (entry 14 vs. entry 7). Further increasing its amount had no effect on the yield (entry 15).
| Entry | Solvent | Temp. (°C) | Molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a) 4a) |
Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: compound 4a (0.6 mmol) and 1 in solvent (3 mL). b Determined by 19F NMR with trifluoromethylbenzene as the internal standard. | ||||
| 1 | p-Xylene | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
26 |
| 2 | Toluene | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28 |
| 3 | DMF | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
| 4 | DCE | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 5 | Cyanobenzene | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 |
| 6 | THF | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
| 7 | 1,4-Dioxane | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 |
| 8 | 1,4-Dioxane | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
23 |
| 9 | 1,4-Dioxane | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 |
| 10 | 1,4-Dioxane | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 |
| 11 | 1,4-Dioxane | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 |
| 12 | 1,4-Dioxane | 100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 |
| 13 | 1,4-Dioxane | 60 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
61 |
| 14 | 1,4-Dioxane | 60 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
66 |
| 15 | 1,4-Dioxane | 60 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 |
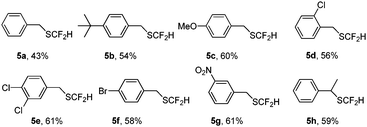
The reaction can be applied to a variety of aliphatic thiols (Table 4). In the case of benzyl aliphatic thiol, a low isolated yield was obtained due to the high volatility of the product (5a). Irrespective of whether the aryl group is substituted by an electron-withdrawing or -donating group, the products were obtained in good yields, indicating that the transformation is not sensitive to the electronic effects (5a–5h). The conversion is not only applicable for primary thiols, but also for secondary thiol (5h). Compared with the reported methods,9,17 for which strong basic conditions are required, our method seems more attractive.
| a Reaction conditions: 4 (0.6 mmol), 1 (1.2 mmol) in p-xylene (3 mL) for 5 h at 60 °C. |
|---|
|
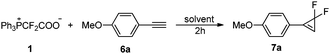
The successful difluoromethylation prompted us to investigate the gem-difluorocyclopropenation. Our initial attempts at the reaction of alkyne 6a with PDFA in p-xylene at 80 °C gave the expected products in 63% yield (entry 1, Table 5). The examination of other solvents suggested that p-xylene was the suitable solvent for this transformation (entries 2–8 vs. entry 1). Elevating the reaction temperature to 110 °C improved the yield slightly (entries 9 and 10), but higher temperature did not give better results (entries 11–13). Using 2 equiv. of PDFA, the yield was increased significantly (entry 14). The concentration of the substrates had no obvious effect on the yield, as evidenced by the observation that the reaction in 3 mL of p-xylene instead of 2 mL gave the desired product in almost the same yield (entry 15).
| Entry | Solvent | Temp. (°C) | Molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a) 6a) |
Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: compound 6a (0.6 mmol) and 1 in solvent (2 mL). b Determined by 19F NMR with trifluoromethylbenzene as the internal standard. c 3 mL of p-xylene was used. | ||||
| 1 | p-Xylene | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63 |
| 2 | Cyclohexane | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39 |
| 3 | Toluene | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45 |
| 4 | DMF | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
| 5 | DG | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19 |
| 6 | Cyanobenzene | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 7 | THF | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 |
| 8 | 1,4-Dioxane | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39 |
| 9 | p-Xylene | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
67 |
| 10 | p-Xylene | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 |
| 11 | p-Xylene | 120 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 |
| 12 | p-Xylene | 130 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 |
| 13 | p-Xylene | 140 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 |
| 14 | p-Xylene | 110 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 15c | p-Xylene | 110 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
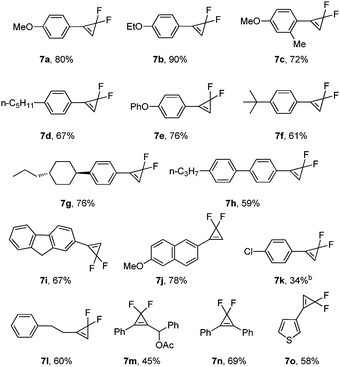
We then explored the substrate scope for the gem-difluorocyclopropenation of alkynes with PDFA under these optimal reaction conditions (Table 6). The electronic effects are important for the transformation. The substrates substituted by electron-donating groups on the phenyl ring can be converted well into the expected products in good yields (7a–7j), but in the case of substrates substituted by electron-withdrawing groups, low yield was afforded (7k). The reaction of aliphatic alkynes was also successful in affording the products in moderate yields (7l). Besides terminal alkynes, internal alkynes are also suitable for this conversion (7m–7n).
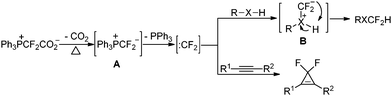
On the basis of the above results and related reports,14a,15a we propose that the reaction mechanism as shown in Scheme 1 is plausible. Decarboxylation of PDFA generates phosphonium ylide A,14a the further dissociation of which produces difluorocarbene.15a Difluorocarbene can be readily trapped by the X–H group (X = N, O or S) to give intermediate B, which undergoes a 1,2-hydride migration to afford the final difluoromethylation product. For the gem-difluorocyclopropenation reaction, the direct cyclization of difluorocarbene with alkyne furnishes the desired product.
In summary, difluoromethylene phosphobetaine (Ph3P+CF2CO2−, PDFA) has been found to be an efficient difluorocarbene precursor in the difluoromethylation of the activated X–H bond (X = N, O, S) and aliphatic thiols, and gem-difluorocyclopropenation of alkynes. All of these reactions proceeded smoothly under neutral conditions without the addition of any other additive or base. This decarboxylative protocol represents an efficient method for the transformation of difluorocarbene due to the operational convenience and the high stability of PDFA.
This work was financially supported by the National Natural Science Foundation (21172240, 21421002, 21472222), the National Basic Research Program of China (2015CB931900, 2012CBA01200), and the Chinese Academy of Sciences.
Notes and references
- (a) P. Kirsch, Modern fluoroorganic chemistry Synthesis, reactivity, applications, WILEY-VCH, Weinheim, 2004 Search PubMed; (b) K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013 CrossRef CAS PubMed; (c) A. M. Thayer, Chem. Eng. News, 2006, 84, 15 CrossRef PubMed; (d) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (e) X.-L. Qiu, X. Yue and F.-L. Qing, in Chiral Drugs, ed. G.-Q. Lin, Q.-D. You and J.-F. Cheng, John Wiley & Sons, Inc., Hoboken, New Jersey, 2011, p. 195 Search PubMed.
- (a) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (b) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (c) U. Hennecke, Angew. Chem., Int. Ed., 2012, 51, 4532 CrossRef CAS PubMed; (d) C. Hollingworth and V. Gouverneur, Chem. Commun., 2012, 48, 2929 RSC; (e) A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef CAS PubMed; (f) X. F. Wu, H. Neumann and M. Beller, Chem. – Asian J., 2012, 7, 1744 CrossRef CAS PubMed; (g) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (h) C.-P. Zhang, Q.-Y. Chen, Y. Guo, J.-C. Xiao and Y.-C. Gu, Coord. Chem. Rev., 2014, 261, 28 CrossRef CAS PubMed.
- For recent reviews, please see: (a) C. Ni and J. Hu, Synthesis, 2014, 842 Search PubMed; (b) D. Brahms and W. Dailey, Chem. Rev., 1996, 96, 1585 CrossRef CAS PubMed; (c) W. Dolbier and M. Battiste, Chem. Rev., 2003, 103, 1071 CrossRef CAS PubMed . For recent examples, please see: ; (d) J. M. Lenhardt, M. T. Ong, R. Choe, C. R. Evenhuis, T. J. Martinez and S. L. Craig, Science, 2010, 329, 1057 CrossRef CAS PubMed; (e) M. D. Kosobokov, A. D. Dilman, V. V. Levin and M. I. Struchkova, J. Org. Chem., 2012, 77, 5850 CrossRef CAS PubMed; (f) V. V. Levin, A. A. Zemtsov, M. I. Struchkova and A. D. Dilman, Org. Lett., 2013, 15, 917 CrossRef CAS PubMed; (g) G. Liu, X. Wang, X.-H. Xu, X. Lu, E. Tokunaga, S. Tsuzuki and N. Shibata, Org. Lett., 2013, 15, 1044 CrossRef CAS PubMed; (h) G. M. Lee, D. J. Harrison, I. Korobkov and R. T. Baker, Chem. Commun., 2014, 50, 1128 RSC.
- M. Hudlicky and A. E. Pavlath, Chemistry of Organic Fluorine Compounds II, American Chemical Society, Washington, DC, 1995 Search PubMed.
- For review, please see: (a) A. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford and P. J. H. Scott, Chem. Sci., 2014, 5, 4545 RSC . For examples, please see: ; (b) M. Huiban, M. Tredwell, S. Mizuta, Z. Wan, X. Zhang, T. L. Collier, V. Gouverneur and J. Passchier, Nat. Chem., 2013, 5, 941 CrossRef CAS PubMed; (c) D. Born, C. Sewing, J. D. M. Herscheid, A. D. Windhorst, R. V. A. Orru and D. J. Vugts, Angew. Chem., Int. Ed., 2014, 53, 11046 CrossRef PubMed; (d) P. Ivashkin, G. Lemonnier, J. Cousin, V. Grégoire, D. Labar, P. Jubault and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 9514 CrossRef CAS PubMed; (e) T. Ruhl, W. Rafique, V. T. Lien and P. J. Riss, Chem. Commun., 2014, 50, 6056 RSC.
- (a) D. L. Reger and M. D. Dukes, J. Organomet. Chem., 1978, 153, 67 CrossRef CAS; (b) G. R. Clark, S. V. Hoskins and W. R. Roper, J. Organomet. Chem., 1982, 234, C9 CrossRef CAS; (c) G. R. Clark, S. V. Hoskins, T. C. Jones and W. R. Roper, J. Chem. Soc., Chem. Commun., 1983, 719 RSC; (d) G. M. Lee, D. J. Harrison, I. Korobkov and R. T. Baker, Chem. Commun., 2014, 50, 1128 RSC; (e) D. J. Harrison, G. M. Lee, M. C. Leclerc, I. Korobkov and R. T. Baker, J. Am. Chem. Soc., 2013, 135, 18296 CrossRef CAS PubMed.
- (a) F. Wang, T. Luo, J. Hu, Y. Wang, H. S. Krishnan, P. V. Jog, S. K. Ganesh, G. K. S. Prakash and G. A. Olah, Angew. Chem., Int. Ed., 2011, 50, 7153 CrossRef CAS PubMed; (b) P. W. Chia, D. Bello, A. M. Z. Slawin and D. O'Hagan, Chem. Commun., 2013, 49, 2189 RSC; (c) G. K. S. Prakash, S. Krishnamoorthy, S. K. Ganesh, A. Kulkarni, R. Haiges and G. A. Olah, Org. Lett., 2014, 16, 54 CrossRef CAS PubMed.
- F. Wang, W. Zhang, J. Zhu, H. Li, K. Huang and J. Hu, Chem. Commun., 2011, 47, 2411 RSC.
- L. Li, F. Wang, C. Ni and J. Hu, Angew. Chem., Int. Ed., 2013, 52, 12390 CrossRef CAS PubMed.
- D. L. S. Brahms and W. P. Dailey, Chem. Rev., 1996, 96, 1585 CrossRef CAS PubMed.
- (a) D. Seyferth, S. P. Hopper and K. V. Darragh, J. Am. Chem. Soc., 1969, 91, 6536 CrossRef CAS; (b) I. Nowak, J. F. Cannon and M. J. Robins, J. Org. Chem., 2007, 72, 532 CrossRef CAS PubMed.
- (a) J. M. Birchall, G. W. Cross and R. N. Haszeldine, Proc. Chem. Soc., London, 1960, 81 CAS; (b) Y. Fujioka and H. Amii, Org. Lett., 2008, 10, 769 CrossRef CAS PubMed; (c) M. Ando, T. Wada and N. Sato, Org. Lett., 2006, 8, 3805 CrossRef CAS PubMed; (d) V. P. Mehta and M. F. Greaney, Org. Lett., 2013, 15, 5036 CrossRef CAS PubMed.
- K. Oshiro, Y. Morimoto and H. Amii, Synthesis, 2010, 2080 CAS.
- (a) J. Zheng, J. Cai, J.-H. Lin, Y. Guo and J.-C. Xiao, Chem. Commun., 2013, 49, 7513 RSC; (b) V. V. Levin, A. L. Trifonov, A. A. Zemtsov, M. I. Struchkova, D. E. Arkhipov and A. D. Dilman, Org. Lett., 2014, 16, 6256 CrossRef CAS PubMed; (c) Y. Qiao, T. Si, M. Yang and R. A. Altman, J. Org. Chem., 2014, 79, 7122 CrossRef CAS PubMed.
- (a) J. Zheng, J.-H. Lin, J. Cai and J.-C. Xiao, Chem. – Eur. J., 2013, 19, 15261 CrossRef CAS PubMed; (b) X.-Y. Deng, J.-H. Lin, J. Zheng and J.-C. Xiao, Chin. J. Chem., 2014, 32, 689 CrossRef CAS PubMed; (c) J. Zheng, J.-H. Lin, X.-Y. Deng and J.-C. Xiao, Org. Lett., 2015, 17, 532 CrossRef CAS PubMed.
- (a) K. Fuchibe, Y. Koseki, T. Aono, H. Sasagawa and J. Ichikawa, J. Fluorine Chem., 2012, 133, 52 CrossRef CAS PubMed; (b) W. Xu and Q.-Y. Chen, J. Org. Chem., 2002, 67, 9421 CrossRef CAS PubMed.
- W. Zhang, F. Wang and J. Hu, Org. Lett., 2009, 11, 2109 CrossRef CAS PubMed.
- P. S. Fier and J. F. Hartwig, Angew. Chem., Int. Ed., 2013, 125, 2146 CrossRef PubMed.
- K. Fuchibe, M. Bando, R. Takayama and J. Ichikawa, J. Fluorine Chem., 2015, 171, 133 CrossRef CAS PubMed.
- R. Eujen and B. Hoge, J. Organomet. Chem., 1995, 503, C51 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization of data for all compounds. See DOI: 10.1039/c5cc02736e |
| This journal is © The Royal Society of Chemistry 2015 |