Carbonylative coupling of allylic acetates with aryl boronic acids†
Abstract
The first allylic carbonylation reaction of allylic acetates with aryl boronic acids under carbon monoxide has been developed. Using Pd–PCy3 as a catalyst, a wide spectrum of allylic acetates was carbonylated in the presence of various aryl boronic acids, affording α,β-unsaturated aryl ketones in good to excellent yields. Preliminary studies indicate that carbon monoxide always inserts at the least substituted terminal allylic carbon and the resulting β,γ-unsaturated aryl ketones generally isomerise to the ketones obtained.
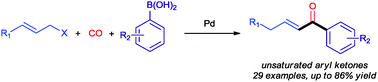


 Please wait while we load your content...
Please wait while we load your content...