Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anion transport across varying lipid membranes – the effect of lipophilicity†
Michael J.
Spooner
and
Philip A.
Gale
 *
*
Chemistry, University of Southampton, Southampton SO17 1BJ, UK. E-mail: philip.gale@soton.ac.uk; Fax: +44 (0)2380 596805; Tel: +44 (0)2380 593332
First published on 23rd February 2015
Abstract
The anion transport properties of a range of alkyl-substituted phenylthioureas were tested in vesicles of different lipid composition. Although changes in the bilayer affected the rate of transport for all compounds in the series, the ‘ideal’ log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for peak activity did not change depending on the composition of the bilayers tested.
P for peak activity did not change depending on the composition of the bilayers tested.
The development of small-molecule transmembrane anion carriers has attracted significant attention recently, as these compounds have potential as future therapeutics for treatment of conditions such as cystic fibrosis in additional to being useful tools for the study of transport processes across cell membranes.1,2 Transport studies are often carried out using large unilamellar vesicles (LUVs) composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC).1 Biological membranes, however, are significantly more complex, containing a wide range of phospholipids, sterols, proteins and other molecules.3,4 Little work has been done to directly compare the performance of synthetic anion transporters in different lipid environments.
The lipophilicity of an anion carrier is known to affect its transport activity5,6 and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (c
P (c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) values (the n-octanol/water partition coefficient) have been shown to be key in determining the transport activity of simple thioureas.7 Additionally, Quesada et al. have shown that for the tambjamine class of transporters, there exists an optimum log
P) values (the n-octanol/water partition coefficient) have been shown to be key in determining the transport activity of simple thioureas.7 Additionally, Quesada et al. have shown that for the tambjamine class of transporters, there exists an optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for transport.8
P for transport.8
It is known that energy barriers exist for solutes crossing lipid bilayers that vary in magnitude depending on lipid composition. In particular, computational studies have shown that the magnitude of these barriers affects the partitioning of solutes into the head or tail regions of the bilayer depend on the hydrophobic nature of the solute.9 We wished to investigate whether similar effects could influence the ideal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for anion receptors in different lipid bilayers therefore we investigated how the transport activity of a series of simple phenylthiourea molecules varied between bilayers formed of different lipids.
P for anion receptors in different lipid bilayers therefore we investigated how the transport activity of a series of simple phenylthiourea molecules varied between bilayers formed of different lipids.
A range of thioureas (Fig. 1) was synthesised with an increasing length of alkyl substituent, such that the set of compounds spanned the full range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values from 2 to 8.5. Thioureas were chosen as they are simple yet highly effective anion transporters.7,10 The length of the alkyl chains was increased in a symmetrical manner, in accordance with the recently described principle of lipophilic balance.10 The compounds were synthesised from the appropriate isothiocyanates and anilines and full synthetic details are given in the ESI.† Compounds 1,112,11312 & 107 have been previously reported.
P values from 2 to 8.5. Thioureas were chosen as they are simple yet highly effective anion transporters.7,10 The length of the alkyl chains was increased in a symmetrical manner, in accordance with the recently described principle of lipophilic balance.10 The compounds were synthesised from the appropriate isothiocyanates and anilines and full synthetic details are given in the ESI.† Compounds 1,112,11312 & 107 have been previously reported.
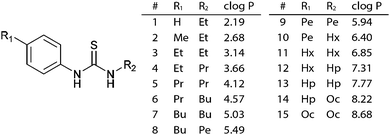 | ||
Fig. 1 General structure and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for the thiourea series.‡ P values for the thiourea series.‡ | ||
The association constants for anion binding were determined for compounds 1, 7 & 15 by 1H NMR titration in DMSO-d6/0.5% H2O using the WINEQNMR2 program.13 The results are shown in Table 1. As expected the binding affinities did not vary significantly across the series, in line with previously reported thioureas.7,10 Any change in relative rate is therefore not likely to be attributable to differences in anion binding.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode. Errors ≤10%. See ESI for details
1 binding mode. Errors ≤10%. See ESI for details
| Guest | Host | ||
|---|---|---|---|
| 1 | 7 | 15 | |
| Cl− | 14 | 17 | 16 |
| NO3− | <10 | <10 | <10 |
| H2PO4− | 180 | 220 | 160 |
| SO42− | 200 | 220 | 250 |
Lipid vesicles were prepared by previously reported methods.14–16 Chloride efflux from the vesicles was measured using a chloride ion-selective electrode. After 5 minutes, the vesicles were lysed to calibrate the readings to 100% efflux. Transport activity was quantified by a linear approximation, as described previously by Quesada et al.8
Initial transport rates were plotted against c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values obtained from the ALOGP17 method (via the VCC Lab web applet,18,19 see Fig. 1). This model was chosen over a selection of other computational methods as it gave the best correlation with retention times obtained by RP-UHPLC (see ESI† for details).20
P values obtained from the ALOGP17 method (via the VCC Lab web applet,18,19 see Fig. 1). This model was chosen over a selection of other computational methods as it gave the best correlation with retention times obtained by RP-UHPLC (see ESI† for details).20
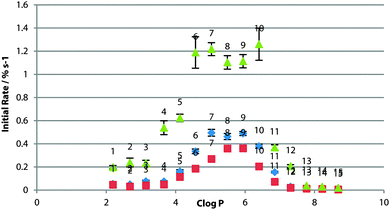
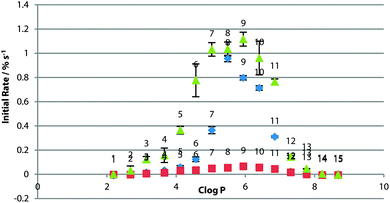
For POPC bilayers, the peak transport rate was observed for c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 5–6 (Fig. 3). The existence of the optimum log
P = 5–6 (Fig. 3). The existence of the optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range has been rationalised as being due to a balance between the receptor being lipophilic enough to cross the tail region of the membrane, without being so lipophilic as for delivery to the bilayer to be an issue.21 It might be expected that if the effect were purely a partitioning effect then the optimum log
P range has been rationalised as being due to a balance between the receptor being lipophilic enough to cross the tail region of the membrane, without being so lipophilic as for delivery to the bilayer to be an issue.21 It might be expected that if the effect were purely a partitioning effect then the optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P would be the same for the same bilayer composition. This result suggests otherwise as it is different from that found in studies of other sets of compounds,8 and presumably the effect is more complex and the optimum log
P would be the same for the same bilayer composition. This result suggests otherwise as it is different from that found in studies of other sets of compounds,8 and presumably the effect is more complex and the optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range is specific to transporter class.
P range is specific to transporter class.
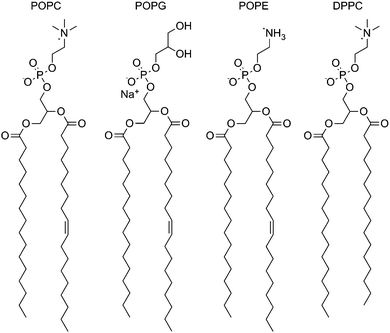
The experiments were also conducted in vesicles of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) (Fig. 2), both having the same tail groups as POPC. PG lipids have a glycerol head-group in the place of the choline moiety in PC lipids and have an overall negative charge in the head-group region. Across the whole series, transport was slower across POPG bilayers (Fig. 3), possibly as the formation of the negatively charged chloride complex was disfavoured. The peak in activity with respect to log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P remained unchanged.
P remained unchanged.
POPE has a similar structure to POPC but lacks the three methyl groups appended to the nitrogen atom (Fig. 2). Vesicles of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPE
1 POPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC ratio were used as POPE will not form vesicles on its own (due to the higher transition temperature for this lipid and the likelihood of forming a combination of gel and liquid crystalline phases at the concentrations used in these experiments22). In contrast to POPG, the transport rate across the mixed lipid bilayer was significantly faster than that across the pure POPC bilayer for the whole series (Fig. 3), where it might be expected that increased head-group – head-group interactions for PE would increase the energy barrier in the polar region of the membrane.9 Instead, the transporter molecules may be relieving some potential energy introduced by inclusion of the non-bilayer-forming lipid in the membrane.3 Again, the peak in activity did not appear to change with respect to log
POPC ratio were used as POPE will not form vesicles on its own (due to the higher transition temperature for this lipid and the likelihood of forming a combination of gel and liquid crystalline phases at the concentrations used in these experiments22). In contrast to POPG, the transport rate across the mixed lipid bilayer was significantly faster than that across the pure POPC bilayer for the whole series (Fig. 3), where it might be expected that increased head-group – head-group interactions for PE would increase the energy barrier in the polar region of the membrane.9 Instead, the transporter molecules may be relieving some potential energy introduced by inclusion of the non-bilayer-forming lipid in the membrane.3 Again, the peak in activity did not appear to change with respect to log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, although 6 & 10 also matched the rate of 7, 8 & 9, suggesting that a different rate-determining step in transport may be operating in this system.
P, although 6 & 10 also matched the rate of 7, 8 & 9, suggesting that a different rate-determining step in transport may be operating in this system.
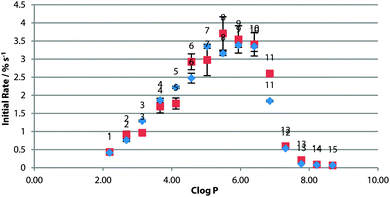
To investigate the effect of changing the lipid tail group, the screening was repeated using a fully-saturated PC lipid DPPC (Fig. 2). These experiments were carried out at higher temperature due to the higher phase transition temperature of DPPC. The rate across the series was not significantly different from the POPC control (Fig. 4). This would suggest that the rate-limiting step for transport is not the diffusion across the tail region of the bilayer.
Experiments were also conducted using POPC vesicles doped with cholesterol. The condensing and ordering effects of cholesterol (Chol) on the membrane are well described,23 hence cholesterol assays have previously been used as evidence for a mobile carrier mechanism in anion transport5,24 (as slower diffusion across a more rigid membrane is evidence that supports a mobile carrier over a channel mechanism).
For this series of thioureas, transport rate increased ubiquitously in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 POPC
3 POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol vesicles (Fig. 5). Crucially, the ideal lipophilicity range remained constant. This provides further evidence that diffusion across the tail region is not the rate-determining step, and in addition to other previous results21,25 that cholesterol assays are not sufficient evidence of a mobile carrier assay mechanism.
Chol vesicles (Fig. 5). Crucially, the ideal lipophilicity range remained constant. This provides further evidence that diffusion across the tail region is not the rate-determining step, and in addition to other previous results21,25 that cholesterol assays are not sufficient evidence of a mobile carrier assay mechanism.
Finally, to explore any effect of the delivery of the compounds into the membrane, experiments in POPC/POPG/POPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC 3
POPC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were repeated in the presence of external NaSO4. The SO42− ion has a high energy of solvation26 and is generally considered not able to be transported through lipid bilayers mediated by small molecule transporters although there are compounds that have been shown to be capable of transporting this anion.6,27 After 2 minutes allowing the transporter to equilibrate with the SO42− suspended vesicles, transport was initiated with the addition of a pulse of NaNO3.
1 were repeated in the presence of external NaSO4. The SO42− ion has a high energy of solvation26 and is generally considered not able to be transported through lipid bilayers mediated by small molecule transporters although there are compounds that have been shown to be capable of transporting this anion.6,27 After 2 minutes allowing the transporter to equilibrate with the SO42− suspended vesicles, transport was initiated with the addition of a pulse of NaNO3.
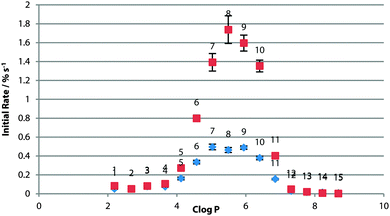
In the three systems, the relative peak in optimal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P was retained in the presence of sulfate (Fig. 6). In the POPG system, transport rates were dramatically reduced for all compounds. It has been proposed that glycerol –OH groups form hydrogen bonds to the lipid phosphate to screen the negative charge, packing lipid molecules closer together.28 It may be that the kosmotropic properties of sulfate are affecting the water lipid interface. More work needs to be done to confirm the origin of this effect.
P was retained in the presence of sulfate (Fig. 6). In the POPG system, transport rates were dramatically reduced for all compounds. It has been proposed that glycerol –OH groups form hydrogen bonds to the lipid phosphate to screen the negative charge, packing lipid molecules closer together.28 It may be that the kosmotropic properties of sulfate are affecting the water lipid interface. More work needs to be done to confirm the origin of this effect.
A degree of Cl− transport was observed in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPE
1 POPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC system before the nitrate spike addition. In further assays, we found no direct evidence of sulfate transport or vesicle leakage. A slight dependence of chloride transport on the present cation was found however, indicating that MCl co-transport may make a minor contribution to the overall efflux of chloride. Slight de-acidification of the vesicle interior was also seen for the most active compounds in an HPTS pH assay, indicating a small amount of possible HCl transport. We suspect these two mechanisms may explain this effect in this system. This data is presented in full in the ESI.†
POPC system before the nitrate spike addition. In further assays, we found no direct evidence of sulfate transport or vesicle leakage. A slight dependence of chloride transport on the present cation was found however, indicating that MCl co-transport may make a minor contribution to the overall efflux of chloride. Slight de-acidification of the vesicle interior was also seen for the most active compounds in an HPTS pH assay, indicating a small amount of possible HCl transport. We suspect these two mechanisms may explain this effect in this system. This data is presented in full in the ESI.†
For the other systems, the compounds in the middle of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range appear to transport faster in the sulfate system. We attribute this to the longer equilibration time allowing majority of transporters to be ideally located within the membrane when transport is initiated, giving a boost to the initial rate. Conversely, those at the hydrophobic and hydrophilic extremes of the series transport more slowly. The more polar compounds may be involved in competitive binding to SO42− at the interface, whilst the hydrophobic compounds are likely further confined to the tail region due to the effect of the presence of kosmotropic sulfate anions at the lipid water interface.
P range appear to transport faster in the sulfate system. We attribute this to the longer equilibration time allowing majority of transporters to be ideally located within the membrane when transport is initiated, giving a boost to the initial rate. Conversely, those at the hydrophobic and hydrophilic extremes of the series transport more slowly. The more polar compounds may be involved in competitive binding to SO42− at the interface, whilst the hydrophobic compounds are likely further confined to the tail region due to the effect of the presence of kosmotropic sulfate anions at the lipid water interface.
We have shown that in the different bilayer systems tested, the ideal range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for maximum transport rate by this series of compounds does not change. We note that the ideal log
P for maximum transport rate by this series of compounds does not change. We note that the ideal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range appears to be a property of the transporter class and not of the membrane.
P range appears to be a property of the transporter class and not of the membrane.
Optimising log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P is still an important factor for maximising transport rate for a given compound series, although it is important to note that other design factors must determine the absolute rate in different lipid systems. We have shown that different lipid environments have a significant influence on transporter activity and it will be important in further work to identify what the limiting steps are for different receptor classes. This will aid more targeted molecular design for natural target membranes and help ensure that future model bilayers accurately represent the target cellular environment to avoid potentially misleading results.
P is still an important factor for maximising transport rate for a given compound series, although it is important to note that other design factors must determine the absolute rate in different lipid systems. We have shown that different lipid environments have a significant influence on transporter activity and it will be important in further work to identify what the limiting steps are for different receptor classes. This will aid more targeted molecular design for natural target membranes and help ensure that future model bilayers accurately represent the target cellular environment to avoid potentially misleading results.
We thank the EPSRC for a DTG studentship (MJS) and for EPSRC Core Capability Funding (EP/K039466/1). PAG thanks the Royal Society and the Wolfson Foundation for a Research Merit Award.
Notes and references
- N. Busschaert and P. A. Gale, Angew. Chem., Int. Ed., 2013, 52, 1374–1382 CrossRef CAS PubMed.
- J. M. Tomich, U. Bukovnik, J. Layman and B. D. Schultz, Channel Replacement Therapy for Cystic Fibrosis - Cystic Fibrosis - Renewed Hopes Through Research, InTech, 2012 Search PubMed.
- D. E. Vance and J. E. Vance, Biochemistry of Lipids, Lipoproteins and Membranes, 2008 Search PubMed.
- G. van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112–124 CrossRef CAS PubMed.
- C. J. E. Haynes, S. J. Moore, J. R. Hiscock, I. Marques, P. J. Costa, V. Félix and P. A. Gale, Chem. Sci., 2012, 3, 1436–1444 RSC.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed.
- N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Félix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC.
- V. Saggiomo, S. Otto, I. Marque, V. Félix, T. Torroba and R. Quesada, Chem. Commun., 2012, 48, 5274–5276 RSC.
- C. L. Wennberg, D. v. d. Spoel and J. S. Hub, J. Am. Chem. Soc., 2012, 134, 5351–5361 CrossRef CAS PubMed.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Sci., 2014, 5, 1128–1134 RSC.
- W. Weith, Ber. Dtsch. Chem. Ges., 1875, 8, 1523–1530 CrossRef.
- A. Yahyazadeh and Z. Ghasemi, Eur. Chem. Bull., 2013, 2, 573–575 CAS.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- R. C. MacDonald, R. I. MacDonald, B. P. M. Menco, K. Takeshita, N. K. Subbarao and L. Hu, Biochim. Biophys. Acta, 1991, 1061, 297–303 CrossRef CAS.
- B. D. Smith and T. N. Lambert, Chem. Commun., 2003, 2261–2268 RSC.
- A. V. Koulov, T. N. Lambert, R. Shukla, M. Jain, J. M. Boon, B. D. Smith, H. Li, D. N. J. Sheppard, J. Baptiste, J. P. Clare and A. P. Davis, Angew. Chem., Int. Ed., 2003, 42, 4931–4933 CrossRef CAS PubMed.
- V. N. Viswanadhan, A. K. Ghose, G. R. Revankar and R. K. Robins, J. Chem. Inf. Comput. Sci., 1989, 29, 163–172 CrossRef CAS.
- VCCLAB, Virtual Computational Chemistry Laboratory, http://www.vcclab.org.
- I. V. Tetko, J. Gasteiger, R. Todeschini, A. Mauri, D. Livingstone, P. Ertl, V. A. Palyulin, E. V. Radchenko, N. S. Zefirov, A. S. Makarenko, V. Y. Tanchuk and V. V. Prokopenko, J. Comput.-Aided Mol. Des., 2005, 19, 453–463 CrossRef CAS PubMed.
- OECD, OECD Guidelines for the Testing of Chemicals, Section 1, 2004.
- C. J. E. Haynes, N. Busschaert, I. L. Kirby, J. Herniman, M. E. Light, N. J. Wells, I. Marques, V. Félix and P. A. Gale, Org. Biomol. Chem., 2014, 12, 62–72 CAS.
- R. Marinov and E. J. Dufourc, Eur. Biophys. J., 1996, 24, 423–431 CrossRef CAS.
- T. Róg, M. Pasenkiewicz-Gierula, I. Vattulainena and M. Karttunen, Biochim. Biophys. Acta, 2009, 1788, 97–121 CrossRef PubMed.
- P. A. Gale, Acc. Chem. Res., 2011, 44, 216–226 CrossRef CAS PubMed.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC.
- N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 1118–1127 RSC.
- D. E. Elmore, FEBS Lett., 2006, 580, 144–148 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of thiourea synthesis, transport studies and NMR titration data. See DOI: 10.1039/c5cc00823a |
| ‡ Pe = pentyl, Hx = hexyl, Hp = heptyl, Oc = octyl. |
| This journal is © The Royal Society of Chemistry 2015 |