Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymerase synthesis of DNA labelled with benzylidene cyanoacetamide-based fluorescent molecular rotors: fluorescent light-up probes for DNA-binding proteins†
Dmytro
Dziuba
a,
Radek
Pohl
a and
Michal
Hocek
*ab
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead & IOCB Research Center, Flemingovo nam. 2, CZ-16610 Prague 6, Czech Republic. E-mail: hocek@uochb.cas.cz
bDepartment of Organic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 8, CZ-12843 Prague 2, Czech Republic
First published on 11th February 2015
Abstract
Viscosity-sensitive fluorophores, fluorescent molecular rotors based on aminobenzylidene–cyanoacetamide moiety, were tethered to 2′-deoxycytidine triphosphate via a propargylamine linker and incorporated into DNA by polymerases in primer extension, nicking enzyme amplification or PCR. DNA probes incorporating modified nucleosides show a light-up response upon binding to a protein.
Fluorescent probes targeting DNA-binding proteins are of great value for cell biology to study signaling pathways, for medicinal chemistry to develop drug screening assays, and for clinical chemistry to detect biomarkers.1 Although fluorescence spectroscopy is a powerful method for studying biomolecular interactions, the existing methods of detection of DNA–protein binding in solutions using fluorescent reporting groups suffer several limitations. Most of the current DNA-based fluorescent probes are designed for the detection of proteins exhibiting enzymatic activity.1a Examples are nuclease2 and uracil DNA glycosylase3 probes based on artificial emissive nucleoside analogues quenched by stacking interactions with neighbouring nucleobases within the double helix. In contrast to enzymes which modify the chemical structure of DNA, many biologically important DNA-binding proteins, i.e. transcription factors or histones, do not alter the chemical composition of DNA and thus some more sophisticated approaches are required for their detection. Several methods based on molecular beacons1b,4 and environmentally sensitive fluorescent nucleoside analogues5 have been developed for this purpose. These methods usually require de novo synthesis of the probe for each particular target via solid phase phosphoramidite synthesis, which make them cost-consuming and can limit high-throughput applications. In this respect, polymerase construction6 of DNA-based probes using chemically modified deoxynucleoside triphosphates (dNTPs) would be advantageous. In our proof-of-the-principle studies we showed that dNTPs bearing suitable environmentally sensitive fluorescent reporter (either solvatochromic aminophthalimide7 or GFP-fluorophore as a molecular rotor8) can be incorporated into DNA by polymerases. The resulting probes showed a light-up response upon binding to proteins, although the increase of fluorescence was rather low (max. 2.5-fold).7,8 Therefore, development of more sensitive fluorophores is highly desirable.
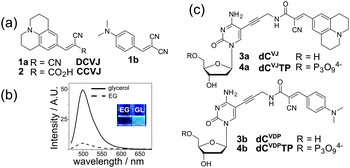
Here we report improved fluorescent dNTPs based on molecular rotors, their enzymatic incorporation into DNA and use for sensing of DNA–protein interactions. Fluorescent molecular rotors (FMRs) are a class of fluorescent dyes sensitive to local viscosity. The most widely used are p-(N,N-dialkylamino)benzylidene-malononitriles DCVJ and CCVJ (1a, 2, Fig. 1a).9,10,14 These FMRs show increased fluorescence intensity in media of high viscosity (Fig. 1b). They found applications in life sciences as fluorescent viscosity probes for microheterogeneous systems, such as cytoplasm and cell membranes,9 but the possibility to probe biomolecular interactions by emission of FMRs is only poorly explored.10 In the field of DNA studies, FMRs were used to study pre-melting of DNA11 and probing of G-quadruplexes.12 Several emissive nucleoside analogues have been shown to be sensitive to the viscosity of the media,13 but none of them was used for the probing of interactions with proteins. In this study we hypothesized that a nucleoside analogue bearing a CCVJ-type FMR attached to a nucleobase via a short linker will be a useful probe for protein binding. CCVJ is known to increase fluorescence upon binding with tight hydrophobic sites of proteins, such as albumins or antibodies.14 Although DNA binding proteins usually do not have hydrophobic pockets, the layer of water molecules next to the protein interface (the hydration shell) exhibits retarded molecular dynamics comparing to water in the bulk.15 This might provide a rational basis for protein-induced fluorescence enhancement (PIFE).16
Our design of FMR-labelled nucleosides is shown in Fig. 1c. We use a short propargylic linker to connect the p-(N,N-dialkylamino)benzylidene-malononitrile to position 5 of cytosine. Two distinct N,N-dialkylamino-aryl groups were used to give a julolidine derivative (dCVJ) and its less bulky N,N-dimethylaniline analogue (dCVDP). The FMR-nucleosides were synthesized using a modular strategy based on the Sonogashira coupling of 5-iodo-dC with the corresponding fluorophore-linked acetylene (Scheme S1 in the ESI†).
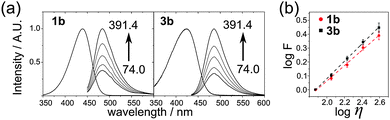
Having in hands the nucleosides we compared their photophysical and viscosity-sensitive properties with reference compounds 1a,b17 (Fig. 2, Table 1). The introduction of a nucleoside moiety blue-shifted the absorption spectra, whereas the positions of emission maxima were not affected. The sensitivity to viscosity was measured using the Forster–Hoffmann theory,9a,17 stating that the logarithm of fluorescence intensity (F) depends on the logarithm of the viscosity of the media (η) as follows:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F = x F = x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η + C η + C |
| Compound | λ abs | λ em | x |
|---|---|---|---|
| a Position in nm of the maximum of the absorption in EG. b Position in nm of the maximum of the emission in EG–glycerol. c Viscosity sensitivity obtained from the Forster–Hoffmann equation. | |||
| 1a | 466 | 503 | 0.594 ± 0.028 |
| 1b | 440 | 486 | 0.554 ± 0.020 |
| 3a (dCVJ) | 452 | 506 | 0.630 ± 0.021 |
| 3b (dCVDP) | 423 | 488 | 0.627 ± 0.022 |
Then we proceeded to the synthesis of DNA. For the enzymatic incorporation, nucleosides 3a and 3b were phosphorylated at 5′ using the procedure of Ludwig18 to give corresponding triphosphates 4a and 4b (Fig. 1c; Scheme S1, ESI†). We examined the possibility of incorporating the modified dNTPs into DNA by enzymatic methods, i.e. primer extension (PEX), nicking enzyme amplification reaction (NEAR) and polymerase chain reaction (PCR).6c,19
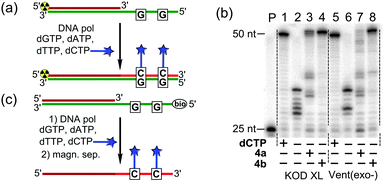
At first we tested the modified dCXTPs in combination with three natural dNTPs in PEX with KOD XL and Vent(exo-) DNA polymerases (Fig. 3a). PAGE analysis of the PEX reaction (Fig. 3b) shows that dCVDPTP was a good substrate for both enzymes tested (lanes 4 and 8) and gave full-length extended products, whereas the bulky dCVJTP was less efficient, giving a number of shorter products (lanes 3 and 7). Nucleotide dCVDP was nicely incorporated into DNA even at a high density (Fig. S1, ESI†). Single-stranded oligonucleotides (ssONs) were prepared by PEX using a biotinylated template and isolated by magnetoseparation with streptavidine-coated magnetic beads (Fig. 3c). MALDI-TOF analysis of the ssON containing modified nucleotides dCx confirmed the correct full-length products (Fig. S1 in the ESI†) and UV-vis spectroscopy confirmed the absence of non-specific binding of dCVDPTP to DNA (Fig. S2, ESI†).
Then, we tested the modified triphosphates in the nicking enzyme amplification reaction (NEAR),19b,c a two-step linear isothermal amplification process for synthesis of short ssONs (Fig. 4a). The results of NEAR with different templates (12-mer ON, containing one or three dCx modifications) are shown in Fig. 4b. The NEAR works well with both modified dCXTPs, but the yield was higher in the case of less dense labelling. Correct incorporation of modified dNTPs was also confirmed by MALDI-TOF spectrometry. The NEAR on a semi-preparative scale was also performed using dCVDPTP followed by HPLC purification, yielding the corresponding 12-mer modified oligonucleotide in 1.9 nmol yield (0.5 mL scale). To further explore the applicability of the FMR-modified dNTPs, we tested them in PCR. A series of optimization experiments have shown that dCVDP (but not dCVJ) can be sufficiently incorporated by KOD XL DNA polymerase using dCVDPTP as the substrate (Fig. 4c), although it requires higher concentration of dCVDPTP, increased number of cycles and longer elongation time. Altogether, these results indicate that dCVDPTP is a good substrate for enzymatic synthesis of DNA using PEX, NEAR and PCR.
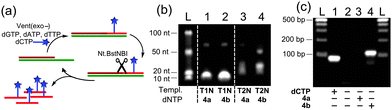 | ||
| Fig. 4 Enzymatic synthesis of FMR-modified DNA by NEAR and PCR. (a) Schematic representation of NEAR; (b) agarose gel analysis of NEAR products; and (c) agarose gel analysis of PCR products. | ||
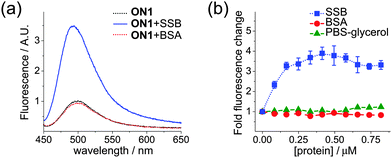
Finally, we examined the usability of fluorescent molecular rotors attached to DNA as reporting groups for protein–DNA interactions. Binding studies in solution were performed using a 30-mer ssDNA probe ON1 (Table S2 in the ESI†) obtained by PEX with magnetoseparation (Fig. 3c) and single-strand binding protein from E. coli (SSB), exhibiting a non-sequence specific binding to ssDNA.20 We observed a significant 4-fold increase of fluorescence upon titration of DNA by SSB with a stoichiometry of interaction 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 5). Since at least 56 nt ssDNA is needed to wrap around the SSB tetramer under the conditions used,21 the short ONs used in our study apparently bind in a higher ratio. Notably, the maximal increase of fluorescence was higher than with our previous probes.7,8
1 (Fig. 5). Since at least 56 nt ssDNA is needed to wrap around the SSB tetramer under the conditions used,21 the short ONs used in our study apparently bind in a higher ratio. Notably, the maximal increase of fluorescence was higher than with our previous probes.7,8
To rule out possible non-specific interaction between the DNA-tethered fluorophores and protein, we also studied the influence of bovine serum albumin (BSA), which does not bind to DNA but possess hydrophobic pockets where FMR can bind.14b No fluorescence enhancement was observed, indicating the absence of non-specific binding. Finally, to exclude the possible effect of components of SSB buffer, we titrated the ssDNA probe with PBS–glycerol, which also did not change the fluorescence (Fig. 5b). These control experiments clearly show that the increase in FMR-DNA fluorescence was induced by its binding to the protein.
To conclude, we have designed, synthesized and characterized new fluorescent dCXTPs bearing viscosity-sensitive fluorophores, fluorescent molecular rotors. While the julolidine derivative (dCVJTP) was too bulky for enzymatic incorporation, the smaller dCVDPTP (4b) was a very good substrate for polymerases in PEX, NEAR and PCR and can be efficiently used in enzymatic construction of DNA probes. The probe containing modified dCVDP nucleotides exhibits a light-up response upon binding to protein, which makes it prospective for probing DNA–protein interactions under homogenous conditions.
This work was supported by the Academy of Sciences of the Czech Republic (RVO: 61388963), by the Czech Science Foundation (P206-12-G151) and by Gilead Sciences Inc. D.D. thanks the IOCB for postdoctoral fellowship.
Notes and references
- (a) N. Dai and E. T. Kool, Chem. Soc. Rev., 2011, 40, 5756–5770 RSC; (b) C.-H. Leung, D. S.-H. Chan, H.-Z. He, Z. Cheng, H. Yang and D.-L. Ma, Nucleic Acids Res., 2012, 40, 941–955 CrossRef CAS PubMed.
- (a) M. Hawkins, Cell Biochem. Biophys., 2001, 34, 257–281 CrossRef CAS; (b) A. S. Wahba, A. Esmaeili, M. J. Damha and R. H. E. Hudson, Nucleic Acids Res., 2010, 38, 1048–1056 CrossRef CAS PubMed; (c) J.-W. Jung, S. K. Edwards and E. T. Kool, ChemBioChem, 2013, 14, 440–444 CrossRef CAS PubMed.
- T. Ono, S. Wang, C.-K. Koo, L. Engstrom, S. S. David and E. T. Kool, Angew. Chem., Int. Ed., 2012, 51, 1689–1692 CrossRef CAS PubMed.
- J. J. Li, X. Fang, S. M. Schuster and W. Tan, Angew. Chem., Int. Ed., 2000, 39, 1049–1052 CrossRef CAS.
- (a) S. Park, H. Otomo, L. Zheng and H. Sugiyama, Chem. Commun., 2014, 50, 1573–1575 RSC; (b) P. A. Hopkins, R. W. Sinkeldam and Y. Tor, Org. Lett., 2014, 16, 5290–5293 CrossRef CAS PubMed; (c) Y. Saito, A. Suzuki, Y. Okada, Y. Yamasaka, N. Nemoto and I. Saito, Chem. Commun., 2013, 49, 5684–5686 RSC; (d) D. Dziuba, V. Y. Postupalenko, M. Spadafora, A. S. Klymchenko, V. Guérineau, Y. Mély, R. Benhida and A. Burger, J. Am. Chem. Soc., 2012, 134, 10209–10213 CrossRef CAS PubMed; (e) M. Weinberger, F. Berndt, R. Mahrwald, N. P. Ernsting and H.-A. Wagenknecht, J. Org. Chem., 2013, 78, 2589–2599 CrossRef CAS PubMed; (f) T. Kanamori, H. Ohzeki, Y. Masaki, A. Ohkubo, M. Takahashi, K. Tsuda, T. Ito, M. Shirouzu, K. Kuwasako, Y. Muto, M. Sekine and K. Seio, ChemBioChem, 2015, 16, 167–176 CrossRef CAS PubMed.
- (a) M. Hocek and M. Fojta, Chem. Soc. Rev., 2011, 40, 5802–5814 RSC; (b) M. Hollenstein, Molecules, 2012, 17, 13569–13591 CrossRef CAS PubMed; (c) M. Hocek, J. Org. Chem., 2014, 79, 9914–9921 CrossRef CAS PubMed.
- J. Riedl, R. Pohl, N. P. Ernsting, P. Orsag, M. Fojta and M. Hocek, Chem. Sci., 2012, 3, 2797–2806 RSC.
- J. Riedl, P. Menova, R. Pohl, P. Orsag, M. Fojta and M. Hocek, J. Org. Chem., 2012, 77, 8287–8293 CrossRef CAS PubMed.
- (a) M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC; (b) M. K. Kuimova, Phys. Chem. Chem. Phys., 2012, 14, 12671–12686 RSC.
- W. L. Goh, M. Y. Lee, T. L. Joseph, S. T. Quah, C. J. Brown, C. Verma, S. Brenner, F. J. Ghadessy and Y. N. Teo, J. Am. Chem. Soc., 2014, 136, 6159–6162 CrossRef CAS PubMed.
- S. Murudkar, A. K. Mora, P. K. Singh and S. Nath, Chem. Commun., 2012, 48, 5301–5303 RSC.
- L. Liu, Y. Shao, J. Peng, C. Huang, H. Liu and L. Zhang, Anal. Chem., 2014, 86, 1622–1631 CrossRef CAS PubMed.
- (a) R. W. Sinkeldam, A. J. Wheat, H. Boyaci and Y. Tor, ChemPhysChem, 2011, 12, 567–570 CrossRef CAS PubMed; (b) M. G. Pawar, A. Nuthanakanti and S. G. Srivatsan, Bioconjugate Chem., 2013, 24, 1367–1377 CrossRef CAS PubMed; (c) Y. Zhang, X. Yue, B. Kim, S. Yao and K. D. Belfield, Chem. – Eur. J., 2014, 20, 7249–7253 CrossRef CAS PubMed; (d) A. A. Tanpure and S. G. Srivatsan, ChemBioChem, 2012, 13, 2392–2399 CrossRef CAS PubMed.
- (a) T. Iwaki, C. Torigoe, M. Noji and M. Nakanishi, Biochemistry, 1993, 32, 7589–7592 CrossRef CAS; (b) Y.-Y. Wu, W.-T. Yu, T.-C. Hou, T.-K. Liu, C.-L. Huang, I. C. Chen and K.-T. Tan, Chem. Commun., 2014, 50, 11507–11510 RSC.
- A. C. Fogarty, E. Duboue-Dijon, F. Sterpone, J. T. Hynes and D. Laage, Chem. Soc. Rev., 2013, 42, 5672–5683 RSC.
- H. Hwang and S. Myong, Chem. Soc. Rev., 2014, 43, 1221–1229 RSC.
- J. Sutharsan, D. Lichlyter, N. E. Wright, M. Dakanali, M. A. Haidekker and E. A. Theodorakis, Tetrahedron, 2010, 66, 2582–2588 CrossRef CAS PubMed.
- J. Ludwig, Acta Biochim. Biophys. Acad. Sci. Hung., 1981, 16, 131–133 CAS.
- (a) V. Raindlová, R. Pohl, M. Šanda and M. Hocek, Angew. Chem., Int. Ed., 2010, 49, 1064–1066 CrossRef PubMed; (b) P. Menova and M. Hocek, Chem. Commun., 2012, 48, 6921–6923 RSC; (c) P. Ménová, V. Raindlová and M. Hocek, Bioconjugate Chem., 2013, 24, 1081–1093 CrossRef PubMed; (d) D. Dziuba, R. Pohl and M. Hocek, Bioconjugate Chem., 2014, 25, 1984–1995 CrossRef CAS PubMed.
- R. D. Shereda, A. G. Kozlov, T. M. Lohman, M. M. Cox and J. L. Keck, Critical Rev. Biochem. Mol. Biol., 2008, 43, 289–318 CrossRef CAS PubMed.
- (a) T. M. Lohman and M. E. Ferrari, Ann. Rev. Biochem., 1994, 63, 527–570 CrossRef CAS PubMed; (b) W. Bujalowski and T. M. Lohman, J. Mol. Biol., 1989, 207, 249–268 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and tables, experimental details, copies of NMR and MALDI spectra. See DOI: 10.1039/c5cc00530b |
| This journal is © The Royal Society of Chemistry 2015 |