A dual FRET based fluorescent probe as a multiple logic system†
Xin
Zhou
ab,
Xue
Wu
b and
Juyoung
Yoon
*a
aDepartment of Chemistry and Nanoscience, Ewha Womans University, Seoul 120-750, Republic of Korea. E-mail: jyoon@ewha.ac.kr
bKey Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, People's Republic of China
First published on 31st October 2014
Abstract
A new fluorescent probe with a unique sequential dual FRET process was designed and constructed. This probe showed distinguished responses towards Fe3+ and Hg2+, making it an ideal candidate for multiple logic operations at the molecular level.
Since the first influential exploration by Silva demonstrating that florescent probes can serve as molecular logic gates,1 the development of such molecular systems capable of performing binary arithmetic and logical operations has attracted continuous attention of academic researchers in chemistry and biology. In the past two decades, a large number of excellent studies related to this field have been carried out.2 To date, there have been important advances toward finding practicality in fluorescent logic gates, such as AND, OR, NOR, INHIBIT, XOR, YES, NOT, and XNOR logic gates. Besides their potential applications in information processing and computing at the molecular level, their novel applications in the design of smart supramolecular materials,3 in drug delivery,4 and in clinical diagnostics5 have also been established. However, most of the logic systems rely on only one output mode.6 Recent interest has been mainly focused on the construction of molecules with multiple fluorescent output logic functions that can be modulated by multi-input combinations. This inspired us to fabricate a logic gate system that can utilize fluorescence via metal ions.2d,7 Such basic logic gates, programmed at a single molecular level, have possible implications in the development of electronic and photonic devices.
Taking these factors into account, we present here our idea for the construction of a novel fluorescent probe 1, possessing sequential dual FRET processes for multiple logic gate operations via the response signals of metal ions. The chemical structure of probe 1 is documented in Scheme 1. As described, probe 1 is composed of a dansylamide group and a rhodamine B moiety, a well known fluorophore pair for the FRET process,8 which were further covalently connected by a tryptophan unit.
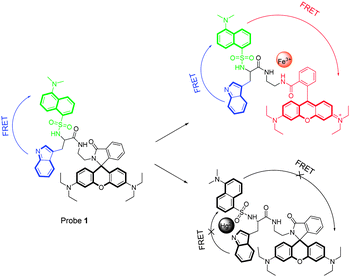 | ||
| Scheme 1 Chemical structure of probe 1 and the proposed multiple sensing mechanism based on sequential dual FRET. | ||
The design concepts of this probe are described as follows. First, as shown in Fig. S1 (ESI†), the emission spectra of the indole group (residue of tryptophan) significantly overlap with the absorbance of the dansylamide group, which means that they are well matched as an energy donor and acceptor.9 As a result, a FRET process between them is possible. Meanwhile, a similar situation is also observed in the case of dansylamide and rhodamine groups, where the emission of the dansylamide group shows a large overlap with the absorbance of the rhodamine moiety. Thus, a sequential dual FRET of probe 1 from the indole group to the rhodamine moiety via the dansylaminde unit is expected. Second, in the case of probe 1, the spirolactam ring of rhodamine can be converted into its open-ring form in the presence of certain metal ions, with the characteristic turn-on fluorescence emission and red color.8 Thus, a dual FRET process of probe 1 induced by metal ions is reasonable. Moreover, as a metal ligand, the indole group could regulate the coordination behaviours of probe 1 with different metal ions, making multiple responses possible. Notably, to our knowledge, there has been no report on such a dual FRET based fluorescent probe to date.
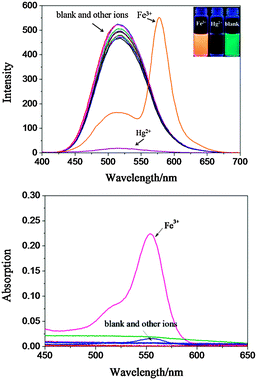
To confirm these above mentioned hypotheses, probe 1 was facilely synthesized by uncomplicated reactions. Its fluorescence properties were examined first. As expected, probe 1 gave a bright green fluorescence emission with a peak at 510 nm, which was attributed to the characteristic emission of the dansylamide group. No red emission corresponding to rhodamine was found, which confirmed that the rhodamine moiety of probe 1 was in its spirolactam-ring form, which is non-fluorescent and colourless. Next, the selective responses of probe 1 (10 μM) to various metal ions, including Ag+, Al3+, Ba2+, Ca2+, Fe3+, Hg2+, K+, Mg2+, Ni2+, Pb2+, Zn2+, and Cu2+, were investigated using fluorescence and absorbance spectra. As shown in Fig. 1, upon the addition of Fe3+, a dramatic fluorescence response was observed, in which a new emission peak at 580 nm emerged, accompanied by an obvious fluorescent color change in probe 1 from green to jacinth (Fig. 1 inset). Meanwhile, a remarkable change was also observed in the absorbance spectra, where a new peak was formed at 550 nm. The corresponding colour of probe 1 also changed from colorless to pink, which was easily distinguished by the naked eye (Fig. S2, ESI†). These fluorescence and colorimetric responses were attributed to a conversion from the spirolactam ring form of rhodamine to its open-ring form in the presence of Fe3+.8b On the other hand, the addition of Hg2+ caused a complete fluorescence quenching, and no peak was observed at around 550 nm in the absorbance spectra, indicating that the open-ring reaction of the rhodamine moiety did not occur in the presence of Hg2+. In contrast, other metal ions could not induce any observable fluorescence or colorimetric changes.
Subsequently, fluorescence titrations using Fe3+ and Hg2+ were examined (Fig. S3 and S4, ESI†). Upon the gradual addition of Fe3+, the emission intensity at 510 nm decreased with the simultaneous gradual appearance of a new emission peak at 580 nm (rhodamine). Notably, the emission intensity reached its maximum after the addition of one equivalent of Fe3+ (Fig. S3 inset, ESI†). A Job plot further proves this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe 1 and Fe3+ (Fig. S5, ESI†). Hg2+ titration against probe 1 was also monitored using fluorescence spectra (Fig. S4, ESI†). Upon the gradual addition of Hg2+, the emission intensity at 510 nm decreased and this decrease saturated after adding one equivalent of Hg2+ (Fig. S4 inset, ESI†). The association constants (Ka) for Fe3+ and Hg2+ binding with probe 1 were determined to be 2.66 × 103 M−1 and 9.29 × 104 M−1, respectively. The detection limit of fluorescent probe 1 for the analysis of Fe3+ and Hg2+ was determined from the plot of fluorescence intensity as a function of the concentrations of Fe3+ and Hg2+ (Fig. S6 and S7, ESI†).10 Moreover, competition experiments indicated that the detection processes of probe 1 for Fe3+ and Hg2+ were not affected by other competitive metal ions (Fig. S8, ESI†).
1 stoichiometry between probe 1 and Fe3+ (Fig. S5, ESI†). Hg2+ titration against probe 1 was also monitored using fluorescence spectra (Fig. S4, ESI†). Upon the gradual addition of Hg2+, the emission intensity at 510 nm decreased and this decrease saturated after adding one equivalent of Hg2+ (Fig. S4 inset, ESI†). The association constants (Ka) for Fe3+ and Hg2+ binding with probe 1 were determined to be 2.66 × 103 M−1 and 9.29 × 104 M−1, respectively. The detection limit of fluorescent probe 1 for the analysis of Fe3+ and Hg2+ was determined from the plot of fluorescence intensity as a function of the concentrations of Fe3+ and Hg2+ (Fig. S6 and S7, ESI†).10 Moreover, competition experiments indicated that the detection processes of probe 1 for Fe3+ and Hg2+ were not affected by other competitive metal ions (Fig. S8, ESI†).
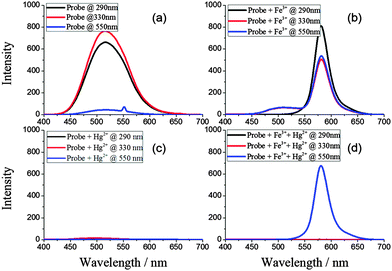
As mentioned above, probe 1 displayed a distinguishing response towards Fe3+ and Hg2+. To further understand this mechanism, a detailed investigation was conducted, in which probe 1 was excited by using the individual excitation wavelengths of its three fluorophores. For instance, 290 nm corresponds to the excitation wavelength of the indole group, 330 nm represents the excitation wavelength of the dansylamide unit and 550 nm is the excitation wavelength of the rhodamine moiety. The results are described in Fig. 2. As shown in Fig. 2a, when probe 1 was excited at 290 nm and 330 nm, respectively, strong green emission peaks at 510 nm with similar intensities were observed. This indicated that a FRET process occurred from the indole group to the dansylamide unit. In contrast, no emission was observed after excitation at 550 nm. Upon the addition of Fe3+, new peaks at 580 nm corresponding to the ring-open form of the rhodamine moiety were found after the three individual excitations. The fluorescence intensities were almost equal under excitations at 290 nm and 330 nm, which demonstrated that a sequential dual FRET process occurred from the indole group to the dansylamide unit and then from the dansylamide unit to the rhodamine moiety upon the excitation at 290 nm, as shown in Scheme 1. In addition, upon the addition of Hg2+, no emission peak was observed, which indicated that the Hg2+ interacted directly with the indole and dansylamide groups and suspended the initial FRET process between them, resulting in fluorescence quenching. In the presence of both Fe3+ and Hg2+, only an emission peak was observed following excitation at 550 nm, and one peak was found after excitation at 290 nm and 330 nm. These results revealed that Fe3+ did induce a ring-open reaction in the rhodamine group, and Hg2+ suspended the initial FRET process.
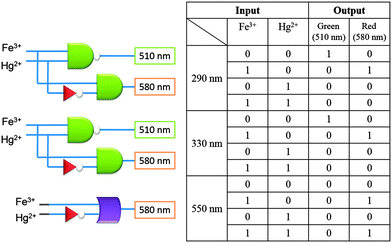
With these satisfactory fluorescence responses in mind, we set out to construct a multiple logic gate system using probe 1. The corresponding truth values are collected in Fig. 3. As described, upon excitation at 290 nm or 330 nm, probe 1 can mimic the INHIBIT logic gate11 (integrated by combining NOT, YES, and AND gates). By combining with the addition of Hg2+ and Fe3+, two individual outputs with different emissions, i.e. at 510 nm and 580 nm, respectively, were unambiguously observed. In addition, under excitation at 550 nm, probe 1 can serve as a cascading system of NOT and an integrated OR gate with a single output at 580 nm.
In summary, we present here the design and construction of a new fluorescent probe with a unique sequential dual FRET process. This probe showed distinguished fluorescence responses to Fe3+ and Hg2+. Fe3+ activated the dual FRET process and resulted in ratiometric and colorimetric responses; Hg2+ blocked the initial FRET process and caused fluorescence quenching. Moreover, these responses were not affected by other competitive metal ions. Finally, after various satisfactory responses, probe 1 was employed as a multiple logic gate system for multiple tandem logic operations.
This research was supported by a grant from the National Creative Research Initiative programs of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2012R1A3A2048814).
Notes and references
- P. A. de Silva, N. H. Q. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42 CrossRef.
- (a) E. T. Ecik, A. Atilgan, R. Guliyev, T. B. Uyar, A. Gumus and E. U. Akkaya, Dalton Trans., 2014, 43, 67 RSC; (b) H. Tian, Angew. Chem., Int. Ed., 2010, 49, 4710 CrossRef CAS PubMed; (c) A. Credi, Aust. J. Chem., 2010, 63, 145 CrossRef CAS; (d) R. Guliyev, S. Ozturk, Z. Kostereli and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 9826 CrossRef CAS; (e) S. Kou, H. N. Lee, D. van Noort, K. M. K. Swamy, S. H. Kim, J. H. Soh, K.-M. Lee, S.-W. Nam, J. Yoon and S. Park, Angew. Chem., Int. Ed., 2008, 47, 872 CrossRef CAS PubMed; (f) D. C. Magri, M. C. Fava and C. J. Mallia, Chem. Commun., 2014, 50, 1009 RSC; (g) K. Szacilowski, Chem. Rev., 2008, 108, 3481 CrossRef CAS PubMed; (h) D. C. Magri, G. J. Brown, G. D. McClean and A. P. de Silva, J. Am. Chem. Soc., 2006, 128, 4950 CrossRef CAS PubMed.
- (a) A. Prasanna de Silva, M. R. James, B. O. F. McKinney, D. A. Pears and S. M. Weir, Nat. Mater., 2006, 5, 787 CrossRef CAS PubMed; (b) S. Angelos, Y.-W. Yang, N. M. Khashab, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11344 CrossRef CAS PubMed; (c) F. Pu, E. Ju, J. Ren and X. Qu, Adv. Mater., 2014, 26, 1111 CrossRef CAS PubMed; (d) D. S. Kim, V. M. Lynch, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 14889 CrossRef CAS PubMed; (e) C. Gao, S. Silvi, X. Ma, H. Tian, M. Venturi and A. Credi, Chem. Commun., 2012, 48, 7577 RSC; (f) M. Dong, Y. Peng, Y.-M. Dong, N. Tang and Y.-W. Wang, Org. Lett., 2011, 14, 130 CrossRef PubMed; (g) Y. Peng, Y.-M. Dong, M. Dong and Y.-W. Wang, J. Org. Chem., 2012, 77, 9072 CrossRef CAS PubMed.
- (a) M. A. Correa-Duarte, J. Pérez-Juste, A. Sánchez-Iglesias, M. Giersig and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2005, 44, 4375 CrossRef CAS PubMed; (b) M. Elstner, K. Weisshart, K. Mullen and A. Schiller, J. Am. Chem. Soc., 2012, 134, 8098 CrossRef CAS PubMed.
- D. Kim and T. S. Lee, Chem. Commun., 2014, 50, 5833 RSC.
- S. Goswami, A. Manna, S. Paul, K. Aich, A. K. Das and S. Chakraborty, Dalton Trans., 2013, 42, 8078 RSC.
- R. Gotor, A. M. Costero, S. Gil, M. Parra, P. Gavina and K. Rurack, Chem. Commun., 2013, 49, 11056 RSC.
- (a) M. H. Lee, H. J. Kim, S. Yoon, N. Park and J. S. Kim, Org. Lett., 2007, 10, 213 CrossRef PubMed; (b) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed.
- (a) L. You and G. W. Gokel, Chem. – Eur. J., 2008, 14, 5861 CrossRef CAS PubMed; (b) K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45, 4562 CrossRef CAS PubMed.
- X. Zhou, X. Jin, G. Sun, D. Li and X. Wu, Chem. Commun., 2012, 48, 8793 RSC.
- J. Cao, X. Ma, M. Min, T. Cao, S. Wu and H. Tian, Chem. Commun., 2014, 50, 3224 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc08245a |
| This journal is © The Royal Society of Chemistry 2015 |