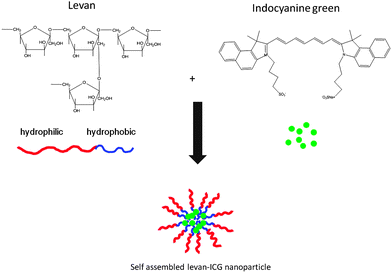
Self-assembled levan nanoparticles for targeted breast cancer imaging†
Sun-Jung
Kim
a,
Pan Kee
Bae
b and
Bong Hyun
Chung
*abc
aBioNanotechnology Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), 125 Gwahak-ro, Yuseong-gu, Daejeon 305-806, Republic of Korea. E-mail: chungbh@kribb.re.kr; Fax: +82-42-879-8594; Tel: +82-42-860-4442
bBioNano Health Guard Research Center, 125 Gwahak-ro, Yuseong-gu, Daejeon 305-806, Republic of Korea
cNanobiotechnology Major, School of Engineering, University of Science and Technology (UST), 125 Gwahak-ro, Yuseong-gu, Daejeon 305-806, Republic of Korea
First published on 10th November 2014
Abstract
We report on the targeted imaging of breast cancer using self-assembled levan nanoparticles. Indocyanine green (ICG) was encapsulated in levan nanoparticles via self-assembly. Levan–ICG nanoparticles were found to be successfully accumulated in breast cancer via specific interaction between fructose moieties in levan and overexpressed glucose transporter 5 in breast cancer cells.
Many types of carbohydrate moieties interact with cell surface lectins and transporters and are useful tools for targeted drug delivery and imaging.1–4 Galactose can specifically bind to the asialoglycoprotein receptor (ASGP-R) on hepatocytes, and mannose interacts with the mannose binding protein on macrophages.5–9 Moreover, glucose interacts with glucose transporters on cancer cells.10,11 Cancer cells and tissues that use more glucose overexpress glucose transporters to obtain the higher amount of energy they need for proliferation compared with normal tissues, which is known as the Warburg effect.12–14 This phenomenon is one of the hallmarks of cancer15 and can be exploited to target cancers via glycoconjugation. Active targeting of cancer refers to interactions between carriers and cancer cells, such as receptor-mediated interactions.16,17 Glycoconjugated materials can be used as active targeting carriers for cancer imaging and therapy because of interactions between glycosides and receptors of cancer cells. Recently, many researchers reported on the glycoconjugation of small molecules for cancer imaging and therapy.14,18
For example, 2-deoxy-2-(18F) fluoro-D-glucose (18F-FDG) is widely used to diagnose tumors clinically via interaction with glucose transporters 1 and 3 (Glut1, Glut3) in tumor tissues.19–21 The advantage of active targeting is that it efficiently targets only cancer cells and reduces side effects.
Fructose is a fruit sugar related to cancer metabolism. Glucose transporter 5 (Glut5), which is a transmembrane protein, is involved in transporting sugars and specifically interacts with fructose. In general, glucose transporters are known to be overexpressed in mammalian cancer cells, and breast cancer cells are known to overexpress Glut5. Therefore, fructose can be specifically transported and taken up by Glut5 in breast cancer cells.22–24
Levan is a biocompatible carbohydrate polymer that consists of β-D-fructofuranose attached by β-(2,6) linkages and is used in biomedical applications.25,26 The applications of levan are limited compared with other carbohydrate polymers such as dextran and gelatin because a difficult process is required to obtain pure levan. However, many attempts have been made to obtain pure and large amounts of levan.27 Levan has the advantages of high biodegradability and biocompatibility, and its amphiphilic nature has been exploited for the simple formation of nanoparticles in water.28 One study reported that levan has biological activities, including anti-tumor activity and an ability to inhibit infection.29 In addition, levan nanoparticles have also been investigated for the delivery of proteins and peptides.30
Indocyanine green (ICG) is well known as a Food and Drug Administration (FDA)-approved imaging probe for the diagnosis of tumors and metastatic lymph nodes. However, the near-infrared (NIR) fluorescence signal from ICG rapidly disappears. To overcome some of these problems, conjugation to nanomaterials has been used to improve the optical properties and stability of NIR dyes during blood circulation in the body.31 The stable and correct diagnosis of tumors is important for increasing patient survival rates.
In this study, ICG-encapsulating self-assembled levan nanoparticles were formed for targeted imaging of breast cancers. Levan is amphiphilic in nature and contains a hydrophobic moiety which is the CH2 group in the furanoside moieties.28 Because of its amphiphilicity, levan easily forms nanoparticles in water. ICG has two hydrophobic polycyclic parts in addition to hydrophilic sulfate groups. Because of these properties, ICG is easily encapsulated by levan via hydrophobic interactions during nanoparticle self-assembly (Scheme 1).
The morphology of the levan nanoparticles was observed using transmission electron microscopy (TEM), which showed that the levan–ICG nanoparticles were spherical (Fig. 1(B)). The hydrodynamic size of the nanoparticles was determined by dynamic light scattering (DLS) (Fig. 1(A)). The hydrodynamic size of the low molecular weight levan particles (estimated MW < 2000 kDa) in aqueous solutions was 57.1 ± 14.6 nm, and the size of the levan–ICG assembled nanoparticles which are fabricated at 200 rpm was 138.5 ± 34.1 nm. These results confirm that levan and ICG successfully formed self-assembled particles. The sizes of the high-molecular-weight levan (estimated MW > 2000 kDa) and levan–ICG particles which are fabricated at 200 rpm were 129.1 ± 32.8 and 202.1 ± 46.8 nm, respectively. The high-molecular-weight levan formed larger particles than the low-molecular-weight levan particles in aqueous solutions. The agitation speed also affected the size of nanoparticles. The size of low-molecular-weight levan–ICG nanoparticles which are manufactured at 1000 rpm was 229.2 ± 41.5 nm (Table S1, ESI†). It appears that larger particle formation at more vigorous agitation might result for more ICG encapsulation. To get an efficient imaging of breast cancer, we selected the low-molecular-weight-levan–ICG nanoparticles which are fabricated at 200 rpm for the following experiments.
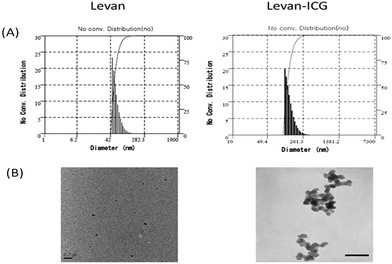 | ||
| Fig. 1 Morphology and size of self-assembled nanoparticles. (A) Size and distribution of nanoparticles measured by DLS. (B) TEM images of nanoparticles (scale bar: 0.2 μm). | ||
The encapsulation of ICG by levan was also confirmed using ultraviolet-visible (UV–vis) spectroscopy (Fig. S1, ESI†). The absorbance peak of levan–ICG appeared at 780 nm, which was similar to the absorbance peak of ICG alone. These results also show that ICG was successfully encapsulated in the levan nanoparticles. The efficiencies of ICG encapsulation using low and high molecular weight levans were 14.275% and 12.65%, respectively. (Table S1, ESI†). The concentration of levan was varied for ICG encapsulation. The absorbance of ICG at 780 nm decreased in self-assembled nanoparticles that were prepared with a low concentration of levan. Because the amount of hydrophobic groups was reduced at low levan concentrations, the amount of ICG encapsulation was reduced. The encapsulated ICG was found to be released from nanoparticles. The results show that 70% of ICG was released from levan nanoparticles within 1 h and an additional 10% of ICG was released within 48 h (Fig. S2, ESI†). The initial burst release does not seem to be a problem for imaging. However this initial burst might lead to a problem in drug delivery. Therefore, to use the drug delivery, further improvement may be required. In 0.1% Triton X-100 solution, the absorption spectrum of the self-assembled nanoparticles was red-shifted, and the maximum adsorption wavelength was increased by approximately 6-fold. Moreover, the size of the assembled levan–ICG nanoparticles in Triton X solution was not measurable. These results indicate that the self-assembled nanoparticles were formed by non-covalent interactions and completely disassembled and disappeared in the Triton X solution. These results also confirm that ICG was encapsulated in the levan particles via hydrophobic interactions.
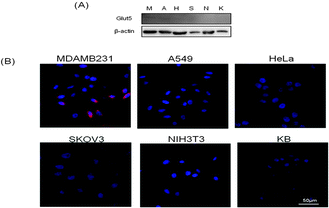
To evaluate cellular cytotoxicity, the MTT assay was performed (Fig. S3, ESI†). Various concentrations of levan–ICG nanoparticles were incubated with MDAMB231 cells (1 × 104 cells per well) for 24 h; high concentrations of nanoparticles were not cytotoxic. Thus, these results demonstrate that levan–ICG nanoparticles are a safe probe for breast cancer imaging. Intracellular uptake of the nanoparticles by various cells was observed by confocal microscopy. Breast cancer (MDAMB231), lung cancer (A549), ovarian cancer (SKOV3), cervical cancer (HeLa), nasopharyngeal cancer (KB) and mouse fibroblast cells (NIH3T3) were incubated with levan–ICG nanoparticles for 1 h. Fig. 2(B) shows that the levan–ICG nanoparticles were more highly taken up by the MDAMB231 breast cancer cells than all of the other cell lines. Overexpression of glucose transporter 5 (Glut5) in the breast cancer cells was confirmed by western blot analysis (Fig. 2(A)). We hypothesized that the levan–ICG nanoparticles would strongly interact with Glut5 on the MDAMB231 cells.
One study reported on the use of 1-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]-1-deoxy-D-fructose (1-NBDF) and 1-Cy5.5-1-deoxy-D-fructose (1-Cy5.5-DF), which are fluorescent fructose derivatives, to image Glut5-overexpressing breast cancer.21 Therefore, we also hypothesized that internalization of levan nanoparticles by MDAMB231 breast cancer cells occurred via interaction with Glut5.
We confirmed that levan has a higher affinity for Glut5 than fructose monosaccharide by carrying out a modified ELISA (Fig. S4(A), ESI†). The results show that levan bound more glucose 5 recombinant protein than fructose and bovine serum albumin (BSA). We also carried out a modified ELISA assay using a variety of concentrations of levan. As expected, levan at high concentration interacted more strongly with Glut5 than levan at low concentration (Fig. S4(B), ESI†). To confirm the interaction of levan–ICG nanoparticles with Glut5, we performed an inhibition assay using mercuric chloride, which is an inhibitor of glucose transporters (Fig. S5(A), ESI†). The result shows that a large number of levan–ICG nanoparticles were taken up by MDAMB231 cells. However, the levan–ICG nanoparticles were not taken up by cells after treatment with mercuric chloride. Because the interaction of levan and Glut5 was decreased by mercuric chloride, these results demonstrate that levan–ICG is specifically taken up by breast cancer cells via Glut5. Moreover, we carried out an inhibition assay by preincubation of fructose, levan and Glut5 antibody. The uptake of levan nanoparticles was found to be decreased after treatment of fructose, levan and Glut5 antibody (Fig. S5(B), ESI†). Especially, the intracellular uptake of nanoparticles was found to be more highly inhibited by preincubation of levan than preincubation of fructose. These results demonstrate that levan more strongly interacted with Glut5 than fructose.
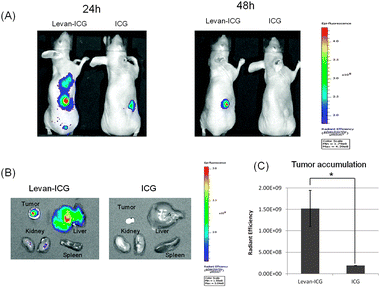
For imaging with the levan–ICG nanoparticles, breast cancer-bearing mice were prepared by injecting MDAMB231 cells subcutaneously. After the establishment of tumors in the mice, 2 mg of levan–ICG nanoparticles were injected intravenously. The mice were subsequently observed using an IVIS imaging system. As a control, ICG alone was injected into the tumor-bearing mice. The results show that the signals of both ICG and the levan–ICG nanoparticles appeared throughout the body immediately after injection. After 24 h had passed, the levan–ICG nanoparticles had accumulated in the tumors. However, the signal from the control ICG treatment was not observed in the tumors; rather, it showed accumulation in the liver. After 48 h, the levan–ICG nanoparticles had accumulated completely in the tumors, whereas the control ICG had been discharged out of the mice (Fig. 3(A)). Ex vivo characterization confirmed the accumulation of the levan–ICG nanoparticles in breast cancer (Fig. 3(B)). The biodistribution results for the levan–ICG nanoparticles showed accumulation in tumor and liver tissue 48 h after injection. Moreover, the near infrared fluorescence signal intensity (NIRF signal) of the tumors was higher after injection of levan–ICG than that with ICG alone (Fig. 3(C)). These results indicate that the levan–ICG nanoparticles interacted highly with the breast cancer cells. ICG is a NIR fluorescent probe that is FDA-approved for the detection of cancer and lymph node metastasis. However, the fluorescence signal of ICG quickly disappeared in the blood. In contrast, the signal from ICG fluorescence in the breast tumors was continually maintained for a long time by encapsulation in levan.
To observe the presence of nanoparticles in the tumor site, histochemical analysis was performed on cryosectioned tumor specimens (Fig. S6, ESI†). The samples from tumors treated with the levan–ICG nanoparticles showed that the indocyanine fluorescence signal was observed in almost all of the MDAMB 231 breast cancer cells. However, the samples treated by injecting ICG alone only showed weak fluorescence signals in the tumor cells. These results indicate that levan–ICG nanoparticles can be a useful tool for breast cancer imaging.
In summary, ICG encapsulated levan nanoparticles were easily formed by self-assembly. The size of the levan–ICG nanoparticles was about 138 nm. The levan–ICG nanoparticles selectively interacted with breast cancer cells, which were positive for glucose transporter 5. In addition, in vivo imaging showed that the levan–ICG nanoparticles successfully targeted breast tumors in mice. Therefore, levan–ICG nanoparticles have the advantage of being useful for the targeting and imaging of breast tumors. Moreover, the amphiphilic nature of levan can easily be exploited to encapsulate hydrophobic materials, such as contrast agents, drugs and nanoparticles. Therefore, these levan nanoparticles could be useful for biomedical applications that involve drug delivery and molecular imaging.
This work was supported by the BioNano Health-Guard Research Center, funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea as a Global Frontier Project (Grant Number H-GUARD_2013M3A6B2078950). This research was also supported by the KRIBB Initiative Research Program, Republic of Korea.
Notes and references
- N. Yamazaki, S. Kojima, N. V. Bovin, S. André, S. Gabius and H. J. Gabius, Adv. Drug Delivery Rev., 2000, 30, 225–244 CrossRef.
- W. Yu, N. Zhang and C. Li, Curr. Pharm. Des., 2009, 15, 3826–3836 CrossRef CAS.
- B. Lepenies, J. Lee and S. Sonkaria, Adv. Drug Delivery Rev., 2013, 65, 1271–1281 CrossRef CAS PubMed.
- Z. Liu, Y. Jiao, Y. Wang, C. Zhou and Z. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1650–1662 CrossRef CAS PubMed.
- S. E. Cook, I. K. Park, E. M. Kim, H. J. Jeong, T. G. Park, Y. J. Choi, T. Akaike and C. S. Cho, J. Controlled Release, 2005, 105, 151–163 CrossRef CAS PubMed.
- M. Hashida, S. Takemura, M. Nishikawa and Y. Takakura, J. Controlled Release, 1998, 53, 301–310 CrossRef CAS.
- W. Wijagkanalan, S. Kawakami, M. Takenaga, R. Igarashi, F. Yamashita and M. Hashida, J. Controlled Release, 2008, 125, 121–130 CrossRef CAS PubMed.
- G. J. Bernardes, R. Kikkeri, M. Maglinao, P. Laurino, M. Collot, S. Y. Hong, B. Lepenies and P. H. Seeberger, Org. Biomol. Chem., 2010, 8, 4987–4996 CAS.
- L. Gallego-Yerga, M. Lomazzi, F. Sansone, C. O. Mellet, A. Casnati and J. M. Garcia Fernández, Chem. Commun., 2014, 50, 7440–7443 RSC.
- L. S. Gritters, I. R. Francis and R. L. Wahl, J. Nucl. Med., 1993, 34, 1420–1427 CAS.
- K. H. Park, R. Takei, M. Goto, A. Maruyama, A. Kobayashi, K. Kobayashi and T. Akaike, J. Biochem., 1997, 121, 997–1001 CrossRef CAS.
- O. Warburg, F. Wind and E. Negelein, J. Gen. Physiol., 1927, 8, 519–530 CrossRef CAS.
- O. Warburg, Science, 1956, 123, 309–314 CAS.
- E. C. Calvaresi and P. J. Hergenrother, Chem. Sci., 2013, 4, 2319–2333 RSC.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed.
- J. D. Byme, T. Betancourt and L. Brannon-Peppas, Adv. Drug Delivery Rev., 2008, 60, 1615–1626 CrossRef PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- G. D. Benjamin, J. Chem. Soc., Perkin Trans. 1, 1999, 3215–3237 Search PubMed.
- A. M. Groves, M. Shastry, M. Rodriguez-Justo, A. Malhotra, R. Endozo, T. Davidson, T. Kelleher, K. A. Miles, P. J. Ell and M. R. Keshtgar, Eur. J. Nucl. Med. Mol. Imaging, 2011, 38, 46–52 CrossRef CAS PubMed.
- M. M. Abouzied, E. S. Crawford and H. A. Nabi, J. Nucl. Med. Technol., 2005, 33, 145–155 Search PubMed.
- T. C. Yen, L. C. See, C. H. Lai, C. W. Yah-Huei, K. K. Ng, S. Y. Ma, W. J. Lin, J. T. Chen, W. J. Chen, C. R. Lai and S. Hsueh, J. Nucl. Med., 2004, 45, 22–29 CAS.
- J. Levi, Z. Cheng, O. Gheysens, M. Patel, C. T. Chan, Y. Wang, M. Namavari and S. S. Gambhir, Bioconjugate Chem., 2007, 18, 628–634 CrossRef CAS PubMed.
- M. Wuest, B. J. Trayner, T. N. Grant, H. S. Jans, J. R. Mercer, D. Murray, F. G. West, A. J. McEwan, F. Wuest and C. I. Cheeseman, Nucl. Med. Biol., 2011, 38, 461–475 CrossRef CAS PubMed.
- B. J. Trayner, T. N. Grant, F. G. West and C. I. Cheeseman, Bioorg. Med. Chem., 2009, 17, 5488–5495 CrossRef CAS PubMed.
- Y. W. Han, Microbial levan. Adv. Appl. Microbiol., 1990, 35, 171–194 CAS.
- S. A. Kang, K. H. Jang, J. W. Seo, K. H. Kim, Y. H. Kim, D. Rairakhwada, M. Y. Seo, J. O. Lee, S. D. Ha, C. H. Kim and S. K. Rhee, in Microbial production of biopolymers and polymer precursors, ed. B. H. Rehm, Academic Press, Caister, 2009, ch. 6, pp. 145–162 Search PubMed.
- K. B. Song and S. K. Rhee, Biotechnol. Lett., 1994, 16, 1305–1310 CAS.
- I. J. Vereyken, V. Chupin, R. A. Demel, S. C. M. Smeekens and B. D. Kruijff, Biochim. Biophys. Acta, 2001, 1510, 307–320 CrossRef CAS.
- S. H. Yoo, E. J. Yoon, J. Cha and H. G. Lee, Int. J. Biol. Macromol., 2004, 34, 37–41 CrossRef CAS PubMed.
- A. D. Sezer, H. Kazak, E. T. Öner and J. Akbuğa, Carbohydr. Polym., 2011, 84, 358–363 CrossRef CAS PubMed.
- P. K. Bae, J. Jung and B. H. Chung, Nano Convergence, 2014, 1, 6 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc07679f |
| This journal is © The Royal Society of Chemistry 2015 |