Post-assembly transformations of porphyrin-containing metal–organic framework (MOF) films fabricated via automated layer-by-layer coordination†
Monica C.
So
a,
M. Hassan
Beyzavi
a,
Rohan
Sawhney
a,
Osama
Shekhah
b,
Mohamed
Eddaoudi
b,
Salih S.
Al-Juaid
c,
Joseph T.
Hupp
*a and
Omar K.
Farha
*ac
aDepartment of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208, USA. E-mail: j-hupp@northwestern.edu; o-farha@northwestern.edu
bAdvanced Membranes and Porous Materials Research Center, 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia
cDepartment of Chemistry, King Abdulaziz University, Jeddah, Saudi Arabia
First published on 17th October 2014
Abstract
Herein, we demonstrate the robustness of layer-by-layer (LbL)-assembled, pillared-paddlewheel-type MOF films toward conversion to new or modified MOFs via solvent-assisted linker exchange (SALE) and post-assembly linker metalation. Further, we show that LbL synthesis can afford MOFs that have proven inaccessible through other de novo strategies.
Metal–organic frameworks (MOFs)1 are hybrid materials composed of inorganic vertices connected to organic linkers. Due to the plethora of available linkers and vertices, MOFs can be easily tuned towards specific applications such as catalytic activity,2 chemical sensing,3 and light harvesting.4,5 Certain applications capitalizing on these behaviors require MOFs to be fabricated into thin films, ideally of well-defined and predetermined thickness and crystallographic orientation. Among the most versatile and useful approaches to MOF film synthesis is layer-by-layer (LbL) coordination, also called liquid-phase epitaxy (Scheme 1a).5,6 This approach successively and repetitively introduces solutions of each framework building block into contact with functionalized substrates and subsequently the growing MOF film. The advantage of LbL over other techniques is that MOF layer thicknesses can be controlled with molecular-scale precision. Notably, the composition of an added layer need not match that of preceding layers.5,7 Thus, complex, multifunctional crystalline MOF structures are, in principle, obtainable.
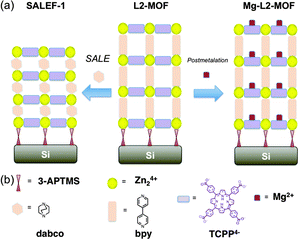 | ||
| Scheme 1 (a) Summary of the transformations performed on L2-MOF. (b) Representation of structural components used in the experiments. | ||
One of the current areas of focus of MOF research is the development of methods for obtaining frameworks that are inaccessible via conventional solvothermal methods, but that would be stable or meta-stable if they could indeed be made. Many desired, but conventionally inaccessible, MOFs are direct structural analogues of materials that, in contrast, are readily obtained via standard synthesis routes. For example, the widely studied compound ZIF-8 (or MAF-4) consisting of Zn(II) and 2-methylimidazolate in a sodalite topology,8 is easily obtained by solvothermal synthesis, whereas the methyl-free analogue, SALEM-2, is obtainable only indirectly.9 Defining an additional set of examples is the observation that traditional batch-solvothermal synthesis methods typically preclude the direct incorporation of free-base porphyrins as linkers in MOFs because the linkers spontaneously recruit metal ions present as building blocks for nodes.10
A promising partial solution to the aforementioned problem is post-synthesis elaboration,11 subsequently renamed and generalized to post-synthesis modification (PSM),12,13i.e. chemical alteration (atom, ion or functional group addition, subtraction, or transformation) of the framework without changing the parent topology. Indeed, the challenge of obtaining MOFs featuring metal-free porphyrin- or salen-based linkers has been met by first assembling metalated-linker versions of the compounds and then replacing nonstructural (i.e. linker-localized) metal ions with pairs of protons. A second PSM step permits new metal ions to be introduced, including catalytic metals that are incompatible with de novo MOF synthesis.14
Closely related to PSM is the concept of MOF building-block replacement.15 One approach to building block replacement is solvent-assisted linker exchange (SALE).9,16 Implementation typically entails exposing MOF crystals to a solution of a candidate replacement linker in a carefully selected solvent. Notably, SALE occurs by ligand exchange within intact MOFs, rather than by dissolution and recrystallization. Consequently, the topology of the parent MOF is replicated in the daughter structure. Following the pioneering work of Choe and co-workers with a pillared paddlewheel MOF,17 SALE has been demonstrated for a range of MOFs,16–19 including seemingly inert compounds such as ZIFs20 (e.g., ZIF-8 conversion to SALEM-2)9 and, even more remarkably, to UiO-66,19,21 a material that is characterized by unusually strong metal(node)-linker bonds. Perhaps most striking and illustrative has been recent work by Cohen and co-workers; they showed that SALE could be used (with UiO-66) to incorporate catechol-presenting linkers and, subsequently, that PSM in the form of metal-ion binding by linker-based catecholates could be used to render the linker-exchanged UiO compound catalytic for selected organic oxidation reactions.22
While significant effort has been devoted to applying PSM to bulk crystals of MOFs,12 its application towards thin films of MOFs is limited.23 Given the versatility and practical utility of both PSM and building-block-replacement for gaining access to otherwise difficult-to-synthesize MOFs as bulk materials, we reasoned that their application to MOFs in thin-film form – especially, well-defined LbL-fabricated form – could prove similarly useful. Here we report proof-of-concept demonstrations of: (a) SALE, and (b) PSM (linker post-metalation), with a representative LbL-assembled, thin-film material, L2-MOF, to yield solvent-assisted linker exchanged film (SALEF-1) and Mg-L2-MOF, respectively; see Scheme 1b. Additionally, we report that time-of-flight secondary ion mass spectrometry (TOF-SIMS) can be used to follow the progress of SALE and qualitatively assess LbL film composition. For bulk samples of MOFs, NMR of subsequently dissolved samples is typically the method of choice for documenting SALE or PSM. The quantities of material available in LbL samples, however, are generally too low for evaluation by NMR. Finally, we show that the unusually mild conditions associated with LbL construction of pillared-paddlewheel-type MOFs enable otherwise easily demetalated magnesium porphyrins to be incorporated in growing films without degradation.
We previously reported the LbL synthesis of oriented thin-films of the porous coordination polymer, L2-MOF.5 As shown in Scheme 1, this material contains: (a) 5,10,15,20-(4-carboxyphenyl)porphyrin (TCPP4−) units as tetratopic linkers that coordinate pairs of Zn(II) ions in paddlewheel fashion, and (b) 4,4′-bipyridine (bpy) linkers that serve as Zn(II)-ligating spacers or pillars between TCCP-defined layers. As in our previous report,5 films were LbL-grown on either silicon or indium-doped tin oxide (ITO) substrates. We used films prepared via automated LbL-growth cycles. Profilometry indicated L2-MOF thicknesses of about 60 nm.
As a candidate linker for exchange and replacement of the bpy pillars (7.1 Å) of L2-MOF, we selected 1,4-diazabicyclo[2.2.2]octane (dabco, 2.65 Å). We have noted elsewhere that single-stage SALE of pillar-type linkers tends to proceed essentially to completion when the pKa of the conjugate acid of the incoming linker exceeds by more than a pH unit or two the pKa of the conjugated acid of the linker being replaced.24 pKa values for conjugate acids are likely to be good surrogates for relative strengths of bonds formed by the corresponding bases with zinc ions. For bpy-H+ and dabco-H+, respectively, pKa values are 4.9 and 9.8.
Typical conditions for film-based SALE consisted of several hours of exposure at 43 °C to a stirring solution of 2 mM of dabco. As noted above, the small absolute amount of material incorporated into or displaced from thin MOF films presents challenges for monitoring reaction progress. We found, however, that time-of-flight secondary ion mass spectrometry (TOF-SIMS) works well for this purpose. TOF-SIMS relies upon an ion beam (in our case 69Ga+ ion) to bombard a surface and eject surface/film components – generally as small, ionic fragments.25
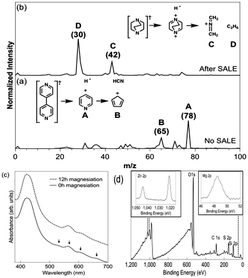
Fig. 1a shows a portion of the TOF-SIMS spectrum of an LbL-grown film of L2-MOF. The largest peaks appear at m/z 78 (A) and at m/z 65 (B) and are attributable, respectively, to pyridinium, bpy fragmentation product, and (with less certainty) 1,3-cyclopentadiene, a rearrangement product. Shown in Fig. 1b is a portion of the TOF-SIMS spectrum for a film following 13 hours of exposure to dabco. Consistent with conversion of L2-MOF to SALEF-1via pillar exchange, peaks attributable to bpy fragmentation and rearrangement are absent. Instead, the spectrum is dominated by peaks assignable to N-methylene-methaniminium C and ethane D and consistent with fragmentation of dabco. Notably, for thinner films of L2-MOF, TOF-SIMS measurements show that less time is needed to complete their conversion to SALEF-1.
If LbL-grown films of L2-MOF are oriented as indicated in Scheme 1, complete exchange of bpy pillars for dabco pillars should decrease the film thickness. For a 45-cycle film (61 nm), the anticipated decrease is 15 nm. The experimentally observed decrease (via profilometry) is 15 ± 0.8 nm, implying that the extent of exchange is ca. 100% (Fig. S3, ESI†). Further, SALEF-1 shows lower porosity than L2-MOF (Table S2, ESI†); the actual relative change in porosity is in good agreement with the calculated value.
Since the LbL-grown films contain free-base porphyrins, we hypothesized that post-metalation (i.e. film-based PSM) of the porphyrin units would be possible. After 12 hours of reaction in excess amounts of magnesium bromide diethyl etherate (MgBr2(OEt)2), free-base porphyrin-containing films, grown on quartz slides, were converted to metalloporphyrin form. Visible-region electronic absorption spectra confirm the metalation. Thus, the pattern of four Q-bands (530, 563, 607, and 659 nm) that are characteristic of free-base porphyrin sites collapses to two Q bands (566 and 608 nm), as expected for a metalation-driven increase in porphyrin symmetry from D2h to D4h (Fig. 1c).26 The observed minimal changes in full-width-at-half-maximum (fwhm) and intensity for porphyrin B-band absorption point to the stability of films under the selected conditions for post-assembly metalation.
X-ray photoelectron spectroscopy (XPS) analysis of post-metalated films yielded peaks for Zn 2p at 1021.6 eV and 1044.6 eV, and Mg 2p at 48.5 eV (Fig. 1d). Based on the integration of Zn 2p and Mg 2p peak areas, the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio for the magnesium-exposed material is 69
Mg ratio for the magnesium-exposed material is 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31, which is consistent with the expected value of 67
31, which is consistent with the expected value of 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 for a pillared-paddlewheel Mg-porphyrin-based MOF having zinc nodes (Mg-L2-MOF). To demonstrate another example of post-assembly linker metalation, we also formed Zn-L2-MOF as confirmed by UV-vis (Fig. S4, ESI†) and XPS data (Fig. S5, ESI†). Nearly no relative change in % porosity was detected (Table S2, ESI†), suggesting that porosity is fully retained.
33 for a pillared-paddlewheel Mg-porphyrin-based MOF having zinc nodes (Mg-L2-MOF). To demonstrate another example of post-assembly linker metalation, we also formed Zn-L2-MOF as confirmed by UV-vis (Fig. S4, ESI†) and XPS data (Fig. S5, ESI†). Nearly no relative change in % porosity was detected (Table S2, ESI†), suggesting that porosity is fully retained.
Inspired by the post-magnesiation of the LbL films, we wondered how the synthetic conditions might affect the magnesiation of porphyrins in MOFs. Under solvothermal conditions (i.e. dimethylformamide at 80 °C),27 less than 3% of initially linker-sited magnesium(II) remained in bulk samples of MOFs similar to L2-MOF. From inductively coupled plasma (ICP) measurements of bulk MOF samples, we determined that ca. 98% of the initially magnesiated tetratopic porphyrins enlisted as MOF building blocks lost Mg2+ upon completion of the MOF synthesis – a finding consistent with the slightly acidic nature of the solvothermal milieu and the low stability of Mg-porphyrins under these conditions (Table S1, ESI†). It is known that weakly bound Mg2+ completely exchanges with Zn2+ under MOF solvothermal synthesis conditions.28
We hypothesized, given the mild conditions afforded by the LbL approach to MOF growth (i.e., comparatively low reaction temperature and repetitive removal of acetic acid by rinsing), that the magnesium(II) would remain porphyrin-coordinated throughout LbL growth. Indeed, after LbL growth, samples constructed from pre-magnesiated porphyrins, retained two Q bands in their electronic spectra (Fig. S6, ESI†). By XPS, we confirmed that magnesium is retained in such films. The observed Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio is 2
Mg ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as expected for a Zn(II)-paddlewheel MOF containing fully magnesiated porphyrin linkers (Fig. S7, ESI†). To our knowledge, no direct route to MOFs containing magnesiated porphyrins has previously been described. These results, and the mild synthesis conditions of LbL coordination, point to the broader potential of LbL methods to produce thin films of MOFs that would be difficult or impossible to directly synthesize under the harsher conditions typically used for synthesis of bulk samples (e.g. crystalline powder samples).
1, as expected for a Zn(II)-paddlewheel MOF containing fully magnesiated porphyrin linkers (Fig. S7, ESI†). To our knowledge, no direct route to MOFs containing magnesiated porphyrins has previously been described. These results, and the mild synthesis conditions of LbL coordination, point to the broader potential of LbL methods to produce thin films of MOFs that would be difficult or impossible to directly synthesize under the harsher conditions typically used for synthesis of bulk samples (e.g. crystalline powder samples).
The LbL-fabricated, thin-film material, L2-MOF, has proven to be sufficiently robust to participate, without detectable degradation, in both building-block replacement and PSM reactions. Thus, the dipyridyl pillars of the MOF can be readily and essentially completely replaced with dabco pillars via single-stage SALE from a 2 mM DMF solution of the replacement linker. Depending on film thickness, SALE equilibrium is achievable within 13 hours – with thinner films equilibrating more rapidly. TOF-SIMS measurements offer a convenient way of assessing SALE in samples such as LbL films that contain only tiny absolute amounts of materials. Complete post-assembly metalation of free-base-porphyrin-containing LbL films, proved possible, even with weakly coordinated Mg(II) ions. Additionally, using mild LbL conditions, we that these weakly bound metal ions could be fully retained during film assembly – an outcome not achievable, in our experience, with MOF synthesis via conventional high-temperature solvothermal methods. While beyond the scope of our initial investigation, we believe that the ability of the known metal lability of magnesiated porphyrins28 should facilitate incorporation of other metal ions, including weakly bound ions, potentially displaying interesting redox, catalytic, or photophysical behavior. The findings effectively diversify the arsenal of techniques that can be deployed to obtain desired MOF films for sensing, catalysis, and energy conversion schemes.
O.K.F and J.T.H gratefully acknowledge funding from the Army Research Office (project number W911NF-13-1-0229). M.C.S. acknowledges support from the National Defense Science & Engineering Graduate Fellowship Program. M.H.B. acknowledges support from the Institute for Sustainability and Energy at Northwestern University. The authors are grateful for the help of Dr Diego Gomez-Gualdron for simulation of single crystals of the MOFs and the use of experimental facilities at the Integrated Molecular Structure Education and Research Center and the Northwestern University Atomic- and Nanoscale Characterization Experimental Center (NUANCE, Keck-II).
Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef CAS PubMed; J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC; H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef PubMed; O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166–1175 CrossRef PubMed; S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef PubMed.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS PubMed; J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404–14405 CrossRef CAS PubMed; S. Hermes, M.-K. Schröter, R. Schmid, L. Khodeir, M. Muhler, A. Tissler, R. W. Fischer and R. A. Fischer, Angew. Chem., Int. Ed., 2005, 44, 6237–6241 CrossRef PubMed; L. E. Kreno, J. T. Hupp and R. P. Van Duyne, Anal. Chem., 2010, 82, 8042–8046 CrossRef PubMed; Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2011, 112, 1126–1162 CrossRef PubMed.
- S. Jin, H.-J. Son, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 955–958 CrossRef CAS PubMed; C. Y. Lee, O. K. Farha, B. J. Hong, A. A. Sarjeant, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2011, 133, 15858–15861 CrossRef PubMed; H.-J. Son, S. Jin, S. Patwardhan, S. J. Wezenberg, N. C. Jeong, M. So, C. E. Wilmer, A. A. Sarjeant, G. C. Schatz, R. Q. Snurr, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2012, 135, 862–869 CrossRef PubMed.
- M. C. So, S. Jin, H.-J. Son, G. P. Wiederrecht, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 15698–15701 CrossRef CAS PubMed.
- A. Bétard and R. A. Fischer, Chem. Rev., 2011, 112, 1055–1083 CrossRef CAS PubMed; E. Biemmi, C. Scherb and T. Bein, J. Am. Chem. Soc., 2007, 129, 8054–8055 CrossRef PubMed; S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2005, 127, 13744–13745 CrossRef PubMed; O. Shekhah, W. Hui, M. Paradinas, C. Ocal, B. Schüpbach, A. Terfort, D. Zacher, R. A. Fischer and C. Wöll, Nat. Mater., 2009, 8, 481–484 CrossRef PubMed; O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef PubMed; M. Tu, S. Wannapaiboon and R. Fischer, Inorg. Chem. Front., 2014, 1, 442–463 RSC; D. Zacher, R. Schmid, C. Wöll and R. A. Fischer, Angew. Chem., Int. Ed., 2011, 50, 176–199 CrossRef PubMed; V. Stavila, J. Volponi, A. M. Katzenmeyer, M. C. Dixon and M. D. Allendorf, Chem. Sci., 2012, 3, 1531–1540 RSC; A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon and M. D. Allendorf, Science, 2014, 343, 66–69 CrossRef PubMed; L. E. Kreno, J. T. Hupp and R. P. Van Duyne, Anal. Chem., 2010, 82, 8042–8046 CrossRef PubMed; G. Lu, O. K. Farha, L. E. Kreno, P. M. Schoenecker, K. S. Walton, R. P. Van Duyne and J. T. Hupp, Adv. Mater., 2011, 23, 4449–4452 CrossRef PubMed; P. Szilágyi, R. Westerwaal, R. van de Krol, H. Geerlings and B. Dam, J. Mater. Chem. C, 2013, 1, 8146–8155 RSC.
- D. Zacher, K. Yusenko, A. Bétard, S. Henke, M. Molon, T. Ladnorg, O. Shekhah, B. Schüpbach, T. de los Arcos and M. Krasnopolski, Chem. – Eur. J., 2011, 17, 1448–1455 CrossRef CAS PubMed; O. Shekhah, K. Hirai, H. Wang, H. Uehara, M. Kondo, S. Diring, D. Zacher, R. Fischer, O. Sakata and S. Kitagawa, Dalton Trans., 2011, 40, 4954–4958 RSC; M. Tu and R. A. Fischer, J. Mater. Chem. A, 2014, 2, 2018–2022 Search PubMed.
- X.-C. Huang, Y.-Y. Lin, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed; K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef PubMed; J.-P. Zhang, A.-X. Zhu, R.-B. Lin, X.-L. Qi and X.-M. Chen, Adv. Mater., 2011, 23, 1268–1271 CrossRef PubMed.
- O. Karagiaridi, M. B. Lalonde, W. Bury, A. A. Sarjeant, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 18790–18796 CrossRef CAS PubMed.
- H. Chung, P. M. Barron, R. W. Novotny, H.-T. Son, C. Hu and W. Choe, Cryst. Growth Des., 2009, 9, 3327–3332 Search PubMed; E.-Y. Choi, P. M. Barron, R. W. Novotny, H.-T. Son, C. Hu and W. Choe, Inorg. Chem., 2008, 48, 426–428 CrossRef CAS PubMed.
- T. Gadzikwa, B.-S. Zeng, S. T. Nguyen and J. T. Hupp, Books of Abstracts, 233rd ACS National Meeting, Chicago, IL, March 25–29, 2007; American Chemical Society: Washington, DC, 2007; INOR 331.
- K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498–519 RSC; Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- Z. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2008, 48, 296–306 CrossRef CAS PubMed; K. K. Tanabe, Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2008, 130, 8508–8517 CrossRef PubMed.
- A. M. Shultz, A. A. Sarjeant, O. K. Farha, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2011, 133, 13252–13255 CrossRef CAS PubMed; W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef PubMed; Z. Zhang, L. Zhang, L. Wojtas, P. Nugent, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 134, 924–927 CrossRef PubMed.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 53, 4530–4540 Search PubMed; O. Karagiaridi, W. Bury, J. E. Mondloch, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2014, 53, 4530–4540 CrossRef CAS PubMed; C. K. Brozek and M. Dinca, Chem. Soc. Rev., 2014, 43, 5456–5467 RSC; J. D. Evans, C. J. Sumby and C. J. Doonan, Chem. Soc. Rev., 2014, 43, 5933–5951 RSC.
- W. Bury, D. Fairen-Jimenez, M. B. Lalonde, R. Q. Snurr, O. K. Farha and J. T. Hupp, Chem. Mater., 2013, 25, 739–744 CrossRef CAS.
- B. J. Burnett, P. M. Barron, C. Hu and W. Choe, J. Am. Chem. Soc., 2011, 133, 9984–9987 CrossRef CAS PubMed.
- S. Takaishi, E. J. DeMarco, M. J. Pellin, O. K. Farha and J. T. Hupp, Chem. Sci., 2013, 4, 1509–1513 RSC; T. Li, M. T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed; A. F. Gross, E. Sherman, S. L. Mahoney and J. J. Vajo, J. Phys. Chem. A, 2013, 117, 3771–3776 CrossRef PubMed.
- M. Kim, J. F. Cahill, H. Fei, K. A. Prather and S. M. Cohen, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- M. Kim, J. F. Cahill, Y. Su, K. A. Prather and S. M. Cohen, Chem. Sci., 2012, 3, 126–130 RSC; D. H. Hong and M. P. Suh, Chem. – Eur. J., 2014, 20, 426–434 CrossRef CAS PubMed.
- H. Fei, J. Shin, Y. S. Meng, M. Adelhardt, J. Sutter, K. Meyer and S. M. Cohen, J. Am. Chem. Soc., 2014, 136, 4965–4973 CrossRef CAS PubMed.
- Z. Wang, J. Liu, H. K. Arslan, S. Grosjean, T. Hagendorn, H. Gliemann, S. Bräse and C. Wöll, Langmuir, 2013, 29, 15958–15964 CrossRef CAS PubMed; O. Shekhah, H. K. Arslan, K. Chen, M. Schmittel, R. Maul, W. Wenzel and C. Wöll, Chem. Commun., 2011, 47, 11210–11212 RSC.
- O. Karagiaridi, W. Bury, E. Tylianakis, A. A. Sarjeant, J. T. Hupp and O. K. Farha, Chem. Mater., 2013, 25, 3499–3503 CrossRef CAS.
- H. Fei, J. F. Cahill, K. A. Prather and S. M. Cohen, Inorg. Chem., 2013, 52, 4011–4016 CrossRef CAS PubMed.
- M. Gouterman, J. Chem. Phys., 1959, 30, 1139–1161 CrossRef CAS PubMed; C. Weiss, H. Kobayashi and M. Gouterman, J. Mol. Spectrosc., 1965, 16, 415–450 CrossRef.
- O. K. Farha, A. M. Shultz, A. A. Sarjeant, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2011, 133, 5652–5655 CrossRef CAS PubMed.
- M. Speckbacher, L. Yu and J. S. Lindsey, Inorg. Chem., 2003, 42, 4322–4337 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Procedures, materials, and instrumentation; characterization (1H NMR, MS-MALDI, ICP-OES, XPS, UV-vis, profilometry, ellipsometry). See DOI: 10.1039/c4cc05727a |
| This journal is © The Royal Society of Chemistry 2015 |