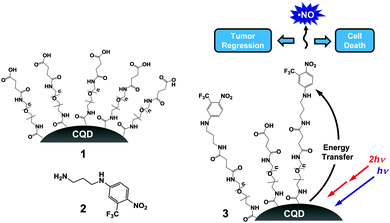
Carbon quantum dot–NO photoreleaser nanohybrids for two-photon phototherapy of hypoxic tumors†
Colin
Fowley
a,
Anthony P.
McHale
a,
Bridgeen
McCaughan
a,
Aurore
Fraix
b,
Salvatore
Sortino
*b and
John F.
Callan
*a
aBiomedical Sciences Research Institute, University of Ulster, Coleraine, Northern Ireland BT521SA, UK. E-mail: j.callan@ulster.ac.uk
bLaboratory of Photochemistry, Department of Drug Sciences, University of Catania, I-95125 Catania, Italy. E-mail: ssortino@unict.it
First published on 5th November 2014
Abstract
We report a conjugate between carbon quantum dots and a NO photoreleaser able to photogenerate the anticancer NO radical via an energy transfer mechanism. This nanohybrid proved toxic to cancer cells in vitro and significantly reduced tumor volume in mice bearing human xenograft BxPC-3 pancreatic tumors upon two-photon excitation with the highly biocompatible 800 nm light.
In recent years, nitric oxide (NO) has stimulated an explosion of interest due, not only to its pivotal role in the maintenance and bioregulation of vital functions,1 but also to its promising antioxidant, antibacterial, wound healing and anticancer activities.2 These exciting discoveries have made the development of molecular scaffolds and nanomaterials for NO delivery a hot topic in the burgeoning field of nanomedicine, with the intriguing prospect to tackle important diseases.3 With regard to cancer therapy, a major problem is that the biological effects of NO are highly site, concentration and dosage dependent, creating a dual role for this molecule in acting as a tumor enhancer or suppressor, when present in picomolar or nanomolar quantities respectively.2 This dichotomy has made the light-controlled NO donors much more appealing than those based on spontaneous thermolysis as it represents a convenient non-invasive on–off trigger to deliver NO on demand since it allows the accurate control of site, timing and dosage.4 Light is in fact the most elegant and finely tunable tool for the non-invasive introduction of therapeutic agents in a desired bio-environment, mimicking an “optical microsyringe” with exquisite spatiotemporal control.5 Importantly, since the NO release from NO photodonors is independent from O2 availability, the NO-photostimulated treatment modalities may well complement conventional photodynamic therapy (PDT). In fact, the PDT mechanism, which is almost exclusively based on the photoproduction of the cytotoxic singlet oxygen (1O2),6 strictly relies on the presence of molecular oxygen and thus, has serious limitations in hypoxic conditions, which is typical for many solid tumours. Furthermore, the NO radical shares some advantageous features with 1O2, i.e. (i) confinement of its reactivity in the restricted region of space (<200 μm) from where it has been photogenerated, reducing the likelihood of systemic toxicity, (ii) capability to attack various kinds of biological, representing a multitarget species and finally, (iii) does not present multiple drug resistance (MDR) problems encountered with several conventional anticancer drugs that are often target-specific.
The main limitation of several NO photoprecursors is that their absorption window falls below the photo-therapeutic window (≈650–1350 nm),7 where light penetration through human tissue is restricted to only a few millimetres. Two-photon excitation (TPE) offers two main advantages in this regard since it allows excitation in the therapeutic window, where penetration depths in soft tissue of greater than one centimetre can be achieved, and permits high spatial resolution in three dimensions. A limited number of NO photodonors based on suitable organic molecules as two-photon antennas are reported in the literature.4c,8 Carbon quantum dots (CQDs) are a new class of nanomaterials which possess high photostability, tuneable emission and TPE cross-sections three orders of magnitude greater than that of small molecule dyes (∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM).9 We have recently demonstrated that conjugation of CQDs with the 1O2 photosensitizer protoporphyrin IX, provides an effective strategy to activate such sensitisers in the phototherapeutic window using TPE.10
000 GM).9 We have recently demonstrated that conjugation of CQDs with the 1O2 photosensitizer protoporphyrin IX, provides an effective strategy to activate such sensitisers in the phototherapeutic window using TPE.10
In this communication, we report a novel nanoconstruct in which carboxylic acid-terminated CQDs 1 were covalently linked to the nitroaniline derivative NO photodonor 211 to give the nanohybrid 3 (Scheme 1). We demonstrate that photoinduced energy transfer occurs from the CQDs core to the NO photodonor shell, encouraging the NO release. This process can be exploited to overcome the main limitation of the NO photocage, whose absorption is confined below 450 nm. In fact, the high TPE cross section of the CQDs allows the indirect excitation of the NO photodonor by NIR light at 800 nm. The photoinduced toxicity of this nanomedicine upon TPE is evaluated both in vitro and in vivo towards a human tumour cell line and human pancreatic xenograft BxPc-3 tumours respectively, which are known to display hypoxic characteristics.12
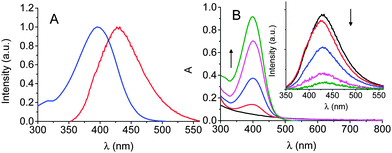
As shown in Fig. 1A, the emission spectrum of the CQDs 1 has considerable overlap with absorption spectrum of the NO photodonor 2 making, in principle, a Förster Resonance Energy Transfer (FRET) between these two components in the conjugate 3 feasible.
2 was then loaded onto the surface of the hydrophilic 1 at concentrations of 30, 50, 100 and 600 μM (with respect to 2) using carbodiimide based coupling (see ESI†) to give the respective CQD–NO photoreleaser conjugates 3a–d. They were freely dispersible in aqueous buffer and exhibited the typical spectral features of the NO photodonor with an absorption maximum at ca. 400 nm, in contrast to the carboxylated CQDs 1 which shows an unstructured absorption (Fig. 1B). The increasing absorbance values observed for the four samples are the result of the increasing number of chromophores on the CQDs surface, i.e. from 3a to 3d. Accordingly, the hydrodynamic diameter of these nanohybrids also increased, being ca. 11, 13, 16 and 18 nm, respectively (Fig. S1, ESI†). The unaltered position of the absorption maximum and the very same spectral profiles with the increasing loading of the NO photodonor suggest the absence of any relevant intra- and inter-chromophoric interactions in the conjugated CQDs. Furthermore, no changes in these spectroscopic properties or evidence of aggregation were observed over a two-month period.
The fluorescence emission spectra of the CQDs significantly decreased with the increased loading of 2 on their surface (inset Fig. 1B).
Neither a trivial energy transfer mechanism nor a filtering effect is responsible for such a quenching. In fact, (i) the samples were diluted 5-fold in order to avoid any re-absorption of the emitted light and (ii) the fluorescence spectra were corrected by the different fraction of absorbed photon by the CQDs at the excitation wavelength. Besides, no fluorescence quenching was observed when 1 and 2 were mixed together at the same concentrations used in the CQDs–NO photodonor conjugates. Therefore, the quenching observed is most likely the result of an energy transfer process from the excited CQDs core to the NO photodonor shell in which a FRET mechanism may play a key role. Increasing the number of energy acceptors on a single energy donor is known to increase the effective overlap integral and thus the efficiency of the energy transfer process.13 Due to the non-emissive properties of the NO photodonor it was not possible to follow a complementary activation of the acceptor fluorescence. However, the NO photorelease experiments reported confirm energy transfer as a mechanism involved in fluorescence quenching, ruling out the involvement of any potential photoinduced electron transfer (PET) between the core and the shell of the CQDs–NO photodonor conjugates (vide infra).
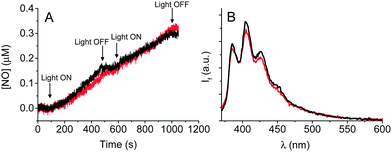
The most convenient methodology to demonstrate NO generation is the direct and real-time monitoring of this radical species. To this end, we used an ultrasensitive NO electrode, which directly detects NO concentration by an amperometric technique. Fig. 2A shows the results of two experiments carried out by exciting at λexc = 350 nm the CQD conjugate 3b‡ and, for comparison, an optically matched solution of the free NO photodonor 2. This single photon wavelength was chosen as it was not possible to configure the two-photon source for use in this experiment. In both experiments, we observed linear photogeneration of NO, which promptly stopped when the light was turned off and restarted as the illumination was turned on again, obtaining unambiguous evidence for the exclusively light-controlled generation of NO. Interestingly, the rate of NO photorelease was very similar for the two samples although in the case of the 3bca. 50% of the light was absorbed by the CQDs core. These results were not affected by the excitation wavelength, as confirmed by the photoamperograms recorded at λexc = 405 nm (Fig. S2, ESI†). These data were also supported by means of the sensitive and selective chemical method for NO detection based on the reaction between the poorly fluorescent 2,3-diaminonaphthalene (DAN) with N2O3 (formed by reaction of NO with O2) to give the highly fluorescent 2,3-naphthotriazole (ESI†). As shown in Fig. 2B, a comparable fluorescence intensity was observed for 3b and 2 after irradiation.
Collectively, these results provide additional evidence for the occurrence of an energy transfer process between the CQDs and the NO photodonor components in the nanohybrid which are in very good agreement with the fluorescence quenching results discussed before. Furthermore, no significant changes in the size of the CQDs nanohybrid were noted after the NO photorelease experiments, indicating the sole release of NO with no other change to the nanohybrid surface.
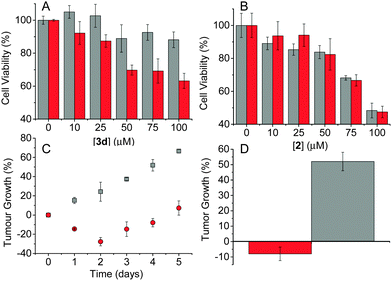
To determine the ability of these nanohybrids to produce a cytotoxic effect in cancer cells upon irradiation in the therapeutic window, HeLa cells were incubated with 3d and, for comparison, with 2 alone, at different concentrations for 24 h. Afterwards the samples were either kept in the dark or irradiated with two-photon light at 800 nm for 30 s. Cell cytotoxicity was determined using the MTT assay 24 h after the completion of the irradiation. Fig. 3A reveals a concentration-dependent reduction in viability for those cells treated with 3d and TPE compared to those that were non-irradiated. In contrast, cells treated with 2 alone showed no noticeable difference in viability with or without light exposure (Fig. 3B). These results suggest the CQDs are mandatory to absorb TPE and trigger cytotoxicity through the generation of NO. Furthermore, 3d showed significantly less dark toxicity than 2, a feature we have also observed when other photosensitisers were attached to CQDs.10
To determine the therapeutic efficiency of 3din vivo, human pancreatic BxPC-3 tumours were induced in Balb/c SCID mice. These tumours are relatively hypoxic, exhibiting a pO2 of 2 mmHg. Once the tumours had reached 100 mm3, the mice received a 50 μL aliquot of 3dvia intra-tumoural injection. The tumours were then treated with TPE at 800 nm for three 3 min periods with a 1 min lag between each treatment. The results shown in Fig. 3C reveal a significant reduction in tumour size only for those animals that received both 3d and TPE compared to those that were treated with 3d and kept in the dark. Indeed, 4 days post treatment, tumours on animals which had received the conjugate and exposure to TPE were found to be 8% smaller than the original pre-treatment size while the control group grew by 52% over the same period (Fig. 3D).
In conclusion, we have demonstrated that photoinduced energy transfer occurs from CQDs and a NO photoreleaser covalently linked at their surface. The high two-photon absorption cross section of the CQDs core can be utilised to efficiently absorb two-photons in the therapeutic NIR region and transfer this excitation energy to the NO photodonor. The CQDs–NO photoreleaser conjugates were quite biocompatible in the dark and were able to exert cytotoxic effects on HeLa cells in vitro and effectively reduce tumour volume in mice bearing hypoxic BxPC-3 tumours upon 800 nm TPE. This offers the potential of activating these nanohybrids more deeply within human tissue expanding their potential biomedical use. Furthermore, anchoring the NO photoreleaser to the CQDs can also take advantage of the enhanced permeation and retention (EPR) effect characteristic of nanoparticles as they are known to preferentially accumulate in tumour tissue relative to healthy tissue improving the passive targeting of these conjugates to solid tumours.
In a previous communication, we demonstrated the capability of CQDs to indirectly excite the 1O2 sensitizer protoporphyrin IX attached to their surface by TPE.10 In addition, we have also proved the amplified therapeutic effects based on the simultaneous one-photon generation of 1O2 and NO by porphyrinoid sensitizers combined with the NO photoreleaser used herein, in many nanoconstructs.14 On this basis and given the results presented herein, fabrication of hybrid CQDs integrating both the porphyrin and NO photodonor units at their surface should benefit from bimodal photoaction triggered by TPE in the biocompatible NIR region, an area we hope to report on soon.
We thank Norbrook Laboratories Ltd, AIRC (IG-12834) and the MIUR (PRIN 2011) for financial support.
Notes and references
-
Nitric Oxide: Biology and Pathobiology, ed. L. J. Ignarro, Elsevier Inc., 2010 Search PubMed
.
- A. W. Carpenter and M. H. Schoenfisch, Chem. Soc. Rev., 2012, 41, 3731 RSC
.
- D. A. Riccio and M. H. Schoenfisch, Chem. Soc. Rev., 2012, 41, 3742 RSC
.
-
(a) S. Sortino, Chem. Soc. Rev., 2010, 39, 2903 RSC
; (b) P. C. Ford, Nitric Oxide, 2013, 34, 56 CrossRef CAS PubMed
; (c) P. C. Ford, Acc. Chem. Res., 2008, 41, 190 CrossRef CAS PubMed
; (d) N. L. Fry and P. K. Mascharak, Acc. Chem. Res., 2011, 44, 289 CrossRef CAS PubMed
.
- S. Sortino, J. Mater. Chem., 2012, 22, 301 RSC
.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535 CrossRef CAS PubMed
.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710 CrossRef CAS PubMed
.
-
(a) H. Nakagawa, K. Hishikawa, K. Eto, N. Ieda, T. Namikawa, K. Kamada, T. Suzuki, N. Miyata and J. Nabekura, ACS Chem. Biol., 2013, 8, 2493 CrossRef CAS PubMed
; (b) K. Hishikawa, H. Nakagawa, T. F. K. Fukuhara, H. Tsumoto, T. Suzuki and N. Miyata, J. Am. Chem. Soc., 2009, 131, 7488 CrossRef CAS PubMed
; (c) V. Kirejev, N. Kandoth, R. Gref, M. B. Ericson and S. Sortino, J. Mater. Chem. B, 2014, 2, 1190 RSC
.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS PubMed
.
- C. Fowley, N. Nomikou, A. P. McHale, B. McCaughan and J. F. Callan, Chem. Commun., 2013, 49, 8934 RSC
.
-
(a)
S. Conoci, S. Petralia and S. Sortino, EP2051935A1/US20090191284, 2006
; (b) E. B. Caruso, S. Petralia, S. Conoci, S. Giuffrida and S. Sortino, J. Am. Chem. Soc., 2007, 129, 480 CrossRef CAS PubMed
; (c) F. L. Callari and S. Sortino, Chem. Commun., 2008, 1971 RSC
.
- A. S. Lalani, S. E. Alters, A. Wong, M. R. Albertella, J. L. Cleland and W. D. Henner, Clin. Cancer Res., 2007, 13, 2216 CrossRef CAS PubMed
.
- I. L. Medintz and H. Mattoussi, Phys. Chem. Chem. Phys., 2009, 11, 17 RSC
.
-
(a) A. Fraix, N. Kandoth, I. Manet, V. Cardile, A. C. E. Graziano, R. Gref and S. Sortino, Chem. Commun., 2013, 49, 4459 RSC
; (b) N. Kandoth, V. Kirejev, S. Monti, R. Gref, M. B. Ericson and S. Sortino, Biomacromolecules, 2014, 15, 1768 CrossRef CAS PubMed
; (c) N. Kandoth, E. Vittorino, M. T. Sciortino, I. Colao, A. Mazzaglia and S. Sortino, Chem. – Eur. J., 2012, 18, 1684 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic and experimental procedures. See DOI: 10.1039/c4cc07827f |
| ‡ We chose the CQDs–NO photodonor conjugate 3b for these experiments because in this case, the excitation light is significantly absorbed not only by the NO photodonor shell but also from the CQDs core. This enables confirmation that the CQDs absorption triggers NO release. |
| This journal is © The Royal Society of Chemistry 2015 |