Supramolecular chirality in peptide microcrystals†
Dmitry
Kurouski
a,
Joseph D.
Handen
a,
Rina K.
Dukor
b,
Laurence A.
Nafie
bc and
Igor K.
Lednev
*a
aDepartment of Chemistry, University at Albany, 1400 Washington Ave., Albany, USA. E-mail: ilednev@albany.edu; Fax: +1 518 442 3462; Tel: +1 518 591 8863
bBioTools, Inc., 17546 Beeline Hwy, Jupiter, FL, USA. E-mail: rkdukor@aol.com; Fax: +1 561 625 0717; Tel: +1 561 625 0133
cDepartment of Chemistry, Syracuse University, 1-014 CST, Syracuse, NY, USA. E-mail: lnafie@syr.edu; Tel: +1 315 443 2925
First published on 20th October 2014
Abstract
The vibrational circular dichroism (VCD) spectra of microcrystals of fibril-forming peptides have been measured for the first time. VCD spectra were measured and compared for microcrystals and fibrils formed from the same peptide, human islet amyloid polypeptide (IAPP, amylin). Structural information related to the supramolecular chirality of both the microcrystals and the fibrils, as well as the VCD enhancement mechanisms in fibrils and microcrystals, is obtained from these spectral comparisons. It is concluded that strongly enhanced VCD does not require braiding of two or more filaments that is permitted in fibrils but not microcrystals.
Direct structural studies of amyloid fibrils, protein aggregates that have a cross β-core structure, are complicated by their non-crystalline and highly aggregated nature. Eisenberg and co-authors have proposed that short peptide sequences that form amyloid-like fibrils and microcrystals may be good models of full-length protein fibrillar cores. This approach involves several steps: first, putative fibril forming short peptide segments are identified within various proteins using experimental evidence1 together with 3D profile molecular modelling.2 The segments obtained by modelling are subsequently screened for crystallization. Finally, the atomic structures of the microcrystals are obtained by X-ray diffraction.3
This approach is attractive because the elucidation of the structure of microcrystals may shed light on the structural organization of the full-length protein fibrils. It is additionally important since the resolution of the fibril structure on the atomic level allows designing peptide optomers that bind to the fibrils and prevent their propagation.4 Several experimental studies demonstrate that microcrystals are able to seed fibrils, and frequently the two structures form simultaneously.5,6 Previously we have demonstrated that full-length protein fibrils exhibit supramolecular chirality by means of unusually intense vibrational circular dichroism (VCD).7 Further, it has been shown that this sensitivity of VCD to supramolecular chirality originates at a deeper level of fibril organization that occurs at the fibril filament level rather than from any apparent fibril handedness observed in AFM or SEM images.8–10 Therefore, if polypeptide microcrystals mimic structural organization of full-length protein fibrils, one can expect that they also ought to exhibit a distinct supramolecular chirality. A test of this hypothesis is the main objective of this work.
In a recent study, Dryzun et al. describe the general lack of assignments of chirality to many crystals.11 This leads to an interesting question of whether we should expect these microcrystals to belong to a chiral or achiral space group. Avnir offers a simple rule: If the space group contains only proper symmetry operations, the crystal is chiral.11 Eisenberg's group reported space groups of P212121 and C2 for crystals of peptides NNFGAIL and SSTNVG, respectively.6 The space groups of these two peptide microcrystals contain only proper operations, so it is expected that they will be chiral.
Vibrational circular dichroism (VCD) is a powerful spectroscopic technique,12 which has been shown to have enhanced sensitivity to amyloid-like fibril motifs.7–10,12,13 In these publications it is shown that the observed VCD intensities from fully developed fibrils are one to two orders of magnitude larger than VCD intensities observed from solutions of non-fibrillar proteins. Furthermore, it has been unambiguously demonstrated that these enhanced VCD intensities arise from the long-range supramolecular chirality of fibril structure. This sensitivity makes VCD a unique solution-phase, stereo-specific probe of protein fibril structure, development and morphology. It can be argued from basic theoretical concepts that enhanced VCD spectra of this magnitude can originate only from supramolecular aggregates that have a long-distance chiral organization.9
We report here the use of VCD to investigate the supramolecular chirality of peptide microcrystals grown from two segments of human islet amyloid polypeptide (IAPP, amylin) with sequences NNFGAIL and SSTNVG. These spectra were then compared to VCD spectra of fibrils grown from the same peptides under the same buffered solution conditions with which their corresponding crystals were grown. Details of the conditions are reported in the ESI.† Briefly, crystals were grown using a hanging drop vapour diffusion setup, while the corresponding fibrils were formed in bulk solution in microcentrifuge tubes. Both microcrystals and fibrils were measured dispersed in their respective solution of origin.
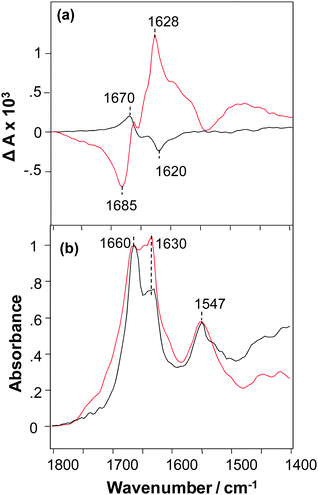
The VCD spectrum of NNFGAIL peptide microcrystals exhibits a strong signal with ΔA in the range +2 to −2 × 10−4 for a maximum solvent-corrected IR absorbance of unity (A = 1) at 1660 cm−1 and has a normal-sense VCD sign pattern (Fig. 1a).9 An intense VCD was previously observed for fibrils prepared from various proteins and polypeptides,13 polyglutamine (polyQ, Qn for 18 < n < 45),14 and AKY8 peptide fibrils.15 This indicates that peptide microcrystals have a level of supramolecular chirality similar to that previously observed for protein fibrils. Unexpectedly, we found that fibrils formed from the NNFGAIL peptide exhibit VCD intensities approximately 3 times more intense, and with the opposite chirality (reversed VCD), compared to the VCD of NNFGAIL microcrystals as illustrated in Fig. 1a.
NNFGAIL microcrystals and fibrils exhibit IR spectra with the nearly the same peak frequencies and some relative intensity differences (Fig. 1b) with strong bands at ∼1630 and 1660 cm−1, indicative of β-sheet structure.16,17 The intensities of IR bands at 1630 and 1660 cm−1 are similar for NNFGAIL microcrystals and fibrils, although the band at 1660 cm−1 is noticeably more intense compared to the one at 1630 cm−1 in the IR spectrum of microcrystals. Since peptide crystallization is a relatively slow process compared to its fibrillation, it could be expected that the amount of free, solution-state, unordered NNFGAIL peptide might be higher in the microcrystal solution. Note that any free NNFGAIL peptides in the solution containing microcrystals will add to the IR spectrum but have negligibly small effect on the much more intense VCD intensity of the dispersed microcrystals. At the same time the 1660 cm−1 band may be associated with nonordered or loop secondary structure of a fully formed cross-β-core of both fibrils and crystals.13 Additional experiments are required to elucidate the origin of this band, which is beyond the scope of the current work.
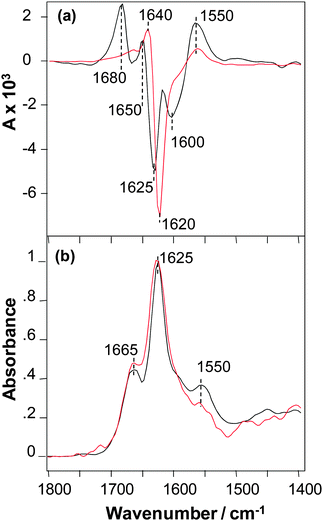
The VCD spectra of SSTNVG microcrystals and fibrils are shown in Fig. 2a. The VCD intensities for this peptide have similar very intense VCD that are both roughly a factor of ∼4 larger than the intense VCD of NNFGAIL fibrils shown in (Fig. 1a), and hence roughly an order of magnitude times larger than the VCD intensity of NNFGAIL microcrystals. In addition, the VCD sign patterns for microcrystals and fibrils of SSTNVG are very similar that is again in contrast to VCD of NNFGAIL microcrystals and fibrils (Fig. 1a). Furthermore, the VCD spectrum of SSTNVG microcrystals shows additional major band maxima relative to the fibril VCD. In particular, there is enhanced positive VCD on the high and low frequency ends of the spectrum at 1680 and 1550 cm−1, followed by three relative sign changes between the two VCD spectra at 1650, 1625 and 1600 cm−1. These sign changes can arise from either band frequency shifts or band splittings in going from the fibril to the microcrystal VCD spectrum. Alternatively, some of the additional features in the microcrystal VCD may arise from enhancements of band features that are much weaker in the fibril VCD. Regardless of the origin of the additional complexity of the microcrystal VCD, a likely source is the additional structural order of the three-dimensional microcrystal peptide environment relative to the one or two-dimensional long range order of a fibril. Additional spatial order can give rise to regular close proximity of carbonyl groups18 on neighbouring peptides, such as adjacent peptide end groups, present in the microcrystal but either not present or orientally averaged in the fibril, and these interactions can give rise to splittings or frequency shifts in the VCD. It is likely that these additional intense VCD features imply a high supramolecular chiral organization of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the C-terminus carbonyls that is in addition to chiral organization of the main-chain amide chromophores. VCD spectra of SSTNVG microcrystals and fibrils have a similar overall pattern including a positive peak near 1550 cm−1 in the amide II region and a positive–negative couplet centred in the region 1650–1625 cm−1 of the amide I mode, which indicate a similar supramolecular organization of amide chromophores. A small difference in the amide I VCD peak position found for the microcrystals and fibrils could be due to the overlap with the terminal carbonyl couplet described above.
O groups of the C-terminus carbonyls that is in addition to chiral organization of the main-chain amide chromophores. VCD spectra of SSTNVG microcrystals and fibrils have a similar overall pattern including a positive peak near 1550 cm−1 in the amide II region and a positive–negative couplet centred in the region 1650–1625 cm−1 of the amide I mode, which indicate a similar supramolecular organization of amide chromophores. A small difference in the amide I VCD peak position found for the microcrystals and fibrils could be due to the overlap with the terminal carbonyl couplet described above.
SSNTVG microcrystals exhibit a high level of VCD intensity previously observed only for mature protein and some peptide fibrils.13,15 It is worth noting here that SSNTVG microcrystals cannot braid and hence do not have a multifilament structure, in contrast to mature fibrils. This suggests that the twisting of two or more filaments into a braided fibril structure is not required for developing a large VCD signal, but rather the degree of twist of individual filaments along the fibril axis most likely is the critical factor for the magnitude of VCD enhancement.
Aside from some small relative intensity differences of bands in the amide I region, the IR spectra of SSTNVG microcrystals and fibrils are nearly the same as shown in Fig. 2b. Since all fibrils are known to contain some degree of cross-β-core structure, the similarity of the VCD implies that SSTNVG microcrystals also contain this same cross-β-core structure. The IR data also demonstrates that there are differences in the overall structural organization of NNFGAIL and SSNTVG fibrils, as previously reported by Wiltzius et al.6 Note that according to the crystallographic data both of these peptides have parallel β-sheet structure, which was proposed to be a pre-requisite for VCD enhancement.15
In the case of both NNFGAIL and SSTNVG microcrystals, there is no evidence of a significant contribution of fibrils to the VCD spectra. The main reason is that the AFM images of SSTNVG fibrils and microcrystals (Fig. S4, ESI†), and NNFGAIL fibrils and microcrystals (data not shown), each show evidence of a single species (only crystals or fibrils). Secondly, there is no spectral evidence of fibril VCD in the VCD of the microcrystals which therefore puts a lower limit on the amount of fibrils that might be present in the microcrystal samples.
Peptides NNFGAIL and SSNTVG, when combined into the IAPP37-residue peptide (amylin) as NNFGAIL–SSNTVG, form a β-sheet zipper-like fibril with SSNTVG comprising the inner chiral axis.13 According to the β-sheet zipper model, the inner chiral axis has a left-handed twist.6 Previously we have reported that all protein fibrils that exhibit VCD sign pattern similar to SSNTVG (++−++) have a left-handed twist,13 which is in agreement with the proposed 3D structure.6 It is likely that the fibril formed from the smaller SSNTVG peptide shares the same chiral axis structure, since when two such peptides interact back-to-back they do so in a self-complimentary manner with respect to the interlocking hydrophobic side chains of their β-sheet structure.6,9 Because the VCD spectra of SSNTVG microcrystals and fibrils exhibit nearly the same major features in terms of signs and intensities, one can conclude that the fibril core structure must be close to the microcrystal structure in agreement with Eisenberg's hypothesis. The huge VCD intensity seen for SSNTVG microcrystals and fibrils indicates strongly that the origin of the VCD is supramolecular in its chiral extent involving vibrational coupling well beyond the limits of, for example, a single, crystal unit cell. On the other hand, NNFGAIL microcrystals show much smaller (∼15 times smaller) enhanced VCD than SSNTVG microcrystals, and therefore have a lower level supramolecular chirality associated with the crystal structure. This peptide is not associated with fibril core of the combined NNFGAIL–SSNTVG peptide but rather has a place as a flanking next layer in the model fibril structure.9 The VCD spectra presented here clearly show that helical sense of twist of the proto-filaments comprising the fibrils NNFGAIL and SSNTVG are opposite. In addition, we note that even though the VCD spectra of microcrystals and fibrils of NNFGAIL have a significant difference in intensity scale, and with opposite signs, the shape of the VCD spectra, are nearly the same. For VCD spectra that are so sensitive to stereo-structural features of molecules, nearly identical VCD spectra (aside from magnitude and opposite signs), strongly implies that they share the same but mirror-image local stereo structure. In the simplest case this can be explained by two structures that are the same aside from a direction and degree of twist between monomeric units of structure, in this case a single β-sheet strand running perpendicular to the fibril axis for the fibril and the long range twist of the unit cells in the microcrystal.15
The similarity of the relative intensities and frequencies of the IR and VCD (aside from sign and magnitude) of NNFGAIL and SSNTVG strongly implies that the strongest interactions between peptides (parallel in this case) in the fibril and crystal structures are those within the plane of individual β-sheets. For fibrils, the next most important interaction is between upper and lower sheets in the cross-β-core, canonically separated by 10 Angstroms. For crystals, there are not only interactions between sheets but also end-end interactions between peptides. Previous studies19,20 have considered the effects of peptide interactions between β-sheets, which have predicted effects on amide I frequencies, but these considerations appear to be secondary to the primarily focus on intra-sheet interactions defining a single β-sheet and the long-range twist of such sheets in fibrils and microcrystals.
While the degree of twist within a unit cell or twists about an axis over larger distances in fibrils may be the most important measure in understanding the size of the observed VCD, there may be additional factors of similar or even greater importance. One such factor is the degree of lateral association perpendicular to the helical growth axis of a fibril. In particular, associations of fibril filaments, two or more, to make braided, multi-filament fibrils or mature fibrils, is one way in which increases in the lateral association of chiral filament structures may result in an increase in VCD intensity without an increase in IR intensity.
A central question arising from the experimental results presented in this paper is the source of the VCD intensity enhancements observed for the peptide microcrystals of SSTNVG and NNFGAIL, the former significantly greater than the latter. One can hypothesize that it can be caused by a slight misalignment of the unit cells within the crystal, a phenomenon known as mosaicity. In a recent extensive review, Shtukenberg, Kahr et al. describe the bending and twisting of single crystals as a relatively common, normally overlooked, phenomena wherein the coordinates of a single unit cell obtained by standard X-ray crystallography contain no information regarding long-range chiral spatial order of the whole crystal.21 Commonly protein and polypeptide crystals contain a variety of local defects, dislocations, and disclinations that may change the long-range order of crystal lattices, causing formation of twists and bend points.21 For protein and polypeptide crystals, such a degree of long-range deviation of the unit cells from perfect rectilinear alignment generally ranges from 0.25 to 0.50 degrees.22 Previously, the presence of such twists was reported for silk fibroin23 and deoxyhemoglobin S crystals.24 Such a long-range twisting of otherwise rectilinear unit cells in the microcrystals of NNFGAIL and SSTVG is most likely the source of unusual VCD enhancement that mimics that observed in the corresponding non-crystalline fibrils of these same two peptides. As a result, a thorough understanding of VCD enhancements in these peptide microcrystals may be reached either by additional measurements under different conditions with the same or different peptides where correlations to known crystal structures, as presented here, can be made. Another path to a more complete level of understanding of giant VCD intensities is ab initio density functional theory quantum mechanical calculations on realistic structures of these peptides or suitably simplified model structures.
By use of VCD we demonstrate that microcrystals formed from short peptide segments of human IAPP indeed have supramolecular chirality, as expected from the theory. NNFGAIL fibrils show an order of magnitude larger VCD enhancement and opposite sign compared to the VCD of microcrystals grown from the same peptide. In contrast, microcrystals and fibrils grown from the SSTNVG peptide segment both show similar huge VCD intensity and nearly the same VCD patterns, though the microcrystals crystals show more detailed structural organization. In essence, in the case of SSTNVG, the microcrystal appears to be simply a crystal of the fibril with the same long-range filament supramolecular chirality and giant VCD.
This work was supported by National Institute on Aging, National Institutes of Health, Grant R01AG033719 (I.K.L.) and the National Science Foundation SBIR Phase II Grant IIP-0945484 (R.K.D and L.A.N.). We are grateful to Ludmila Popova for providing AFM images.
Notes and references
- M. Balbirnie, R. Grothe and D. S. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2375 CrossRef CAS PubMed; M. I. Ivanova, M. R. Sawaya, M. Gingery, A. Attinger and D. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 10584 CrossRef PubMed; M. I. Ivanova, M. J. Thompson and D. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4079 CrossRef PubMed.
- M. J. Thompson, S. A. Sievers, J. Karanicolas, M. I. Ivanova, D. Baker and D. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4074 CrossRef CAS PubMed.
- M. R. Sawaya, S. Sambashivan, R. Nelson, M. I. Ivanova, S. A. Sievers, M. I. Apostol, M. J. Thompson, M. Balbirnie, J. J. W. Wiltzius, H. T. McFarlane, A. Ø. Madsen, C. Riekel and D. Eisenberg, Nature, 2007, 447, 453 CrossRef CAS PubMed.
- S. A. Sievers, J. Karanicolas, H. W. Chang, A. Zhao, L. Jiang, O. Zirafi, J. T. Stevens, J. Munch, D. Baker and D. Eisenberg, Nature, 2011, 475, 96 CrossRef CAS PubMed.
- N. Rubin, E. Perugia, S. G. Wolf, E. Klein, M. Fridkin and L. Addadi, J. Am. Chem. Soc., 2010, 132, 4242 CrossRef CAS PubMed; M. I. Ivanova, S. A. Sievers, M. R. Sawaya, J. S. Wall and D. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18990 CrossRef PubMed.
- J. J. W. Wiltzius, S. A. Sievers, M. R. Sawaya, D. Cascio, D. Popov, C. Riekel and D. Eisenberg, Protein Sci., 2008, 17, 1467 CrossRef CAS PubMed.
- S. Ma, X. Cao, M. Mak, A. Sadik, C. Walkner, T. B. Freedman, I. K. Lednev, R. K. Dukor and L. A. Nafie, J. Am. Chem. Soc., 2007, 129, 12364 CrossRef CAS PubMed.
- D. Kurouski, R. A. Lombardi, R. K. Dukor, I. K. Lednev and L. A. Nafie, Chem. Commun., 2010, 46, 7154 RSC.
- D. Kurouski, R. Dukor, X. Lu, L. A. Nafie and I. K. Lednev, Biophys. J., 2012, 103, 522 CrossRef CAS PubMed.
- D. Kurouski, R. K. Dukor, X. Lu, L. A. Nafie and I. K. Lednev, Chem. Commun., 2012, 48, 2837 RSC.
- C. Dryzun and D. Avnir, Chem. Commun., 2012, 48, 5874 RSC.
- L. A. Nafie, Vibrational Optical Activity: Principles and Applications, Wiley, Chichester, 2011 Search PubMed.
- D. Kurouski, X. Lu, L. Popova, W. Wan, M. Shanmugasundaram, G. Stubbs, R. Dukor, I. K. Lednev and L. A. Nafie, J. Am. Chem. Soc., 2014, 136, 2302 CrossRef CAS PubMed.
- D. Kurouski, K. Kar, R. Wetzel, R. K. Dukor, I. K. Lednev and L. A. Nafie, FEBS Lett., 2013, 587, 1638 CrossRef CAS PubMed.
- T. J. Measey and R. Schweitzer-Stenner, J. Am. Chem. Soc., 2010, 133, 1066 CrossRef PubMed.
- S. Krimm, J. Mol. Biol., 1962, 4, 528 CrossRef CAS.
- J. Kong and S. Yu, Acta Biochim. Biophys. Sin., 2007, 39, 549 CrossRef CAS PubMed.
- S. Stewart and P. M. Fredericks, Spectrochim. Acta, Part A, 1999, 55, 1615 CrossRef.
- C. Lee and M. Cho, J. Phys. Chem. B, 2004, 108, 20397 CrossRef CAS.
- R. Schweitzer-Stenner, J. Phys. Chem. B, 2012, 116, 4141 CrossRef CAS PubMed.
- A. G. Shtukenberg, Y. O. Punin, A. Gujral and B. Kahr, Angew. Chem., Int. Ed., 2014, 53, 672 CrossRef CAS PubMed.
- H. D. Bellamy, E. H. Snell, J. Lovelace, M. Pokross and G. E. Borgstahl, Acta Crystallogr., 2000, 56, 986 CrossRef CAS PubMed.
- B. Lotz, A. Gonthier-Vassal, A. Brack and J. Magoshi, J. Mol. Biol., 1982, 156, 345 CrossRef CAS.
- T. E. Wellems and R. Josephs, J. Mol. Biol., 1980, 137, 443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig S1–S4 and details of the sample preparation and experimental conditions provided. See DOI: 10.1039/c4cc05002a |
| This journal is © The Royal Society of Chemistry 2015 |