Hyperbranched aromatic poly(ether ketone) functionalized with TEMPO as a heterogeneous catalyst for aerobic oxidation of alcohols
Ying Shi,
Yuta Nabae*,
Teruaki Hayakawa and
Masa-aki Kakimoto
Department of Organic and Polymeric Materials, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 S8-26 Ookayama, Meguro-ku, Tokyo 152-8552, Japan. E-mail: nabae.y.aa@m.titech.ac.jp; Tel: +81-3-5734-2429
First published on 28th November 2014
Abstract
A hyperbranched aromatic poly(ether ketone) functionalized with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO/HBPEK) was investigated as a novel heterogeneous catalyst for aerobic oxidation of alcohols. The TEMPO/HBPEK contained 2 mmol g−1 TEMPO and had a turnover number (TON) of 46 compared to commercial TEMPO with a TON of 60, indicating good catalytic activity and selectivity for the aerobic oxidation of benzyl alcohol. This material can be used as a recyclable heterogeneous catalyst by grafting it onto insoluble supports such as carbon black (TEMPO/HBPEK/CB) or polyimide nanoparticles (TEMPO/HBPEK/PI), whereas the bare TEMPO/HBPEK acts as a homogeneous catalyst. These heterogeneous catalysts had TONs of 27 and 22, respectively, indicating greater catalytic activity than the commercially available heterogeneous catalyst, TEMPO immobilized on polystyrene, which had a TON less than 1.
1. Introduction
The oxidation of primary alcohols to aldehydes is an important basic procedure for the synthesis of chemicals and intermediates.1,2 The aerobic oxidation of alcohols is of interest because of its potential for environmentally friendly (“green”) chemistry, because the reaction consumes pure oxygen or air as the oxidant and produces only water as the byproduct.3 For this purpose, a nitroxyl radical with low toxicity and reversible redox behavior, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), has been studied for its catalytic ability.4,5 Several studies utilized catalytic systems involving TEMPO and salts of transition metals such as Cu6,7 and Fe;8 these systems possessed good catalytic performance under mild reaction conditions. In addition, Hu and coworkers9 reported the transition-metal-free system, TEMPO/Br2/NaNO2 and derivatives, as environmentally friendly. In this system, TEMPO oxidizes the alcohol, and the reduced form of TEMPO is re-oxidized by O2 via intermediation of the Br2/Br− and NO2/NO redox couples.10,11 However, this system has the disadvantages of Br-containing byproducts and contamination from the NOx species.12 A nitric acid-assisted carbon-catalyzed oxidation system (NACOS) using carbon-based materials as metal-free catalysts has also been reported13 and it was demonstrated that TEMPO enhanced the oxidation.14 This oxidation protocol did not involve halogen-containing byproducts and resulted in good yield and selectivity for various alcohols under solvent-free or minimal-solvent conditions.The high cost of TEMPO and the purity of the products necessitate the development of heterogeneous TEMPO-based catalysts. A typical strategy involved immobilization of TEMPO onto a support such as mesoporous silica,15,16 silica sol–gels,17,18 cross-linked polystyrene resins,19,20 or polyolefin fibers.21 The supported TEMPO can be collected easily after each cycle by filtration. However, most of these immobilized TEMPO systems possess low catalytic activity or poor chemical stability of the polymer backbone under severe oxidative conditions.
Therefore, chemically and thermally stable polymers, such as poly(ether ketone), have been investigated as backbones to immobilize the TEMPO-based terminals. In addition, hyperbranched structures have the potential to maximize the catalytic activity of the TEMPO-based terminals. Since hyperbranched structures have a low degree of entanglement, the end-groups are exposed and sufficiently accessible for reactants. The catalytic performance of sulfonic acid terminals on hyperbranched poly(ether sulfone)22 and carboxylic acid terminals on hyperbranched poly(ether ketone)23 has been demonstrated.
This reports describes a novel catalyst, hyperbranched poly(ether ketone) functionalized with TEMPO (TEMPO/HBPEK), for the aerobic oxidation of benzyl alcohol. Since HBPEK is fairly soluble under the reaction conditions, immobilization of TEMPO/HBPEK on carbon black (TEMPO/HBPEK/CB) or polyimide particles (TEMPO/HBPEK/PI) has also been studied in order to utilize this polymer as a heterogeneous catalyst.
2. Experimental
2.1. Materials synthesis
The synthetic routes to TEMPO/HBPEK, TEMPO/HBPEK/CB, and TEMPO/HBPEK/PI are shown in Scheme 1. Preparation of a symmetrical AB2 monomer, 4,4′-(m-phenylenedioxy)-bis(benzenecarboxylic acid),24,25 and its polycondensation to form HBPEK23 have been reported previously; this procedure was used with slight modifications.To immobilize TEMPO on the HBPEK, the HBPEK (350 mg, 1.0 mmol) was mixed with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (186 mg, 1.2 mmol) in 5 mL dichloromethane and stirred for 30 min. The 4-amino-TEMPO (188 mg, 1.1 mmol) and 4-dimethylaminopyridine (DMAP) (13 mg, 0.1 mmol) were dissolved in 3 mL dichloromethane and then added to the previous mixture, followed by stirring at 40 °C for 24 h. After reaction, the solvent was removed by evaporation and the product washed with water and acetone.
To immobilize HBPEK on carbon black, HBPEK (300 mg) was dissolved in 20 mL DMF in the presence of Ketjen Black (EC600JD, 60 mg) and PPMA26 (10 mL), stirred under N2 at 110 °C for 24 h, and then filtered. The product was stirred in DMF at 100 °C for 24 h, and then filtered. This washing process was repeated to obtain HBPEK/CB.
To immobilize TEMPO on HBPEK/CB, the HBPEK/CB (777 mg, corresponding to 1.0 mmol of terminal COOH groups) was mixed with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (186 mg, 1.2 mmol) in 5 mL dichloromethane, followed by stirring for 30 min. The 4-amino-TEMPO (188 mg, 1.1 mmol) and 4-dimethylaminopyridine (DMAP) (13 mg, 0.1 mmol) were dissolved in 3 mL dichloromethane and then added to the previous mixture. After stirring at 40 °C for 24 h, the solvent was removed by evaporation and the product washed with water and acetone.
Polyimide particles were synthesized by the precipitation polymerization method.27 A solution of PMDA in acetone was added to a solution of ODA in acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molecular ratio of PMDA
1 molecular ratio of PMDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODA) all at once under vigorous stirring at 0 °C. After 2 h, the solvent was evaporated and the product heated in a vacuum at 220 °C overnight. To immobilize HBPEK on polyimide particles, HBPEK (300 mg), polyimide particles (60 mg), and PPMA (10 mL) were mixed with nitrobenzene (20 mL) and heated at 110 °C under N2 for 24 h, followed by reflux in THF for 24 h twice to remove polymer that was not immobilized.
ODA) all at once under vigorous stirring at 0 °C. After 2 h, the solvent was evaporated and the product heated in a vacuum at 220 °C overnight. To immobilize HBPEK on polyimide particles, HBPEK (300 mg), polyimide particles (60 mg), and PPMA (10 mL) were mixed with nitrobenzene (20 mL) and heated at 110 °C under N2 for 24 h, followed by reflux in THF for 24 h twice to remove polymer that was not immobilized.
To immobilize TEMPO on HBPEK/PI, the HBPEK/PI (921 mg, corresponding to 1.0 mmol of terminal COOH groups) was mixed with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (186 mg, 1.2 mmol) in 5 mL dichloromethane and stirred for 30 min. The 4-amino-TEMPO (188 mg, 1.1 mmol) and 4-dimethylaminopyridine (DMAP) (13 mg, 0.1 mmol) were dissolved in 3 mL dichloromethane and then added to the previous mixture, followed by stirring at 40 °C for 24 h. After reaction, the solvent was removed by evaporation and the product washed with water and acetone.
To determine the inductive effect by the polymer backbone on the catalytic activity of TEMPO, a model compound, 4-benzylamino-TEMPO, also was prepared from benzoic acid (122 mg, 1.0 mmol), and purified using column chromatography, with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 CH2Cl2
3 CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate as the eluent to yield an orange powder.
ethyl acetate as the eluent to yield an orange powder.
TEMPO immobilized on polymer (1% cross-linked with divinylbenzene, TEMPO/PS) with a TEMPO loading of 1.00 mmol g−1 was purchased from Aldrich and used as received.
2.2. Measurements
Thermogravimetric analysis (TGA) was performed under nitrogen using a SII TGA 6200 system at a heating rate of 10 °C min−1. The CHN elemental analysis was performed using a Perkin Elmer 2400-II analyzer. Fourier-transform infrared (FT-IR) spectroscopy was performed using a spectrophotometer (Jasco, 6100) on KBr pellets. Scanning electron microscopy (SEM) was performed using a Hitachi SU9000 microscope operated at 6.0 kV. Gel permeation chromatography (GPC) was performed using a Viscotek GPC-1000 system equipped with a TDA 302 triple detector and a TSK-GEL α-M column. Dimethylformamide (DMF) with 0.05 M LiBr were used as the eluent. The weight average molecular weight (Mw) was calculated based on the light-scattering data.The ion exchange capacity (IEC) of HBPEK and HBPEK/CB was determined by titration as follows. Sample powder (30 mg) was stirred in 0.1 M NaOH (3 mL) overnight, and then filtered. The filtrate was diluted to 10 mL with deionized water, and titrated with 0.05 M HCl.
2.3. Catalytic reaction
The catalytic performance of the materials was determined using a Shibata Chemist Plaza CP100 multi-reactor system equipped with 6 mL reactors. Benzyl alcohol (10 mmol), nitric acid (67%, 0.2 mmol), and the catalyst (TEMPO content: 0.08 mmol) were stirred at 90 °C for 2 h with an oxygen balloon. The products were quantified using a Shimadzu GCMS-QP2010 Plus instrument equipped with a TC-FFAP column (0.25 mmφ × 30 m). The conversion was calculated based on the peak area ratio of benzyl alcohol against the internal standard (naphthalene). The product yield was calculated based on the peak area ratio of benzaldehyde against the internal standard.For catalyst recycling, the reaction mixture was diluted to 40 mL with acetone, filtered, and washed with acetone, and then the residue used for the next cycle after drying and weighing.
3. Results and discussion
3.1. Synthesis of TEMPO/HBPEK
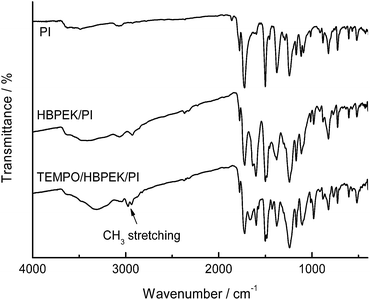
The synthesized HBPEK had a weight-average molecular weight (Mw) of 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, as measured by GPC, and an ion exchange capacity (IEC) of 2.5 mmol g−1 as calculated by titration. The FT-IR spectra of HBPEK and TEMPO/HBPEK are shown in Fig. 1. After loading TEMPO, the OH broad band (2500–3000 cm−1) decreased and the CH3 stretching band (2900–3000 cm−1) appeared, which suggests a decrease in the number of terminal carboxylic acid groups and the inclusion of TEMPO. The CHN elemental analysis results for HBPEK and TEMPO/HBPEK are shown in Table 1. The TEMPO loading rate was calculated from the nitrogen content of the catalyst. The TEMPO/HBPEK had a TEMPO loading of more than 2 mmol g−1.
000, as measured by GPC, and an ion exchange capacity (IEC) of 2.5 mmol g−1 as calculated by titration. The FT-IR spectra of HBPEK and TEMPO/HBPEK are shown in Fig. 1. After loading TEMPO, the OH broad band (2500–3000 cm−1) decreased and the CH3 stretching band (2900–3000 cm−1) appeared, which suggests a decrease in the number of terminal carboxylic acid groups and the inclusion of TEMPO. The CHN elemental analysis results for HBPEK and TEMPO/HBPEK are shown in Table 1. The TEMPO loading rate was calculated from the nitrogen content of the catalyst. The TEMPO/HBPEK had a TEMPO loading of more than 2 mmol g−1.
| Catalyst | Elemental analysis wt% | TEMPO loading/mmol g−1 | ||
|---|---|---|---|---|
| C | H | N | ||
| HBPEK | 68.8 | 4.2 | 0.2 | — |
| TEMPO/HBPEK | 69.1 | 5.3 | 5.9 | 2.04 |
3.2. Synthesis of TEMPO/HBPEK/CB
HBPEK was immobilized on CB using the Friedel–Crafts reaction. The IEC of HBPEK/CB was determined by titration to be 1.5 mmol g−1, whereas that of HBPEK was 2.5 mmol g−1. This IEC value corresponds to 60 wt% of the polymer loading on CB, which represents a 30% yield of the grafted polymer. It was found to be difficult to obtain FT-IR spectra for HBPEK/CB and TEMPO/HBPEK/CB. The CHN elemental analysis results for CB, HBPEK/CB, and TEMPO/HBPEK/CB are shown in Table 2. Both the CB and HBPEK contained a low level of nitrogen. The TEMPO loading was 1.32 mmol g−1. Fig. 2 shows SEM images of carbon black and HBPEK/CB. The surfaces of the HBPEK/CB and carbon black are clearly different. Compared to carbon black, HBPEK/CB had a smooth surface, probably formed by the polymer layer covering the rough surface of the carbon black. These experimental results suggest that HBPEK was successfully grafted onto carbon black, and its terminals were converted to TEMPO units.| Catalyst | Elemental analysis wt% | TEMPO loading/mmol g−1 | ||
|---|---|---|---|---|
| C | H | N | ||
| CB | 90.2 | 0.3 | 0.3 | — |
| HBPEK/CB | 86.3 | 1.5 | 0.2 | |
| TEMPO/HBPEK/CB | 81.1 | 2.5 | 3.9 | 1.32 |
3.3. Synthesis of TEMPO/HBPEK/PI
The CHN elemental analysis for CB, HBPEK/CB, and TEMPO/HBPEK/CB are shown in Table 3. A comparison of the nitrogen content in PI and in HBPE/PI indicated that the HBPEK loading rate was 40 wt%, which corresponds to a 13% yield of the grafted polymer. The TEMPO loading of TEMPO/HBPEK/PI was 0.96 mmol g−1, calculated by comparing the nitrogen content before and after loading TEMPO. The FT-IR spectra of PI, HBPEK/PI, and TEMPO/HBPEK/PI are shown in Fig. 3. Compared to PI, an OH broad band (2500–3000 cm−1) was observed in the spectra of HBPEK/PI. For TEMPO/HBPEK/PI, a CH3 stretching band (2900–3000 cm−1) appeared, which suggests the presence of TEMPO. Fig. 4 shows SEM images of polyimide particles and HBPEK/PI. The surfaces of HBPEK/PI and polyimide particles were different. The polyimide particles had a diameter of 150–200 nm with a smooth surface, while the surface of HBPEK/PI appeared rough. This difference suggests the successful grafting of HBPEK onto polyimide particles.| Catalyst | Elemental analysis wt% | TEMPO loading/mmol g−1 | ||
|---|---|---|---|---|
| C | H | N | ||
| PI | 65.5 | 1.9 | 6.5 | — |
| HBPEK/PI | 67.2 | 2.9 | 4.0 | — |
| TEMPO/HBPEK/PI | 66.5 | 4.0 | 6.7 | 0.96 |
3.4. Thermal stability
The thermal stability of HBPEK, TEMPO/HBPEK, TEMPO/HBPEK/CB, and TEMPO/HBPEK/PI was examined using TGA under a nitrogen atmosphere (Fig. 5). The weight loss of those materials began at 150 °C, and was maintained up to 600 °C. The weight loss of TEMPO/HBPEK occurred most rapidly in the first stage. Meanwhile, TEMPO/HBPEK/PI showed a sharp weight loss beginning at 550 °C. The initial weight losses were likely due to decomposition of the terminal functional groups, resulting in a weight loss near 400–600 °C due to the backbone of poly(ether ketone). Because the TEMPO group is heavier than a carboxylic acid group, the weight loss of TEMPO/HBPEK was more rapid than that of HBPEK during the initial stage.3.5. Catalytic performance
The prepared catalysts were tested for activity in the aerobic oxidation of benzyl alcohol. Table 4 compares the performance of several TEMPO-based catalysts. The turnover number (TON) of the TEMPO unit was calculated from the yield of benzaldehyde. A control test without catalyst resulted in a low yield, while HBPEK showed slight catalytic activity. Commercial TEMPO, which is a homogeneous system, showed the highest activity with a TON of 60. 4-Benzylamino-TEMPO showed similar catalytic activity (TON of 56), suggesting little influence of the inductive effect by aromatic amide backbone on the catalytic activity of the TEMPO unit. Another control experiment with a mixture of TEMPO and HBPEK yielded a result similar to that with TEMPO, suggesting that the presence of HBPEK does not affect the catalytic activity of the TEMPO unit. The catalytic activity of TEMPO/HBPEK, which is a homogenous system, was slightly lower, likely due to the lower mobility of the TEMPO terminal units on HBPEK compared to those of molecular TEMPO. The TEMPO/PS, which is a commercial heterogeneous catalyst, did not possess good catalytic activity. In contrast, two synthesized heterogeneous catalysts, TEMPO/HBPEK/CB and TEMPO/HBPEK/PI, possessed much stronger catalytic activity than the control test and TEMPO/PS, although the catalytic activities were still less than those of the homogeneous systems. The performance of TEMPO/HBPEK/CB was slightly better than that of TEMPO/HBPEK/PI, perhaps due to synergy between TEMPO and CB. Kuang et al. proposed that CB absorbs and activates HNO3 in the nitric acid-assisted carbon-catalyzed oxidation system (NACOS).14 Note that TEMPO/HBPEK/CB and TEMPO/HBPEK/PI showed much better catalytic activity than TEMPO/PS, likely due to high reactivity of the hyperbranched polymer terminals, which have good affinity toward the reactant phase.| Entry | Catalyst | TEMPO unit/mmol | Yield/% | Conversion/% | TON |
|---|---|---|---|---|---|
| a Benzyl alcohol (10 mmol), nitric acid (67%, 0.2 mmol) and catalyst (TEMPO content: 0.08 mmol), 90 °C, 2 h, with an oxygen balloon. | |||||
| 1 | Blank | 0 | 1.0 | 2.0 | — |
| 2 | HBPEK | 0 | 5.4 | 5.7 | — |
| 3 | TEMPO | 0.08 | 47.7 | 48.6 | 60 |
| 4 | 4-Benzylamino-TEMPO | 0.08 | 32.1 | 31.9 | 56 |
| 5 | HBPEK + TEMPO | 0.08 | 47.8 | 49.8 | 60 |
| 6 | TEMPO/PS | 0.08 | 1.23 | 4.15 | <1 |
| 7 | TEMPO/HBPEK | 0.08 | 36.7 | 37.5 | 46 |
| 8 | TEMPO/HBPEK/CB | 0.08 | 22.9 | 26.7 | 27 |
| 9 | TEMPO/HBPEK/PI | 0.08 | 18.4 | 17.4 | 22 |
3.6. Recycling tests
Recyclability of TEMPO/HBPEK, TEMPO/HBPEK/CB, TEMPO/HBPEK/PI, and TEMPO/PS was tested by repeating the catalytic reactions. The conversion of each run was controlled to less than 50% to avoid TEMPO unit over-dose. Recycling of the catalyst of TEMPO/HBPEK was attempted by precipitation into acetone because HBPEK is insoluble in acetone. The other catalysts were collected by filtration and used for the next run after washing with acetone. Fig. 6 shows catalyst yields after each run of the recycling tests, and Fig. 7 shows the benzaldehyde yield of each run. | ||
| Fig. 7 Yield of benzaldehyde with the fresh catalyst (1st run) and the recycled catalysts (2–6th run). | ||
As shown in Fig. 6, TEMPO/HBPEK was difficult to collect at a high yield. The catalytic activity of the recycled TEMPO/HBPEK in the second run was still high; however, it decreased significantly in the third run. These experimental results suggest that TEMPO/HBPEK, as a heterogeneous catalyst or an easily recyclable homogeneous catalyst, is difficult to recycle.
The collected yield of TEMPO/HBPEK/CB was excellent even after the sixth run. The yield of TEMPO/HBPEK/CB gradually decreased in the first 3 runs; however, it remained steady after that.
The collected yield of TEMPO/HBPEK/PI gradually decreased throughout the 6 runs, similar to that of TEMPO/PS. However, this decrease was not due to an inherent instability of TEMPO/HBEPK/PI, but from collection loss during the experimental procedure because this catalyst powder tended to stick to glassware, making it difficult to collect. The yield of TEMPO/HBPEK/PI showed a trend similar to that of TEMPO/HBPEK/CB, but the degradation was not significant; therefore, the yield after 3 runs was better with TEMPO/HBEPK/PI.
Fig. 8 shows SEM images of fresh and recycled TEMPO/HBEPK/CB and TEMPO/HBEPK/PI after the sixth run. The surface of recycled TEMPO/HBEPK/CB (Fig. 8c) appeared smooth with a cover layer, similar to that of TEMPO/HBPEK/CB (Fig. 8a). The surface of recycled TEMPO/HBEPK/PI (Fig. 8d) was rough, similar to that of TEMPO/HBPEK/PI (Fig. 8b). These results indicate that the catalyst did not change after the recycling reaction.
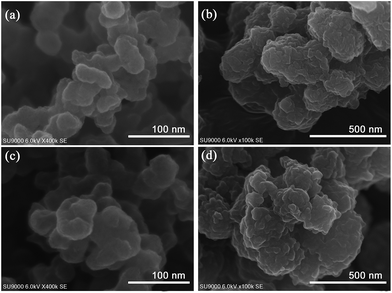 | ||
| Fig. 8 SEM images of (a) fresh TEMPO/HBPEK/CB, (b) fresh TEMPO/HBPEK/PI, (c) recycled TEMPO/HBPEK/CB, and (d) recycled TEMPO/HBPEK/PI after the sixth run. | ||
Compared to commercially available immobilized TEMPO materials, TEMPO/HBPEK/CB and TEMPO/HBPEK/PI exhibit good durability.
4. Conclusions
A novel hyperbranched aromatic poly(ether ketone) functionalized with TEMPO was synthesized as a catalyst for aerobic oxidation of benzyl alcohol. The TEMPO/HBPEK possessed good catalytic activity in the selective oxidation, but could not be collected after the reaction. After immobilizing it onto carbon black and polyimide particles, it formed a heterogeneous system and became recyclable. These heterogeneous catalysts showed much better catalytic activity than the commercially available heterogeneous catalyst, TEMPO immobilized on polystyrene.Acknowledgements
This study was supported by JSPS KAKENHI (26870183). The SEM analysis was performed in the Center for Advanced Materials Analysis at the Tokyo Institute of Technology. M.K. was supported by a Visiting Professorship for Senior International Scientists from the Chinese Academy of Science.Notes and references
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- G. Tojo and M. Fernandez, Oxidations of Alcohols to Aldehydes and Ketones, Springer, New York, 2006 Search PubMed.
- B.-Z. Zhan and A. Thompson, Tetrahedron, 2004, 60, 2917 CrossRef CAS PubMed.
- R. A. Sheldon and I. W. C. E. Arends, Adv. Synth. Catal., 2004, 346, 1051 CrossRef CAS.
- S. S. Wang, Z. Popovic, H. H. Wu and Y. Liu, ChemCatChem, 2011, 3, 1208 CrossRef CAS.
- J. U. Ahmad, M. T. Raisanen, M. Kemell, M. J. Heikkila, M. Leskela and T. Repo, Appl. Catal., A, 2012, 449, 153 CrossRef CAS PubMed.
- J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357 CrossRef CAS PubMed.
- L. Wang, J. Li, Y. Lv, G. Zhao and S. Gao, Appl. Organomet. Chem., 2012, 26, 37 CrossRef CAS.
- R. Liu, X. Liang, C. Dong and X. Hu, J. Am. Chem. Soc., 2004, 126, 4112 CrossRef CAS PubMed.
- J. S. Stamier, D. J. Singel and J. Loscalzo, Science, 1992, 258, 1898 Search PubMed.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. Janczuk, Chem. Rev., 2002, 102, 1091 CrossRef CAS PubMed.
- X. J. He, Z. L. Shen, W. M. Mo, N. Sun, B. X. Hu and X. Q. Hu, Adv. Synth. Catal., 2009, 351, 89 CrossRef CAS.
- Y. Kuang, N. M. Islam, Y. Nabae, T. Hayakawa and M. Kakimoto, Angew. Chem., Int. Ed., 2010, 49, 436 CrossRef CAS PubMed.
- Y. Kuang, H. Rokubuichi, Y. Nabae, T. Hayakawa and M. Kakimoto, Adv. Synth. Catal., 2010, 352, 2635 CrossRef CAS.
- B. Karimi and E. Badreh, Org. Biomol. Chem., 2011, 9, 4194 CAS.
- B. Karimi, A. Biglari, J. H. Clark and V. Budarin, Angew. Chem., 2007, 119, 7348 CrossRef.
- M. L. Testa, R. Ciriminna, C. Hajji, E. Z. Garcia, M. Ciclosi, J. S. Arques and M. Pagliaro, Adv. Synth. Catal., 2004, 346, 655 CrossRef CAS.
- R. Ciriminna and M. Pagliaro, Adv. Synth. Catal., 2003, 345, 383 CrossRef CAS.
- M. A. Subhani, M. Beigi and P. Eilbracht, Adv. Synth. Catal., 2008, 350, 2903 CrossRef CAS.
- A. Gheorghe, A. Matsuno and O. Reiser, Adv. Synth. Catal., 2006, 348, 1016 CrossRef CAS.
- M. Gilhespy, M. Lok and X. Baucherel, Catal. Today, 2006, 117, 114 CrossRef CAS PubMed.
- Y. Nabae, J. Liang, X. Huang, T. Hayakawa and M. Kakimoto, Green Chem., 2014, 16, 3596 RSC.
- Y. Shi, Y. Nabae, T. Hayakawa, H. Kobayashi, M. Yabushita, A. Fukuoka and M. Kakimoto, Polym. J., 2014, 46, 722 CrossRef CAS.
- R. C. Evers, F. E. Arnold and T. E. Helminiak, Macromolecules, 1981, 14, 925 CrossRef CAS.
- S. Hsiao and C. Chang, Macromol. Chem. Phys., 1996, 197, 1255 CrossRef CAS.
- M. Ueda and M. Sato, Macromolecules, 1987, 20, 2675 CrossRef CAS.
- Y. Nabae, Y. Kuang, M. Chokai, T. Ichihara, A. Isoda, T. Hayakawa and T. Aoki, J. Mater. Chem. A, 2014, 2, 11561 CAS.
| This journal is © The Royal Society of Chemistry 2015 |