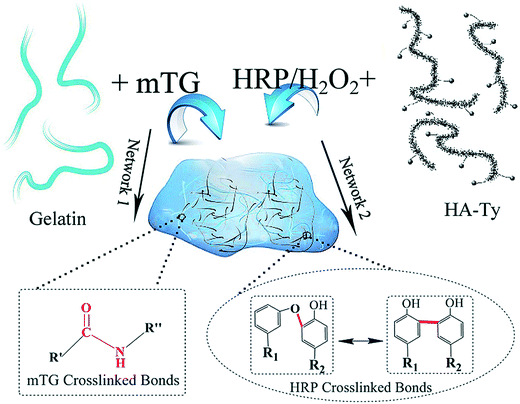
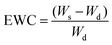Bienzymatically crosslinked gelatin/hyaluronic acid interpenetrating network hydrogels: preparation and characterization
Zhiping Fan,
Yemin Zhang,
Shuo Fang,
Chen Xu and
Xinsong Li*
School of Chemistry and Chemical Engineering, Southeast University, Nanjing 210018, China. E-mail: lixs@seu.edu.cn
First published on 1st December 2014
Abstract
Hydrogels that mimic the extracellular matrix in both composition and crosslinking chemistry have great potential in the development of tissue engineering scaffolds. Natural polymer hydrogels crosslinked by enzymes have excellent biocompatibility due to their natural origin and the mild reaction conditions, but usually suffer from a lack of mechanical strength. In order to improve the mechanical properties, hydrogels of gelatin/hyaluronic acid with interpenetrating network (IPN) structure were prepared by dual enzymatic crosslinking, using transglutaminase (mTG) to crosslink gelatin, and horseradish peroxidase (HRP) to crosslink hyaluronic acid (HA) grafted with tyramine (HA-Ty), respectively. The gelation processes of the gelatin/HA IPN hydrogels were monitored by rheometer. The data confirmed the formation of dual networks: one gelatin network crosslinked by mTG and the other HA network crosslinked by HRP. Hereafter, the results of mechanical test showed the compressive strength of the gelatin/HA-Ty IPN hydrogels was 10 folds higher than that of pure HA hydrogels. Furthermore, the in vitro enzymatic degradation, equilibrium water content, cytotoxicity and cell adhesion of the IPN hydrogels were evaluated in detail. The preliminary biological evaluation revealed the hydrogels could support cell adhesion and proliferation. Summary, the gelatin/HA IPN hydrogels prepared by bienzymatic crosslinking method possess excellent biocompatibility and mechanical properties, which are expected to be novel biomaterials in the field of tissue engineering and in situ wound repair.
1. Introduction
Mimicking the extracellular matrix in composition, structure and fabrication is the most important strategy to develop tissue engineering scaffolds which play an important role in regenerative medicine. In order to achieve this goal, natural polymer hydrogels have been widely investigated as regenerative scaffolds and surgical implantations because of their good biocompatibility and biodegradation.1 However, the poor strength of the hydrogels often limits their clinical applications as biomedical materials. A number of techniques including chemical and physical crosslinking have been developed to improve their mechanical properties as reported in the literature.2–4Interpenetrating network (IPN) hydrogels are polymeric hydrogels possessing unique properties through the formation of interpenetrating networks, utilizing two or more polymers. Two interpenetrating networks entangled with each other and obtained a synergistic effect of mechanical strength. So, the formation of IPN structure is an effective means of enhancing the mechanical properties of hydrogels. Chemical crosslinking methods are commonly used in the preparation of IPN hydrogels,5,6 however the addition of crosslinkers and the presence of residual molecules often reduce the biocompatibility of the hydrogels severely. While, physically crosslinked hydrogels do not have uniform and stable internal structures. Therefore, it remains a challenge to prepare natural polymer hydrogels with excellent biocompatibility and desirable mechanical strength.
Enzyme is available in vivo, which can catalyze specific reactions efficiently. Transglutaminase (mTG) could mediate the chemical reaction between glutamine and lysine residues on gelatin protein, thus providing crosslink point that serves to form hydrogel networks. In the presence of H2O2, phenol-derived polymers could be crosslinked by horseradish peroxidase (HRP). This biochemical reaction has been employed to achieve hydrogels by many research groups in recent years.7–16 Since no small molecule is introduced and the residuals after degradation can also be absorbed in vivo, the enzyme-crosslinked hydrogels always exhibit excellent biocompatibility as biomaterials. However, single enzyme-crosslinked hydrogel has poor mechanical strength and rapid degradation, whose application is often subjected to certain restrictions.17–22
In order to obtain the hydrogels with high mechanical strength and excellent biocompatibility, as described herein, natural polymer based IPN hydrogels were prepared by using mTG to crosslink gelatin and HRP to crosslink hyaluronic acid grafted with tyramine (HA-Ty), respectively. Firstly, modified synthesis method of HA-Ty was described; the formation of the IPN was confirmed by rheometer. After that, mechanical properties, in vitro enzymatic degradation and equilibrium water content were examined in detail. Finally, the cytotoxicity and cell adhesion of the IPN hydrogels were evaluated.
2. Materials and methods
2.1 Materials
The hyaluronic acid (45 kDa) was obtained from Shandong Focuschem Biotech Co., Ltd (Jinan, China). Gelatin (Type A), tyramine hydrochloride (Ty), 1-ethyl-(3-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), horseradish peroxidase (HRP) (260 U mg−1 solid), papain (6000 U mg−1) and hydrogen peroxide were purchased from Sigma Aldrich. Microbial transglutaminase (mTG, 1000 U g−1) was a gift from Yiming Biological Products Co., Ltd (Taixing, China).Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin, L-glutamine, trypsin and 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Invitrogen Co. (Carlsbad, CA). L929 mouse fibroblasts cells were obtained from Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Chinese Academy of Science. The L929 cells were cultured in complete growth culture medium prepared with DMEM supplemented with 10% FBS, 1 mM L-glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in a 5% CO2 atmosphere. All the other chemical reagents were supplied from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
2.2 Synthesis of HA-Ty conjugate
The HA-Ty conjugate was prepared as described previously by EDC/NHS chemistry with some modification to improve grafting rate.23 Briefly, HA (1 g, 2.5 mmol) was dissolved in 50 mL of distilled water followed by addition of tyramine hydrochloride (0.3472 g, 2 mmol). After that, EDC (1.437 g, 7.5 mmol) and NHS (0.863 g, 7.5 mmol) were added to initiate conjugation reaction. As the reaction proceeded, pH of the mixture was maintained at 4.8 with 1 M NaOH and 1 M HCL. The reaction mixture was stirred over night at room temperature and then the pH was brought to 7.0. The solution was transferred to a dialysis bag with a molecular cut-off of 1000 Da. The bag was dialyzed against 100 mM sodium chloride solution for 2 days, a mixture of distilled water and ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 1 day and distilled water for 1 day, successively. The purified solution was lyophilized to obtain HA-Ty. The degree of substitution23 (the number of tyramine molecules per 100 repeating units of HA) was 29% calculated from 1H NMR (Bruker 500 MHz, D2O) by comparing the ratio of the relative peak integrations of the phenyl protons of tyramine (peaks at 7.2 and 6.9 ppm) and the methyl protons of HA (1.9 ppm) as indicated in Fig. 1.
1) for 1 day and distilled water for 1 day, successively. The purified solution was lyophilized to obtain HA-Ty. The degree of substitution23 (the number of tyramine molecules per 100 repeating units of HA) was 29% calculated from 1H NMR (Bruker 500 MHz, D2O) by comparing the ratio of the relative peak integrations of the phenyl protons of tyramine (peaks at 7.2 and 6.9 ppm) and the methyl protons of HA (1.9 ppm) as indicated in Fig. 1.
2.3 Rheological test
Rheology measurements were performed with a Rheometer (HAAKE Rheostress 600, Thermo-Fisher, Hampton, VA), in the oscillatory mode using parallel plate geometry (D = 60 mm). The elastic modulus G′ and viscous modulus G′′ were documented as a function of time at a frequency of 1 Hz at 40 °C. Hydrogel mixtures with different compositions, as shown in Table 1, were quickly transferred to the lower plate. When the upper plate was set to the position, the test was started immediately.| Sample | Gelatin (mg mL−1) | HA-Ty (mg mL−1) | mTG (U mL−1) | HRP (U mL−1) | H2O2 (mmol L−1) |
|---|---|---|---|---|---|
| H5 | 0 | 50 | 0 | 20 | 10 |
| RS1 | 50 | 50 | 0 | 20 | 10 |
| RS2 | 100 | 50 | 0 | 20 | 10 |
| RI1 | 50 | 50 | 10 | 20 | 10 |
| RI2 | 100 | 50 | 10 | 20 | 10 |
2.4 Hydrogel preparation
The preparation of IPN hydrogel is illustrated in Scheme 1, which highlights the crosslinking mechanisms employed in this work. The composition of hydrogels was listed in Table 2. Briefly, an aqueous solution composed of HA-Ty (5%, w/v) and gelatin (3–15%, w/v) was obtained at 37 °C, then the mixture was poured into a 5 mm height cylindrical mold. After addition of mTG, HRP and H2O2 to a final concentration of 10 U mL−1, 20 U mL−1 and 10 mM, respectively, the mixture was quickly stirred to obtain a homogeneous solution. It was allowed to form the IPN hydrogel in 37 °C water bath for 40 min, ensuring the hydrogel to crosslink completely. After release from the molds, the hydrogels, which were further sterilized in 70% ethanol followed by 3 times of deionized water washing, were used for other characterizations.| Sample | Gelatin (mg mL−1) | HA-Ty (mg mL−1) | mTG (U mL−1) | HRP (U mL−1) | H2O2 (mmol L−1) |
|---|---|---|---|---|---|
| H5 | 0 | 50 | 0 | 20 | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Semi-IPN | |||||
| SI1 | 30 | 50 | 0 | 20 | 10 |
| SI2 | 50 | 50 | 0 | 20 | 10 |
| SI3 | 100 | 50 | 0 | 20 | 10 |
| SI4 | 150 | 50 | 0 | 20 | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| IPN | |||||
| I1 | 30 | 50 | 10 | 20 | 10 |
| I2 | 50 | 50 | 10 | 20 | 10 |
| I3 | 100 | 50 | 10 | 20 | 10 |
| I4 | 150 | 50 | 10 | 20 | 10 |
2.5 Equilibrium water content
The hydrogels of H5, SI1, SI2, SI3, I1, I2 and I3 were incubated in distilled water at room temperature for 24 h to reach their equilibrium swollen state. After excess surface water was removed, the swollen weight (Ws) of each hydrogel was recorded. Then the samples were lyophilized to complete dryness and the dry weight (Wd) of each samples were weighed. The equilibrium water content (EWC) was calculated using the following equation:All the samples were tested in triplicates for each group.
2.6 Mechanical properties
Mechanical properties of the IPN hydrogels were characterized by the universal testing system (YL-1109, Yuelian Testing Machines Co., Ltd, Dongguan, China) at room temperature. Cylindrical samples prepared in a mold of 5 mm height and 10.7 mm diameter were subjected to compression test with a compression rate of 20 mm min−1. Individual compressive strength was obtained from the load–displacement curve at the break. All the samples were tested in triplicates for each group.2.7 In vitro enzymatic degradation
To test enzymatic degradation of the IPN hydrogels (SI4 and I4), bulk samples were immersed in PBS (0.01 M) containing papain (0.05 mg mL−1) at 37 °C under constant shaking at 100 rpm for 2, 4, 8, and 24 h. At each time point, the gels were washed with distilled water and then lyophilized. The in vitro degradation rate was calculated by the dry weight after degradation (Wt) divided by the initial weight of the gel (W0) as follows:| Fractional mass remaining = (Wt/W0) × 100% |
All the samples were tested in triplicates for each group.
2.8 In vitro cytotoxicity
Evaluation of the cytotoxicity of the hydrogels was carried out on an extracted solution of the hydrogel via MTT assay according to ISO 10993-5 Standard. Briefly, sterilized hydrogels were extracted using DMEM at an extraction ratio of 1 cm2 mL−1 at 37 °C for 24 h. A 100 μL media suspension containing total of 104 cells and 100 μL extract solution were plated into each well of the 96-well plate and then incubated at 37 °C in 5% CO2 atmosphere. On the 1st, 3rd, and 5th day, 20 μL of MTT (5 mg mL−1 in PBS) was added for 4 h to allow formation of formazan crystal. After removal of the supernatant, 150 μL DMSO was added to each well, and the absorbance was measured at 490 nm using an ELISA reader (Elx800, Bio-Tek Instrument Inc., VT). The results were expressed as percentages relative to the data obtained with blank control. Six samples were tested for each group.2.9 Cell seeding and imaging
L929 mouse fibroblasts were inoculated onto hydrogels surface for observation of cells' morphology on the samples. After ethanol sterilization for 2 h, the hydrogels were rinsed three times with PBS and equilibrated with cell culture medium for 24 h. Cells suspensions were dropped onto the samples in a density of 5 × 104 cells per cm2 and incubated at 37 °C in a 5% CO2 atmosphere with a change of medium every other day. The images of the fibroblasts morphology on the scaffolds at 5th day were recorded using an inverted microscope (LWD200-37T, Cewei Photoelectric Technology Co., Ltd, Shanghai, China).2.10 Statistical analysis
Experiments were performed in triplicate and results are reported as mean ± SD. Comparisons between groups were computed using Origin Pro8 software for paired t tests and significant difference between means was asserted at 95% confidence intervals (p < 0.05).3. Results and discussion
The formation of gelatin/HA-Ty IPN hydrogel via bienzymatic crosslinking was depicted in Scheme 1. Hyaluronic acid grafted with tyramine (HA-Ty) could be crosslinked by HRP in the presence of H2O2. The principle of the reaction can be attributed to the appearance of two types of bonds: (1) C–C bonds between two carbon atoms at the ortho position; (2) C–O bonds between two carbon atoms at the ortho position and the oxygen atom from the phenolic group.24 mTG could mediate the chemical crosslinking reaction between glutamine and lysine residues on adjacent gelatin chains, thus providing amide bonds that serve to reinforce the hydrogel.25 In this hydrogel, one polymer crosslinked network is intertwined with another, resulting in a mechanically reinforced and strengthened hydrogel system. The formation of IPN structure was monitored by rheological study. Additionally, the mechanical properties of the hydrogels were examined by a universal testing machine. Furthermore, the in vitro enzymatic degradation, equilibrium water content, cytotoxicity and cell adhesion of the IPN hydrogels were evaluated in detail.3.1 Rheological study
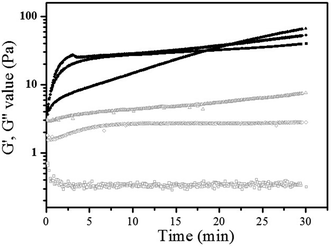
Rheological method was utilized to study the crosslinking mechanism of the interpenetrating network hydrogel. The elastic modulus (G′) and viscous modulus (G′′) of H5, RS1 and RS2 with the composition listed in Table 1 were presented in Fig. 2. The elastic modulus (G′) of H5 increases from 0 to 40.6 Pa in 30 min, which can be attributed to the formation of a hydrogel network through the oxidative coupling of phenol-derived HA under enzymatic conditions.23After the introduction of gelatin, the viscous of the hydrogels increased significantly: G′′ value of RS2 was about 23 times higher than that of H5 (7.61 Pa vs. 0.32 Pa). However, the G′ values of those with different gelatin concentrations at 30 min were very close (40.6 Pa, 53.4 Pa, 66.8 Pa), indicating the gelatin remained in its sol state and no significant physical crosslinking. The obtained hydrogels, in which only HA-Ty crosslinked one network while the gelatin was dispersed within the HA-Ty hydrogel as an emulsion, were considered as semi-IPN hydrogels.
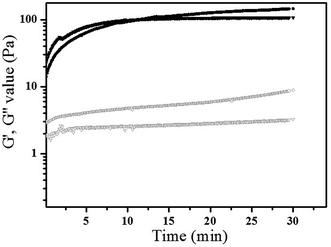
In order to confirm the formation of gelatin network, sample RI1 and RI2 crosslinked under the coexistence of mTG and HRP enzymes were tested in the same manner. As shown in Fig. 3, G′ values increased significantly (107.4 Pa, 146.6 Pa for RI1, RI2) with the increasing of gelatin concentration. The positive correlation between the elastic modulus and gelatin concentration indicated the crosslinked gelatin changed the gel phase properties of gelatin/HA-Ty system. It is due to the transformation of gelatin from sol to gel state in the presence of mTG enzyme.25 According to the curves displayed in Fig. 2 and 3, both HA-Ty and gelatin formed crosslinked networks independent of each other in the case of mTG and HRP enzymes coexistence. The obtained hydrogels with double networks were considered as IPN hydrogels. In order to check whether HRP/H2O2 catalyzes the crosslinking of the tyrosine residues of gelatin, the gelatin solution of 150 mg mL−1 were treated with HRP (20 U mL−1) and H2O2 (10 mmol L−1). It was found that no gelation was occurred and no gel was obtained in the gelatin solutions at 37 °C. According to this result, it can be concluded that HA-Ty formed another network in the presence of HRP/H2O2 within mTG crosslinked gelatin network, and no hybrid crosslinking network was formed in the IPN hydrogels. Therefore, tyrosine residues in gelatin didn't participate the HRP-catalyzed crosslinking significantly.
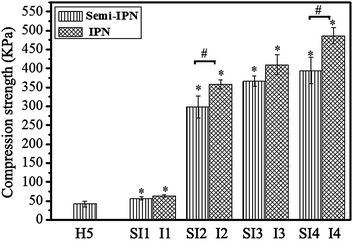
3.2 Mechanical properties
The mechanical properties of gelatin/HA-Ty hydrogels were examined using a compression tester, as shown in Fig. 4. It's found that H5 was easily crushed under a compression of 42.4 kPa, which is significantly lower (p < 0.05) than that of other samples. The compressive strength of I2 and I4 was significantly higher (p < 0.05) than that of SI2 and SI4, respectively. Compared with that of the pure HA-Ty hydrogel, the compressive strength of semi-IPN hydrogels have elevated to a certain extent. Since the effect of intermolecular hydrogen bonding is concerned, the gelatin will form a physically crosslinked hydrogel at room temperature.26 Though there were seemed no statistical differences from the data of semi-IPN and IPN, the contained significances of the data were different essentially. In the semi-IPN system with the gelatin concentration increasing from 30 to 150 mg mL−1, the compressive strength was partial attributed to physically crosslinked gelatin, which is unstable and affected severely by the external environment conditions, especially by the temperature. In the IPN system, the compressive strength was attributed to the bienzymatic crosslinked gelatin and HA-Ty, which is very stable. Although the effect of physical crosslinking could not be excluded completely when the IPN samples temperature returned to room temperature, but that is limited. The compressive strength of I1 is higher than that of H5 and SI1 by 49.3% and 10.9%, respectively (63.3 kPa vs. 42.4 kPa; 63.3 kPa vs. 57.1 kPa), and the compressive strength of I4 is higher than that of H5 and SI4 by 1046.9% and 23.5%, respectively. (486.3 kPa vs. 42.4 kPa; 486.3 kPa vs. 393.9 kPa). The data reveal that, the mechanical enhancement observed in IPN system is much higher than that of semi-IPN system. Furthermore, with the gelatin concentration increasing, the trend of enhancement increases gradually. It can be attributed to the synergistic effect of the bienzymatically crosslinked reinforced IPN structure.These results were consistent with data from the rheological studies basically. It suggested that, in the semi-IPN hydrogel system, the chemical crosslinking of gelatin was not approached. However, in the IPN system, the chemical crosslinking of gelatin was triggered by mTG catalysis.
3.3 In vitro enzymatic degradation
The enzymatic degradation property of the gelatin/HA-Ty hydrogels (SI4, I4 with the composition set out in Table 2) was characterized by measuring the IPN weight change in PBS containing 0.05 mg mL−1 papain. The degradation profiles in Fig. 5 indicated that the weight of semi-IPN decreased by 73.4% within 2 h, 73.8% within 4 h, and 14.4% remained after 24 hours, which showed very irregular trend. The weight of IPN hydrogels decreased by 58.8% within 2 h, 64.1% within 4 h, and 10.6% remained after 24 hours, showing much more regular degradation curve. After the first 2 h degradation, the fractional mass remaining of I4 was significantly higher (p < 0.05) than that of SI4. Apparently, this kind of gelatin/HA-Ty IPN hydrogels own more controllable degradability compared to our previous work.5,27Due to the in vitro enzymatic degradation was performed at 37 °C, so the gelatin in the semi-IPN hydrogel was still in its liquid state. In the initial stage, the unpredictable fast degradation rate of the semi-IPN hydrogel should be attributed to the heterogeneity of the liquid gelatin in the network. The greater fluidity of the gelatin makes it much easier to contact with enzyme and thus degraded rapidly. While in the IPN networks, gelatin and hyaluronic acid form a compact and stable interpenetrating network structure. Enzyme solution penetrates into the IPN hydrogel more regularly, the degradation reaction between the crosslinked gelatin and enzyme process more stable and controllable. It makes this kind of hydrogels gain good potential applications in drug release and protein carries in vivo.
3.4 Equilibrium water content
Fig. 6 illustrated equilibrium water content (EWC) of gelatin/HA-Ty IPN hydrogels. The EWC value of pure HA-Ty hydrogel (H5) is significantly higher (p < 0.05) than that of other samples. In the IPN samples (the composition listed in Table 2), the EWC values decreased with the gelatin content increasing, indicating that the hydrogel network formed more efficiently. Meanwhile, the semi-IPN exhibited similar trend with increasing of gelatin content. Among all these samples, I3 showed the lowest EWC value of 13.67, which can be ascribed to the higher crosslinking degree of the hydrogel. Otherwise the H5 hydrogel was formed at low crosslinking degree, resulting in a relatively loose structure with the highest EWC value of 38. From these data, it seems there was no significant difference (p > 0.05) between the EWC values of semi-IPN and IPN under certain gelatin content. However, the involved principle of EWC value in semi-IPN samples was essentially different from that of the IPN. The equilibrium water content was performed at room temperature. Under this condition, the physically crosslinked gelatin would make the structure of semi-IPN samples more compact, which could reduce the EWC value of semi-IPN samples significantly.3.5 In vitro cytotoxicity
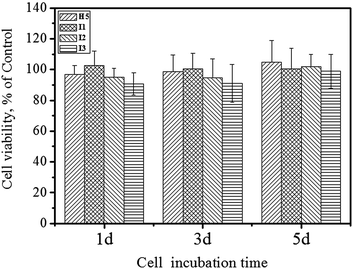
In vitro cytotoxicity of the IPN hydrogels was assessed, using mouse fibroblast cells (L929) incubated with extract solution for 1, 3, 5 days. As shown in Fig. 7, quantitative assessment of the cytotoxicity by MTT assay revealed that there were no significant differences in cytotoxicity among all the hydrogels. The calculated value of RGR, confirmed the excellent biocompatible nature of gelatin/HA-Ty IPN hydrogel, which should meet the requirements of potential materials for biomedical applications.3.6 Cell seeding and imaging
The ability of materials supporting cell adhesion, spreading and proliferation is an important deciding factor for tissue engineering materials. It's reported that mammalian cells are unable to adhere or spread on hyaluronic acid, since the unmodified hyaluronic acid promotes minimal protein adsorption.28–30 The gelatin/HA-Ty IPN hydrogels prepared in this experiment showed excellent performance on cell adhesion and spreading. It was mainly due to the introduction of cell adhesion moieties on the gelatin, which have a good interaction with cells. As shown in Fig. 8, almost all the cells were found to spreading well on the IPN hydrogels prepared by bienzymatic crosslinking method. By the 5th day, the fibroblasts on all the IPN hydrogels with different gelatin concentrations had covered about 90% of the samples' surfaces. The results confirmed that, the hyaluronic acid and gelatin formed IPN hydrogels, not only have no cytotoxicity, but also promote cell spreading and proliferation well. In this system, the introduction of two enzymes did not bring any negative impact and the as-prepared IPN hydrogels possessed good biomedical value. | ||
| Fig. 8 L929 cells inoculated on H5, culture plate, I1, I2, I3 and I4 gels with a density of 5 × 104 cells per cm2: inverted microscope pictures (160×) of cells after 5 days post-seeding. | ||
4. Conclusion
In this paper, a series of gelatin/HA-Ty IPN hydrogels were obtained by bienzymatic crosslinking method. The rheological tests proved the interpenetrating network structure of the IPN system. Compared with pure HA-Ty hydrogel, the compressive strength of IPN hydrogels has more than 10 folds increase. Furthermore, the hydrogels have tunable water content and degradability by adjusting compositions. In addition, cell seeding experiments revealed the IPN hydrogels could promote cell spreading and proliferation well. In general, the bienzymatically crosslinked gelatin/HA-Ty IPN hydrogels are anticipated to have potential in tissue engineering scaffolds, wound repair and drug delivery. Moreover, the bienzymatic crosslinking approach can be applied to other natural polymers, such as fibrinogen and heparin, to extend the library of IPN hydrogel biomaterials and their applications in the biomedical field.Author contributions
X. Li developed the original idea, analyzed data and prepared the manuscript. Z. Fan carried out the experiments, analyzed data and prepared the manuscript. Y. Zhang, S. Fang, C. Xu, contributed to the critical discussion and revision of the manuscript. All authors have given approval to the final version of the manuscript.Acknowledgements
Projects 51073036 and 51373034 were supported by the National Natural Science Foundation of China.Notes and references
- R. S. Ashton, A. Banerjee, S. Punyani, D. V. Schaffer and R. S. Kane, Biomaterials, 2007, 28, 5518–5525 CrossRef CAS PubMed
.
- P. Li, K. Xu, Y. Tan, C. Lu, Y. Li, H. Wang, X. Liang and P. Wang, RSC Adv., 2014, 4, 37812–37815 RSC
.
- D. M. Kirchmajer, C. A. Watson, M. Ranson and M. i. h. Panhuis, RSC Adv., 2013, 3, 1073–1081 RSC
.
- H. Zhang, D. Zhai and Y. He, RSC Adv., 2014, 4, 44600–44609 RSC
.
- C. Wen, L. Lu and X. Li, Macromol. Mater. Eng., 2014, 299, 504–513 CrossRef CAS
.
- L. Liu and H. Sheardown, Biomaterials, 2005, 26, 233–244 CrossRef CAS PubMed
.
- K. M. Park, K. S. Ko, Y. K. Joung, H. Shin and K. D. Park, J. Mater. Chem., 2011, 21, 13180 RSC
.
- R. J. Williams, T. E. Hall, V. Glattauer, J. White, P. J. Pasic, A. B. Sorensen, L. Waddington, K. M. McLean, P. D. Currie and P. G. Hartley, Biomaterials, 2011, 32, 5304–5310 CrossRef CAS PubMed
.
- L.-S. Wang, J. Boulaire, P. P. Y. Chan, J. E. Chung and M. Kurisawa, Biomaterials, 2010, 31, 8608–8616 CrossRef CAS PubMed
.
- L. S. Moreira Teixeira, J. Feijen, C. A. van Blitterswijk, P. J. Dijkstra and M. Karperien, Biomaterials, 2012, 33, 1281–1290 CrossRef CAS PubMed
.
- J. H. Ryu, Y. Lee, M. J. Do, S. D. Jo, J. S. Kim, B.-S. Kim, G.-I. Im, T. G. Park and H. Lee, Acta Biomater., 2014, 10, 224–233 CrossRef CAS PubMed
.
- R. Jin, C. Lin and A. Cao, Polym. Chem., 2014, 5, 391–398 RSC
.
- F. Lee, J. E. Chung and M. Kurisawa, J. Controlled Release, 2009, 134, 186–193 CrossRef CAS PubMed
.
- K. S. Kim, S. J. Park, J. A. Yang, J. H. Jeon, S. H. Bhang, B. S. Kim and S. K. Hahn, Acta Biomater., 2011, 7, 666–674 CrossRef CAS PubMed
.
- K. Xu, F. Lee, S. J. Gao, J. E. Chung, H. Yano and M. Kurisawa, J. Controlled Release, 2013, 166, 203–210 CrossRef CAS PubMed
.
- A. Darr and A. Calabro, J. Mater. Sci.: Mater. Med., 2009, 20, 33–44 CrossRef CAS PubMed
.
- B. P. Lee, J. L. Dalsin and P. B. Messersmith, Biomacromolecules, 2002, 3, 1038–1047 CrossRef CAS PubMed
.
- Y. K. Joung, S. S. You, K. M. Park, D. H. Go and K. D. Park, Colloids Surf., B, 2012, 99, 102–107 CrossRef CAS PubMed
.
- E. Lih, J. S. Lee, K. M. Park and K. D. Park, Acta Biomater., 2012, 8, 3261–3269 CrossRef CAS PubMed
.
- L. S. Moreira Teixeira, S. Bijl, V. V. Pully, C. Otto, R. Jin, J. Feijen, C. A. van Blitterswijk, P. J. Dijkstra and M. Karperien, Biomaterials, 2012, 33, 3164–3174 CrossRef CAS PubMed
.
- S. Sakai, Y. Liu, T. Matsuyama, K. Kawakami and M. Taya, J. Mater. Chem., 2012, 22, 1944–1949 RSC
.
- R. Jin, B. Lou and C. Lin, Polym. Int., 2013, 62, 353–361 CrossRef CAS
.
- M. Kurisawa, J. E. Chung, Y. Y. Yang, S. J. Gao and H. Uyama, Chem. Commun., 2005, 4312–4314 RSC
.
- V. Kuzmenko, D. Hägg, G. Toriz and P. Gatenholm, Carbohydr. Polym., 2014, 102, 862–868 CrossRef CAS PubMed
.
- T. Chen, H. D. Embree, E. M. Brown, M. M. Taylor and G. F. Payne, Biomaterials, 2003, 24, 2831–2841 CrossRef CAS
.
- X. Zhang, Y. Yang, J. Yao, Z. Shao and X. Chen, ACS Sustainable Chem. Eng., 2014, 2, 1318–1324 CrossRef CAS
.
- C. Wen, L. Lu and X. Li, J. Appl. Polym. Sci., 2014, 131 DOI:10.1002/app.40975
.
- Y. Lei, S. Gojgini, J. Lam and T. Segura, Biomaterials, 2011, 32, 39–47 CrossRef CAS PubMed
.
- F. Z. Cui, W. M. Tian, S. P. Hou, Q. Y. Xu and I. S. Lee, J. Mater. Sci.: Mater. Med., 2006, 17, 1393–1401 CrossRef CAS PubMed
.
- X. Z. Shu, K. Ghosh, Y. Liu, F. S. Palumbo, Y. Luo, R. A. Clark and G. D. Prestwich, J. Biomed. Mater. Res., Part A, 2004, 68A, 365–375 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |