Pyrrole[3,2-d:4,5-d′]bisthiazole-bridged bis(naphthalene diimide)s as electron-transport materials†
Yulia A.
Getmanenko
a,
Sanjeev
Singh
b,
Bhupinder
Sandhu
c,
Cheng-Yin
Wang
b,
Tatiana
Timofeeva
c,
Bernard
Kippelen
b and
Seth R.
Marder
*a
aSchool of Chemistry & Biochemistry and Center for Organic Photonics and Electronics, Georgia Institute of Technology, 901 Atlantic Drive NW, Atlanta, GA 30332-0400, USA. E-mail: seth.marder@chemistry.gatech.edu
bSchool of Electrical and Computer Engineering and Center for Organic Photonics and Electronics, Georgia Institute of Technology, 777 Atlantic Drive NW, Atlanta, GA 30332-0250, USA
cDepartment of Chemistry, New Mexico Highlands University, Las Vegas, NM 87701, USA
First published on 14th November 2013
Abstract
A series of materials with 2,6-disubstituted-N-alkyl-pyrrole[3,2-d:4,5-d′]bisthiazole (PBTz) with triisopropylsilyl- (TIPS), bromo- and naphthalene diimide (NDI) groups were synthesized. The electronic properties of 2,6-bis-TIPS- and 2,6-dibromo-N-hexyl-PBTz were studied by cyclic voltammetry and by density functional theory (DFT) calculations, and their solid-state packing was examined by the single crystal X-ray structural analysis. DFT calculations and the electrochemical data revealed that this core is both a weak donor and a weak acceptor. Small molecules with bis(NDI)-substituted N-alkyl-PBTz architecture were studied by differential pulse voltammetry, UV-vis absorption spectroscopy, and differential scanning calorimetry, and their electrical properties were examined in n-channel organic field-effect transistors using solution-processed films. The electron mobility value μe as high as 0.13 cm2 V−1 s−1 with a Ion/Ioff ratio of 5 × 105 and threshold voltage Vth = 4.9 V was observed for PBTz-bridged bis(naphthalene diimide) with hexyl chains on pyrrole and NDI nitrogen atoms, while the material with longer dodecyl groups showed μe up to 0.19 cm2 V−1 s−1 with a Ion/Ioff ratio of 7 × 104 and Vth = 7.9 V in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 polystyrene matrix. Finally, compounds with electron-withdrawing acetyl groups at position 6 of the NDI units were examined by electrochemistry and in OFET configurations.
1 polystyrene matrix. Finally, compounds with electron-withdrawing acetyl groups at position 6 of the NDI units were examined by electrochemistry and in OFET configurations.
Introduction
Molecular electron-transporting materials based on rylene diimides, such as perylene diimide and naphthalene diimide (NDI), have been explored extensively over the last decade, and several materials have shown efficient1–4 and, in some cases, air stable performance2–10 in n-channel organic field-effect transistors (OFETs). Our group has recently investigated molecular semiconductors with NDI–bridge–NDI structures,1,11,12 where the bridge was either a strong donor1 or acceptor.11,12 While the bridge has a strong effect on the electrochemical properties, it was found that electron transport can be efficient (electron mobility μe = 0.1–0.2 cm2 V−1 s−1) for a wide variety of bridges, with the highest electron mobility μe (μe = 1.5 cm2 V−1 s−1) observed for ambipolar material1 with the strongest donor, 4-hexyl-4H-dithieno[3,2-b:2′,3′-d]pyrrole (DTP). The previously reported NDI–bridge–NDI semiconductors showed modest Ion/Ioff ratios of 103 to 104 and threshold voltages, Vth, between 3.4 and 16.2 V.1,11,12 In an attempt to maintain high electron mobility μe observed in NDI–DTP–NDI material and to suppress hole mobility (which was achieved for NDI-based materials with donors weaker than DTP1 and moderate-to-strong acceptors as bridges,12 but was also accompanied by one order decrease in μe) we designed materials with a relatively unexplored pyrrole[3,2-d:4,5-d′]bisthiazole (PBTz) bridge.13,14 The substitution of CH in DTP with N in PBTz was expected to lead to an increase in molecular planarity in target materials 6 and possibly delocalization of the LUMO. The electronic properties of the bridge PBTz and its influence on the properties of the target NDI–bridge–NDI semiconductors were studied by cyclic voltammetry (CV), differential pulse voltammetry (DPV) and density functional theory (DFT).Intermediates 4-hexyl-2,6-bis(triisopropylsilyl)-4H-pyrrolo[2,3-d:5,4-d′]bisthiazole (2a) and 2,6-dibromo-4-hexyl-4H-pyrrolo[2,3-d:5,4-d′]bisthiazole (3a) were also studied by the single crystal X-ray analysis. Thermal properties of the new materials 6 were studied by the differential scanning calorimetry (DSC). In order to shift the 1st reduction of NDI–bridge–NDI to a more positive potential we also designed new NDI–tin derivatives 5c and 5d (analogous to one previously reported15) with acetyl electron-withdrawing groups at position 6, and utilized them in the construction of NDI-based materials with a PBTz bridge and, for comparison, with a 7H-cyclopenta[1,2-d:4,3-d′]bisthiazol-7-one bridge.12,16
The electrical properties of the materials 6 and 8 were studied by the fabrication of n-channel top-gate bottom-contact OFETs with CYTOP/Al2O3 bilayer gate dielectric17 and Au source/drain electrodes using solution-processed films.
Results and discussion
Synthesis
Pyrrole[3,2-d:4,5-d′]bisthiazoles containing either N-hexyl (2a) or N-dodecyl (2b) alkyl chains were prepared from 4,4′-dibromo-2,2′-bis(triisopropylsilyl)-5,5′-bithiazole181 by the Buchwald–Hartwig19,20 amination reaction using a modified literature procedure13 (boiling mesitylene under a normal atmosphere was used instead of toluene in a pressurized vessel) (Scheme 1). Triisopropylsilyl (TIPS) groups were removed using tetra-n-butylammonium fluoride (TBAF) in tetrahydrofuran (THF) followed by the double bromination with N-bromosuccinimide (NBS) in anhydrous N,N-dimethylformamide (DMF).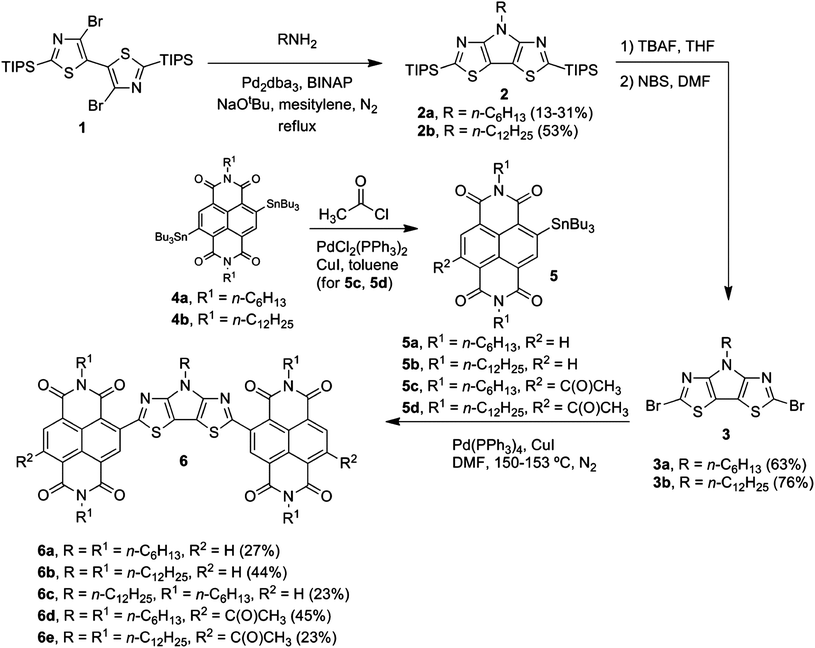 | ||
| Scheme 1 Synthesis of NDI–tin precursors and their use in the preparation of the materials with a pyrrole[3,2-d:4,5-d′]bisthiazole bridge. | ||
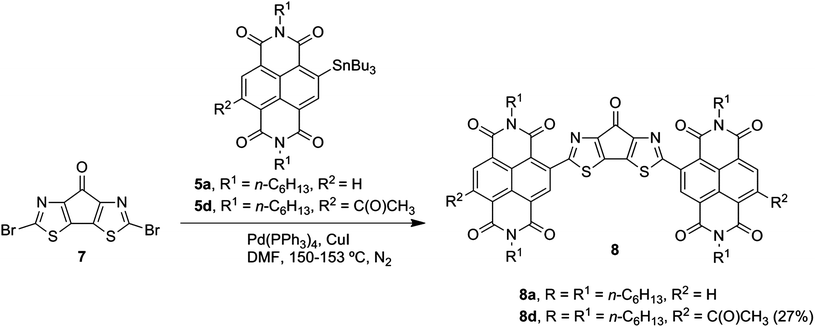
New NDI–tin reagents 5c and 5d with acetyl electron-withdrawing groups were prepared from NDI–ditin15 reagents 4 by the Stille coupling21 with acetyl chloride. The target products 6 (Scheme 1) and 8 (Scheme 2) were synthesized via copper-mediated22 Stille coupling of dibromides 3a, 3b and 7, respectively, with NDI–tin reagents 5.
Electrochemical properties of the 4H-pyrrolo[2,3-d:5,4-d′]bisthiazole core and DFT calculations
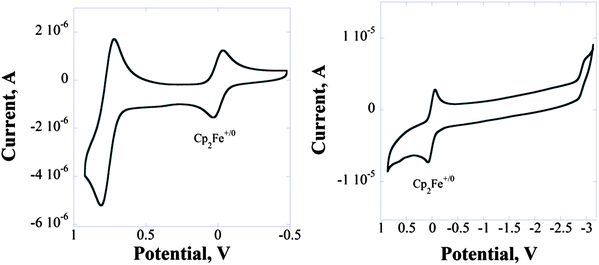
CV analysis of 2a in 0.1 M Bu4PF6 in dichloromethane showed a reversible oxidation wave with the first half-wave reduction potential at +0.76 V vs. ferrocene/ferrocenium Cp2Fe0/+ (no reduction was observed within the solvent window) (Fig. 1). The irreversible reduction process was observed in 0.1 M Bu4PF6 in tetrahydrofuran with a cathodic peak potential Epc at −2.9 V. In comparison with a strong DTP donor (for the 2,6-dibutyl-N-t-butyl derivative23 of DTP E0/+1/2 = +0.23 in 0.1 M Bu4PF6 in dichloromethane) PBTz is a considerably weaker donor, while in comparison with 7H-cyclopenta[1,2-d:4,3-d′]bisthiazol-7-one16 (for the TIPS derivative E0/−1/2 = −1.47 V in 0.1 M Bu4PF6 in dichloromethane) it is a much weaker acceptor.Substitution of TIPS-groups with bromine increased the reduction potential of 3a by ∼0.4 V (Epc = −2.5 V; Fig. S14†) (for 7H-cyclopenta[1,2-d:4,3-d′]bisthiazol-7-one the substitution of the TIPS group with bromine resulted in a reduction which is anodically shifted by 0.26 V (7, E0/−1/2 = −1.21 V (ref. 12))).
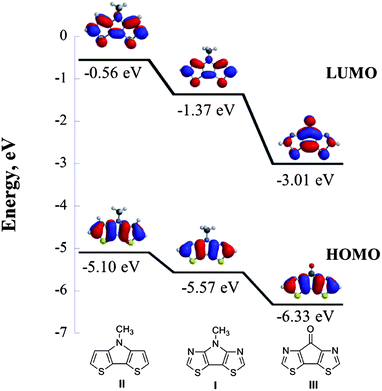
DFT calculations for 4-methyl-4H-pyrrolo[2,3-d:5,4-d′]bis(thiazole) (I), 4-methyl-4H-dithieno[3,2-b:2′,3′-d]pyrrole II‡ and 7H-cyclopenta[1,2-d:4,3-d′]bisthiazol-7-one III§ were performed at the B3LYP/6-31G** level of theory. The HOMO and LUMO wavefunctions of pyrrole derivatives I and II are very similar, while the introduction of nitrogens into I lowers both the HOMO (by 0.47 eV) and LUMO (by 0.81 eV), confirming that the 4-methyl-4H-pyrrolo[2,3-d:5,4-d′]bisthiazole core is a weak donor and a weak acceptor (Fig. 2).
Single crystal X-ray structural analysis of intermediates 2a and 3a
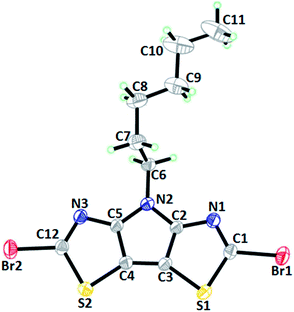
We were able to obtain single crystals of two PBTz derivatives with TIPS (2a) (Fig. S32†) and bromine (3a) groups at positions 2 and 6 (Fig. 3).Compound 2a crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with one molecule per asymmetric unit. The molecule is characterized by a planar tricyclic core as indicated by the dihedral angle between thiazole rings of 1.03(9)°. The molecules stack along the a direction, but the bulky TIPS substituents at 2 and 6 positions and the alkyl group at the N1 position prevent any kind of short intermolecular contacts in this structure (Fig. 4). The neighbouring molecules pack in layers parallel to the bc plane.
space group with one molecule per asymmetric unit. The molecule is characterized by a planar tricyclic core as indicated by the dihedral angle between thiazole rings of 1.03(9)°. The molecules stack along the a direction, but the bulky TIPS substituents at 2 and 6 positions and the alkyl group at the N1 position prevent any kind of short intermolecular contacts in this structure (Fig. 4). The neighbouring molecules pack in layers parallel to the bc plane.
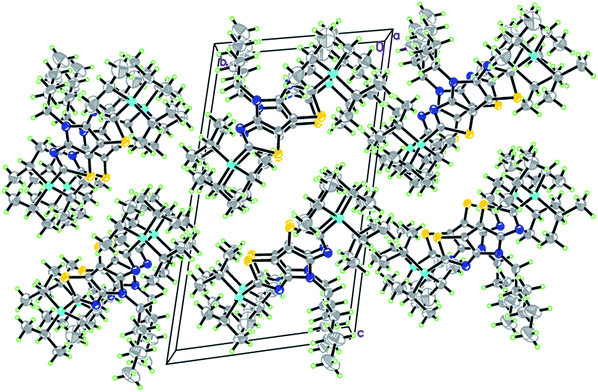
Compound 3a crystallizes in the monoclinic P21/n space group with one molecule per asymmetric unit. The molecule is characterized by a planar tricyclic core as indicated by the dihedral angle between thiazole rings of 2.26(9)°.
The long hexyl chain defines the specific features of self-association in this structure, as the best fitting of neighbouring molecules is provided by their practically perpendicular arrangement with the dihedral angle between their tricyclic cores of 81.91(3)° (Fig. 5). The self-association patterns are generated via three short intermolecular contacts, Br(1)⋯Br(2) = 3.752(5) Å, Br(2)⋯N(1) = 3.279(2) Å and S(2)⋯N(2) = 3.241(2) Å, that results in the herring-bone motif. No pronounced π–π stacking interactions with the short interplanar distances have been found in both structures. The angle between vectors Br(1)–C(1) and Br(2)–C(12) in 3a is 173.1°, which is the closest to the linear arrangement between substituents at positions 2 and 6 in comparison with the series of the tricyclic dihalides investigated previously.12
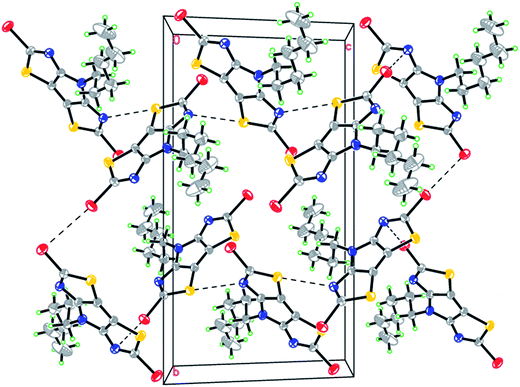 | ||
| Fig. 5 Fragment of crystal packing along the a axis in 3a; Br(2)⋯N(1) = 3.279(2) Å, S(2)⋯N(2) = 3.241(2) Å, Br(1)⋯Br(2) = 3.752(5) Å. | ||
Electrochemical, UV-vis, DSC analyses of the target molecules 6a–e, 8a, and 8b
CV and DPV analyses were used to study the electrochemical properties of the final products 6 and 8 using 0.1 M Bu4NPF6 in tetrahydrofuran (THF) and chloroform (this solvent with a narrow window was used due to a very limited solubility of materials 6a and 6c in THF or dichloromethane). The material 6b showed the first reduction at −1.09 V in 0.1 M Bu4NPF6 in THF vs. Cp2Fe0/+ (Table 1) and was only slightly easier to reduce in comparison with the isolated NDI unit (E0/−1/2 = −1.13 V in 0.1 M Bu4NPF6 in THF vs. Cp2Fe0/+). In materials 6a–c, which only differ by the length of the chains on NDI and PBTz moieties, the first reduction potential determined by DPV in chloroform slightly varied from −1.00 V to −1.05 V. The introduction of the electron-withdrawing acetyl group to the NDI units into 6d and 6e resulted in an anodic shift in the first reduction potential by 0.10 V and 0.15 V in comparison with 6a and 6b, respectively. In 7H-cyclopenta[1,2-d:4,3-d′]bisthiazol-7-one-based materials 8, the incorporation of acetyl groups resulted in a somewhat smaller anodic shift (0.07 V) in chloroform, while in THF 8b with acetyl groups is easier to reduce than 8a by 0.10 V.| Compound | λ max, nm (CHCl3) | Solvent | E red1, V | E red2, V | E red3, V | E red4, V |
|---|---|---|---|---|---|---|
| a Determined by the DPV analysis. b Appears as a shoulder. c Two well resolved maxima were observed for the second reduction. | ||||||
| 6a | 362, 382, 590 | CHCl3 | −1.00 | −1.40 | n/a | n/a |
| 6b | 362, 382, 592 | CHCl3 | −1.04 | −1.42 | n/a | n/a |
| THF | −1.09 | −1.52 | −2.33 | −2.63 | ||
| 6c | 362, 382, 590 | CHCl3 | −1.05 | −1.42 | n/a | n/a |
| 6d | 363, 383, 613 | CHCl3 | −0.90b | −1.00 | −1.44 | n/a |
| 6e | 363, 383, 610 | CHCl3 | −0.89b | −0.96 | −1.36 | n/a |
| 8a | 361, 383, 496 | THF | −0.95 | −1.42, −1.50c | −1.87 | −2.33 |
| CHCl3 | −0.91b | −0.96 | −1.34 | n/a | ||
| 8b | 361, 382, 512 | THF | −0.85 | −1.33 | −1.83 | −2.22 |
| CHCl3 | −0.84 | −1.28 | n/a | n/a | ||
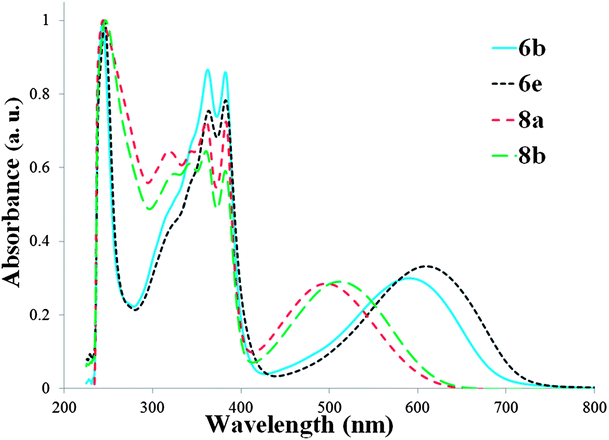
As observed for previously studied NDI–bridge–NDI materials,1,11,12 the UV-vis absorption spectra of the materials 6 and 8 exhibit two major bands: a strong one in the high-energy part of the spectra (this band is almost unaffected by the nature of the bridge) and a weaker one in the visible part of the spectra (this band is assigned to the transition from the HOMO of the bridge to the LUMO predominantly located on the NDI units, and as a result is strongly affected by the bridge) (Fig. 6).
The donor strength of PBTz (E0/+1/2 = +0.76 V) is comparable to the one of 3,5-dihexyl-4H-cyclopenta[1,2-b:5,4-b′]dithiophen-4-one16 (E0/+1/2 = +0.83 V in 0.1 M Bu4NPF6 in dichloromethane vs. Cp2Fe0/+), and λmax values of the low-energy band of NDI derivatives with these bridges are also comparable (592 nm in CHCl3 for 6b; 577 nm in CH2Cl2 for 3,5-dihexyl-4H-cyclopenta[1,2-b:5,4-b′]dithiophen-4-one-based material)12 (a slight red-shift is observed for 6b with the PBTz bridge, which has slightly stronger electron-donating properties in comparison with 3,5-dihexyl-4H-cyclopenta[1,2-b:5,4-b′]dithiophen-4-one bridge). The introduction of electron-withdrawing acetyl groups to the NDI units into 6e and 8b led to a slight red-shift of the low-energy band in comparison with 6b and 8a (by 18 and 16 nm, respectively).
The thermal behavior of the materials was studied by the DSC analysis. With the exception of the material 6b, which showed a melting process on heating and crystallization on cooling (Fig. S19†), materials 6a, 6c, 6d, 6e, 8a, and 8b exhibited no crystallization on cooling (melting events were observed at rather high temperatures in the range from 358 to 415 °C). The lack of crystallization events suggested that the melting process was accompanied by decomposition.
In comparison with known N-dodecyl-NDI-based materials with 4H-cyclopenta[1,2-b:5,4-b′]dithiophen-4-one¶ and 4H-cyclopenta[1,2-b:5,4-b′]dithiazol-4-one bridges,|| the PBTz derivative 6b had a substantially higher reversible melting point at 343 °C,** which provides evidence for improved thermal stability and stronger intermolecular interactions.
OFET characterization
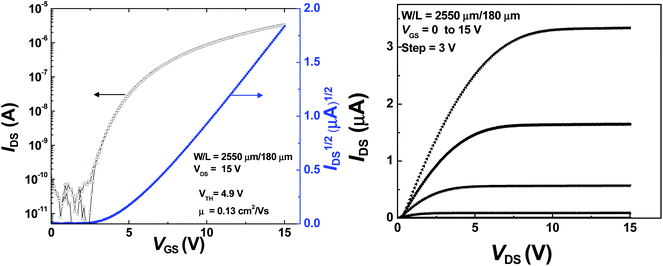
The electrical properties of the materials 6a–d and 8a were investigated by fabricating top-gate, bottom-contact geometry organic field-effect devices with a CYTOP/Al2O3 bilayer gate dielectric and Au source/drain electrodes (Table 2). Three different solvent systems were examined for 6a (1,1,2,2-tetrachloroethane (1,1,2,2-TCE)), chloroform (CF) and a mixture of chlorobenzene (CB) and CF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and it was found that the best device characteristics were obtained for the film spun from TCE with μe reaching 0.13 cm2 V−1 s−1 (Fig. 7), while the use of chloroform resulted in a substantial decrease in μe (down to a value of 1.1 × 10−2 cm2 V−1 s−1 for the best device) and in Ion/Ioff (down to a value of 7 × 103). The use of the mixture of the solvents (high-boiling point CB and low-boiling point CF) for the spin-coating of 6a resulted in slightly better transistor characteristics (Table 2, entry 3) in comparison with the one from CF (Table 2, entry 2).
4), and it was found that the best device characteristics were obtained for the film spun from TCE with μe reaching 0.13 cm2 V−1 s−1 (Fig. 7), while the use of chloroform resulted in a substantial decrease in μe (down to a value of 1.1 × 10−2 cm2 V−1 s−1 for the best device) and in Ion/Ioff (down to a value of 7 × 103). The use of the mixture of the solvents (high-boiling point CB and low-boiling point CF) for the spin-coating of 6a resulted in slightly better transistor characteristics (Table 2, entry 3) in comparison with the one from CF (Table 2, entry 2).
| Entry | Compound | W (μm)/L (μm) | C in (nF cm−2) | Solvent | S/D electrode | N | μ e (cm2 V−1 s−1) | V th (V) | I on/Ioff |
|---|---|---|---|---|---|---|---|---|---|
| a Number of devices used to calculate the average values. b Due to low solubility of the material and poor film quality only one working device was obtained. | |||||||||
| 1 | 6a | 2550/180 | 35.2 | 1,1,2,2-TCE | Au | 4 | 0.128(±0.005) | 4.9(±0.3) | 5 × 105 |
| 2 | 6a | 2550/180 | 35.2 | CF | Au | 9 | 6.4(±4.4) × 10−3 | 4.2(±1.6) | 7 × 103 |
| 3 | 6a | 2550/180 | 35.2 | CB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CF (4 CF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Au | 8 | 2.3(±0.9) × 10−2 | 3.7(±0.8) | 3 × 104 |
| 4 |
6b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS (1 PS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2550/180 | 35.2 | DCB | Au | 7 | 0.15(±0.03) | 7.9(±0.5) | 7 × 104 |
| 5 | 6b | 2550/180 | 35.2 | 1,1,2,2-TCE | Au | 3 | 3.5(±1.4) × 10−2 | 7.6(±0.6) | 3 × 104 |
| 6 | 6c | 2550/180 | 35.2 | 1,1,2,2-TCE | Au | 8 | 7.9(±7.4) × 10−3 | 15.9(±1.6) | 1 × 103 |
| 7 | 6d | 2550/180 | 35.2 | DCB, 1,1,2,2-TCE, CF | Au | Not measurable | n/a | n/a | |
| 8 | 6e | 2550/180 | 35.2 | DCB, 1,1,2,2-TCE | Au | Not measurable | n/a | n/a | |
| 9 | 6e | 2550/180 | 35.2 | CF | Au | 1b | 2.5 × 10−5 | 9.6 | 30 |
| 10 | 8a | 2550/180 | 35.2 | 1,1,2,2-TCE | Au | 8 | 0.10(±0.02) | 6.2(±0.8) | 5 × 104 |
| 11 | 8a | 1000/180 | 35.2 | DCB | Au | 5 | 6.3(±2.6) × 10−3 | 4.9(±1.1) | 1 × 104 |
| 1212 | 8a | 2550/180 | 35.2 | 1,1,1,2-TCE | Au | 8 | 0.059(±0.004) | 7.3(±0.6) | 1 × 104 |
| 13 | 8b | 2550/180 | 35.2 | 1,1,1,2-TCE | Au | 7 | 2.1(±0.5) × 10−3 | 4.1(±0.2) | 3 × 103 |
| 14 | 8b | 2550/180 | 35.2 | DCB | Au | 4 | 8.8(±8.5) × 10−5 | 4.0(±0.8) | 2 × 102 |
To investigate the operational air stability, the continuous dc bias stress of OFET devices fabricated with 1,1,2,2-TCE-processed 6a with VGS = VDS = 15 V was measured over a 1 hour period in ambient air and under a nitrogen atmosphere (Fig. S12†). The decay of the source–drain current IDS(t), normalized by its initial value IDS(0), is identical under both conditions, which indicated that devices remained stable under both conditions within the tested time.
Increasing the alkyl chains from hexyl groups in 6a to longer dodecyl chains in 6b resulted in deterioration of the device characteristics when 6b was processed from TCE (Table 2, entry 5). In order to avoid the dewetting issue observed for the material 6b when DCB was used as a solvent, 6b was blended with polystyrene24,25 (PS) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight) (Table 2, entry 4) and DCB as a solvent, and the devices yielded a performance close to that of the best ones obtained for 6a. The material 6c with a mixed length of the alkyl chains resulted in a larger variation in the device performance (Table 2, entry 6), with the best μe reaching a value of 2.4 × 10−2 cm2 V−1 s−1 and a Ion/Ioff ratio of 104, although the threshold voltage increased substantially to a value of 15.4 V. The introduction of the electron-withdrawing acetyl group on NDI moieties into 6d and 6e, which could lower charge injection barriers, led to a drastic reduction of the electron transport below the sensitivity of our experiments (Table 2, entries 7 and 8). Only one working device (Table 2, entry 9) with poor characteristics was obtained for 6e when the film was processed from CF. Low solubility and, as a result, difficulties with the formation of the high-quality film might be a reason for the poor electrical behaviour of these derivatives.
1 ratio by weight) (Table 2, entry 4) and DCB as a solvent, and the devices yielded a performance close to that of the best ones obtained for 6a. The material 6c with a mixed length of the alkyl chains resulted in a larger variation in the device performance (Table 2, entry 6), with the best μe reaching a value of 2.4 × 10−2 cm2 V−1 s−1 and a Ion/Ioff ratio of 104, although the threshold voltage increased substantially to a value of 15.4 V. The introduction of the electron-withdrawing acetyl group on NDI moieties into 6d and 6e, which could lower charge injection barriers, led to a drastic reduction of the electron transport below the sensitivity of our experiments (Table 2, entries 7 and 8). Only one working device (Table 2, entry 9) with poor characteristics was obtained for 6e when the film was processed from CF. Low solubility and, as a result, difficulties with the formation of the high-quality film might be a reason for the poor electrical behaviour of these derivatives.
Re-investigation of the electric properties of 8a12 (Table 2, entry 12) after extensive purification resulted in improved device characteristics with μe reaching 0.12 cm2 V−1 s−1, Vth = 6.5 V and Ion/Ioff = 5 × 104 (Table 2, entry 10) when the film was processed from 1,1,2,2-TCE. Interestingly, DCB was found to be a superior solvent for PBTZ-based materials 6a and 6b in comparison with TCE, but this solvent led to a substantial decrease in μe down to a value of 9.4 × 10−3 and a Ion/Ioff ratio down to 1 × 104 for 8a (Table 2, entry 11). A similar trend of reduction of μe and Ion/Ioff upon changing from 1,1,2,2-TCE to DCB was also observed for the acetyl derivative 8b, which showed μe values up to 2.8 × 10−3 cm2 V−1 s−1 for the 1,1,2,2-TCE-processed film (Table 2, entry 14) and 2.0 × 10−4 cm2 V−1 s−1 for the DCB-processed film (Table 2, entry 15). These findings yet again emphasize the importance of optimizing the choice of solvent for each individual class of compounds.
Conclusions
Electronic properties of N-alkylpyrrole[3,2-d:4,5-d′]bisthiazole were studied by the cyclic voltammetry and DFT calculations, and it was found that this tricycle has weak donor and weak acceptor properties. The DFT calculations suggest that this core can be used to effectively extend the conjugation of the NDI–bridge–NDI system due to the decreased steric hindrance and as a result, increased planarity. The electron mobilities μe (0.1–0.2 cm2 V−1 s−1) for the solution-processed bis(NDI)-substituted N-alkyl-PBTz derivatives are comparable to the similarly structured semiconductors with thieno[3,2-b]thiophene, dithieno[3,2-b:2′,3′-d]thiophene and 4H-cyclopenta[2,1-b:3,4-b′]dithiophen-4-one as tricyclic bridges, while the Ion/Ioff ratio increased up to 5 × 105 for 6a with hexyl groups and 7 × 104 for 6b with dodecyl groups. The decrease of the threshold voltage Vth down to 4.9 V observed for 6a is another improvement achieved by the use of the PBTz as a bridge.Acknowledgements
Y.A.G. thanks Dr Chad Risko for helpful discussions. Mr Brian Seifried and Ms Taya Khunsupat are thanked for the synthesis of the mixture of mono- and dibromo-NDIs, which were used for the preparation of the NDI–tin reagents 4a,b and 5a,b. B.S. and T.T. thank Prof. Marina Fonari for help with the single crystal X-ray analysis. This work was supported in part by Solvay S.A., by the STC Program of the National Science Foundation under Agreement no. DMR-0120967, and by the PREM Program of the NSF under Agreement no. DMR-0934212.Notes and references
- L. E. Polander, S. P. Tiwari, L. Pandey, B. M. Seifried, Q. Zhang, S. Barlow, C. Risko, J. L. Bredas, B. Kippelen and S. R. Marder, Chem. Mater., 2011, 23, 3408–3410 CrossRef CAS.
- M. Stolte, M. Gsanger, R. Hofmockel, S. L. Suraru and F. Wurthner, Phys. Chem. Chem. Phys., 2012, 14, 14181–14185 RSC.
- Y. B. Hu, Y. K. Qin, X. K. Gao, F. J. Zhang, C. A. Di, Z. Zhao, H. X. Li and D. B. Zhu, Org. Lett., 2012, 14, 292–295 CrossRef CAS PubMed.
- B. J. Jung, K. Lee, J. Sun, A. G. Andreou and H. E. Katz, Adv. Funct. Mater., 2010, 20, 2930–2944 CrossRef CAS.
- H. E. Katz, A. J. Lovinger, J. Johnson, C. Kloc, T. Siegrist, W. Li, Y. Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478–481 CrossRef CAS PubMed.
- J. H. Zhang, L. Tan, W. Jiang, W. P. Hu and Z. H. Wang, J. Mater. Chem. C, 2013, 1, 3200–3206 RSC.
- K. C. See, C. Landis, A. Sarjeant and H. E. Katz, Chem. Mater., 2008, 20, 3609–3616 CrossRef CAS.
- B. A. Jones, A. Facchetti, T. J. Marks and M. R. Wasielewski, Chem. Mater., 2007, 19, 2703–2705 CrossRef CAS.
- W. Yue, A. F. Lv, J. Gao, W. Jiang, L. X. Hao, C. Li, Y. Li, L. E. Polander, S. Barlow, W. P. Hu, S. Di Motta, F. Negri, S. R. Marder and Z. H. Wang, J. Am. Chem. Soc., 2012, 134, 5770–5773 CrossRef CAS PubMed.
- Y. Jung, K. J. Baeg, D. Y. Kim, T. Someya and S. Y. Park, Synth. Met., 2009, 159, 2117–2121 CrossRef CAS PubMed.
- D. K. Hwang, R. R. Dasari, M. Fenoll, V. Alain-Rizzo, A. Dindar, J. W. Shim, N. Deb, C. Fuentes-Hernandez, S. Barlow, D. G. Bucknall, P. Audebert, S. R. Marder and B. Kippelen, Adv. Mater., 2012, 24, 4445–4450 CrossRef CAS PubMed.
- Y. A. Getmanenko, L. E. Polander, D. K. Hwang, S. P. Tiwari, E. Galán, B. M. Seifried, B. Sandhu, S. Barlow, T. Timofeeva, B. Kippelen and S. R. Marder, Journal of Organic Semiconductors, 2013, 1, 7–15 CrossRef CAS.
- M. Al-Hashimi, J. G. Labram, S. Watkins, M. Motevalli, T. D. Anthopoulos and M. Heeney, Org. Lett., 2010, 12, 5478–5481 CrossRef CAS PubMed.
- R. D. Xia, M. Al-Hashimi, W. C. Tsoi, M. Heeney, D. D. C. Bradley and J. Nelson, Sol. Energy Mater. Sol. Cells, 2012, 96, 112–116 CrossRef CAS PubMed.
- L. E. Polander, A. S. Romanov, S. Barlow, D. K. Hwang, B. Kippelen, T. V. Timofeeva and S. R. Marder, Org. Lett., 2012, 14, 918–921 CrossRef CAS PubMed.
- Y. A. Getmanenko, C. Risko, P. Tongwa, E. G. Kim, H. Li, B. Sandhu, T. Timofeeva, J. L. Bredas and S. R. Marder, J. Org. Chem., 2011, 76, 2660–2671 CrossRef CAS PubMed.
- D. K. Hwang, C. Fuentes-Hernandez, J. Kim, W. J. Potscavage, S. J. Kim and B. Kippelen, Adv. Mater., 2011, 23, 1293–1298 CrossRef CAS PubMed.
- Y. A. Getmanenko, P. Tongwa, T. V. Timofeeva and S. R. Marder, Org. Lett., 2010, 12, 2136–2139 CrossRef CAS PubMed.
- J. P. Wolfe, S. Wagaw and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 7215–7216 CrossRef CAS.
- J. Louie and J. F. Hartwig, Tetrahedron Lett., 1995, 36, 3609–3612 CrossRef CAS.
- D. Milstein and J. K. Stille, J. Org. Chem., 1979, 44, 1613–1618 CrossRef CAS.
- L. S. Liebeskind and R. W. Fengl, J. Org. Chem., 1990, 55, 5359–5364 CrossRef CAS.
- S. Barlow, S. A. Odom, K. Lancaster, Y. A. Getmanenko, R. Mason, V. Coropceanu, J. L. Bredas and S. R. Marder, J. Phys. Chem. B, 2010, 114, 14397–14407 CrossRef CAS PubMed.
- S. Goffri, C. Muller, N. Stingelin-Stutzmann, D. W. Breiby, C. P. Radano, J. W. Andreasen, R. Thompson, R. A. J. Janssen, M. M. Nielsen, P. Smith and H. Sirringhaus, Nat. Mater., 2006, 5, 950–956 CrossRef CAS PubMed.
- J. X. Zhang, S. Singh, D. K. Hwang, S. Barlow, B. Kippelen and S. R. Marder, J. Mater. Chem. C, 2013, 1, 5093–5100 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details and characterization for new materials; table with crystallographic data for 2a and 3a; HOMO and LUMO pictorial representations of 4-methyl-4H-pyrrolo[2,3-d:5,4-d′]bisthiazole-bridged NDI IV; Cartesian coordinates of I, II, III and IV; transfer and output characteristics of the n-channel OFET with 6a–c, and 8a and 8b as semiconductors; copies of 1H NMR spectra of 2a,b, 3a,b, 5c,d, 6a–e, 8a,b, copies of 13C{1H} NMR of 2b, 3a,b, 5c,d, 6b, 8b, and copies of DEPT-135 of 2b, 3a, and 5c. CCDC 957527 and 957528. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3tc31677g |
| ‡ 2,6-Dimethyl derivative of II was studied previously.23 |
| § 2,6-Bis-TIPS derivative of III was studied previously.16 |
| ¶ This material exhibited an irreversible melting point at 268.8 °C.12 |
| || This material had irreversible exothermic events at around 300 °C.12 |
| ** During the 2nd heating–cooling cycle the melting and crystallization events were observed at a slightly lower temperature, which might indicate partial decomposition of the sample (the sample was heated up to 350 °C). |
| This journal is © The Royal Society of Chemistry 2014 |