The contribution of chirality and crosslinker concentration to reflection wavelength tuning in structurally chiral nematic gels
Seth A.
Cazzell
ab,
Michael E.
McConney
ab,
Vincent P.
Tondiglia
ac,
Lalgudi V.
Natarajan
ac,
Timothy J.
Bunning
a and
Timothy J.
White
*a
aAir Force Research Laboratory, Materials and Manufacturing Directorate, 3005 Hobson Way, Bldng 651 Rm70, Wright Patterson Air Force Base, Ohio, USA. E-mail: timothy.white.24@us.af.mil
bAzimuth Corporation, Dayton, Ohio, USA
cScience Applications International Corporation, 3005 Hobson Way, Dayton, Ohio, USA
First published on 6th November 2013
Abstract
Tunable reflective material systems are potentially useful in a range of applications including displays, optical filters, photonics, and sensing. Presented here is a systematic study of thermally induced reflection wavelength tuning in structurally chiral nematic gels. The tuning is enabled by a swelling/de-swelling/re-swelling transition driven by order/disorder transitions in the liquid crystal and the liquid crystalline polymer network. By preparing liquid crystal polymer networks from single component liquid crystal monomers we isolate the contribution of chemical chirality and the crosslink density of the liquid crystal polymer network to thermally-induced reflection wavelength tuning. Further elucidating the fundamental relationships between the composition of the polymer network and the resulting optical properties is a key step towards enabling advancement and optimization necessary to realize the utility of this mechanism in devices such as mirrorless lasers.
Introduction
Chiral nematic liquid crystals can exhibit vivid coloration due to the periodic nature of the superstructure. Although the chiral nematic mesophase occurs naturally in certain chiral liquid crystals (such as cholesterols), it is typically studied in formulations composed of a chiral dopant and a nematic liquid crystal host. The chirality of the low molecular weight dopant can be readily transferred to the host mixture.1,2 The output reflection wavelength and bandwidth of the reflection bandgap are both directly proportional to the pitch. The pitch is defined as the lengthscale of one complete rotation of the director. In chiral nematic mixtures, the spectral position of the reflection bandgap (λb) is governed by the average refractive index of the mixture (![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) ) and the pitch length (P) of the chiral nematic as specified in eqn (1):
) and the pitch length (P) of the chiral nematic as specified in eqn (1):λb = ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) P P | (1) |
The pitch, and thus the reflection wavelength, can be varied by adjusting the chiral dopant concentration ([c]) through the following equation:
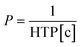 | (2) |
Stimuli-induced reflection wavelength tuning in chiral nematic mixtures has been a topic of considerable interest for nearly fifty years.3 As detailed in our recent review on the subject, reflection wavelength change in these materials has been induced by thermal, electrical, or optical stimuli.4 The focus of this work is on thermally-tunable liquid crystalline material systems. Extensive prior literature has detailed large scale changes in coloration in liquid crystal mixtures upon transition of certain smectic (Sm) mixtures into the chiral nematic (N*) phase.5–8 The large magnitude reflection wavelength change observable near the Sm → N* phase transition have been widely employed in commercial applications for thermometers and mood rings.
We have reported on the ability to generate dynamic changes in reflection wavelength within structurally chiral nematic gels (SCNGs).9,10 These works build upon a burgeoning area of liquid crystal materials science focused on the fabrication and characterization of what is referred to here as “structurally chiral” liquid crystalline materials. The term “structurally chiral” is used here to refer to chiral liquid crystal material systems that derive chirality from interactions with structured materials as opposed to the more conventional translation of chemical chirality. In one of the clearest examples of structural chirality reported to date, Robbie et al. elegantly illustrate that helicoidal structures written into ceramic materials can act as a structural template that forces achiral nematic liquid crystals to align into a structurally chiral nematic phase.11 Other groups have recently reported on similar translation of structure by templating liquid crystal polymer networks in the presence of a chiral nematic12–17 or blue phase.18 Through polymerizing in the chiral nematic phase the resulting polymer network is templated with the chiral structure, thereby forming a structurally chiral polymer template. The original chiral nematic mixture is removed through solvent exchange and the chiral polymer template is refilled with an achiral nematic liquid crystal (or other liquid crystal) to form the SCNG. The resulting SCNG exhibits selective reflection that closely matches the periodicity of the templated liquid crystal formulation, despite the absence of chemical chirality. SCNGs do not follow the pitch relationship presented in eqn (2) but are governed by the translation of the structure of the template polymer network to the backfilled liquid crystal host.
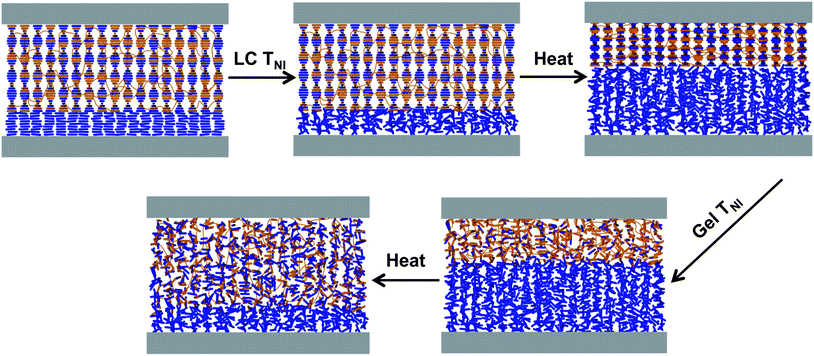
We have recently shown that reducing the crosslinking density of the polymer network can allow large scale changes in the pitch of a swollen polymer network (e.g., the “structure”) that are directly translated into reflection wavelength tuning.9,10 The underlying mechanism builds off the work of Urayama on deswelling/reswelling transitions in nematic gels swollen with nematic liquid crystals.19–22 This unique deswelling/reswelling transition is depicted in Scheme 1 for the systems examined here. The deswelling and reswelling transitions occur in the vicinity of the nematic–isotropic transition temperature of the liquid crystal solvent TSNI and the nematic–isotropic transition temperature of the swollen liquid crystal gel TGNI, respectively. At room temperature, T < TSNI, the anisotropic LC gel interacts favorably with the anisotropic liquid crystal solvent and thus the system is in a swollen state. Upon heating the sample above the liquid crystal solvent transition temperature TGNI > T > TSNI, the mismatch in the orientational interaction energy between the structurally chiral polymer network and the isotropic solvent drives the system through a deswelling transition. This deswelling transition causes a pitch compression that dictates a blue-shift in the reflection wavelength. Upon further heating to a temperature above the gel nematic–isotropic transition temperature T > TGNI the system is driven through a reswelling transition due to the once again favorable interaction between the isotropic gel and the isotropic solvent. This reswelling transition causes a subsequent pitch elongation that forces a red-shift in the reflection wavelength. Contact angle measurements of a droplet of liquid crystal placed upon the structurally chiral swollen gel provided strong support the orientational interaction energy was the driver of the dramatic contraction/expansion of the structurally chiral swollen gel.23
In these prior examinations of reflection wavelength tuning in SCNGs, we employed a commercially available mixture of chiral nematic liquid crystalline monomers of proprietary composition. This work is distinctive from our prior efforts in the isolation of the contribution of compositional variables to assess the effect of the properties of the polymer network on the resulting output optical properties including tuning range and baseline transmission. Towards this end, we examine more than twenty distinguished mixtures formulated from single component monoacrylate and diacrylate liquid crystalline monomers of known chemical structure to yield SCNGs of varying crosslink density, chirality, and connectivity of chiral monomers (pendant, crosslinked). We also examine the repeatability of the large range tuning. Explaining the relationship between the structure of the polymer network and the resulting optical properties is an important step in the development of these materials for dynamic tunable photonic and optics applications including mirrorless lasing.24
Materials and methods
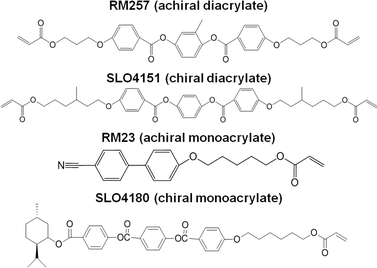
Liquid crystal cells were prepared by spin coating polyimide onto glass substrates. The polyimide-coated glass was then baked at 200 °C for at least 30 minutes to completely imidize the material. The polyimide coated substrates were subsequently rubbed with a velvet surface using a custom rubbing machine to induce a planar orientation in the alignment film. The liquid crystal cell was then assembled by gluing (NOA 68 optical adhesive mixed with 30 μm glass rod spacers) together two pieces of the rubbed, polyimide coated glass with the rubbing directions aligned antiparallel.The formulations examined here are summarized in Tables 1 and 2. The total monomer concentration was held constant at 20 wt% for all samples. As illustrated in Fig. 1, both chiral and achiral monomers were employed including the chiral diacrylate monomer SL04151 (Alpha Micron), an achiral diacrylate monomer RM257 (Merck), a chiral monoacrylate monomer SL04180 (Alpha Micron), and an achiral monoacrylate monomer RM23 (Merck). The chiral dopant R1011 (Merck) was added to blue shift the reflection notch to a measurable transmission notch wavelength (<2400 nm). The compounds were added to the nematic liquid crystal E7 (Merck) which served as the host. Importantly, this sample preparation procedure deviates from our previous work10 by including photoinitiator in the liquid crystal mixtures rather than inducing surface initiated polymerization by embedding photoinitiator in one of the rubbing layers. In the terms of our prior work, the “surface tethering” is achieved during the solvent exchange step by employing gravitational forces to concentrate the polymer network onto one side of the cell upon draining of the liquid crystal mixture. After drying, strong physical forces are sufficient to adhere the porous polymer network to the glass substrate.
| SL04151 | RM257 | RM23 | R1011 | I-907 | E7 | |
|---|---|---|---|---|---|---|
| CL-1.5 | 1.5 | 0 | 18.5 | 1.8 | 3.0 | 75.4 |
| CL-2.0 | 2.0 | 0 | 18.0 | 1.7 | 3.0 | 75.4 |
| CL-2.5 | 2.5 | 0 | 17.6 | 1.7 | 2.9 | 75.3 |
| CL-3.0 | 3.0 | 0 | 17.0 | 1.8 | 2.9 | 75.4 |
| CL-3.5 | 3.5 | 0 | 16.5 | 1.8 | 2.9 | 75.3 |
| C2-A0 | 2.0 | 0 | 18.0 | 1.7 | 3.0 | 75.3 |
| C1.5-A0.5 | 1.5 | 0.5 | 18.0 | 1.7 | 3.0 | 75.3 |
| C1-A1 | 1.0 | 1.0 | 18.0 | 1.7 | 3.0 | 75.4 |
| C0.5-A1.5 | 0.5 | 1.5 | 18.0 | 1.7 | 3.0 | 75.3 |
| C0-A2 | 0 | 2.0 | 18.0 | 1.7 | 3.0 | 75.3 |
| N-2400 | 2.0 | 0 | 18.0 | 1.1 | 3.0 | 76.0 |
| N-1425 | 2.0 | 0 | 18.0 | 1.7 | 3.0 | 75.4 |
| N-795 | 2.1 | 0 | 17.8 | 3.3 | 3.0 | 73.8 |
| N-645 | 1.9 | 0 | 18.1 | 4.1 | 3.0 | 72.9 |
| RM257 | RM23 | SL04180 | R1011 | I-907 | E7 | |
|---|---|---|---|---|---|---|
| Ch-7 | 2.4 | 9.8 | 7.1 | 1.7 | 3.0 | 76.1 |
| Ch-10 | 2.7 | 8.1 | 9.9 | 1.7 | 2.9 | 74.7 |
| Ch-12.6 | 2.4 | 4.7 | 12.6 | 1.7 | 3.0 | 75.7 |
| Ch-15.3 | 2.7 | 2.5 | 15.3 | 1.7 | 2.9 | 75.0 |
| Ch-17.3 | 2.4 | 0.0 | 17.3 | 1.7 | 3.0 | 75.7 |
| ChCL-1.4 | 1.4 | 11.7 | 7.1 | 1.7 | 2.8 | 75.2 |
| ChCL-2.4 | 2.4 | 9.8 | 7.1 | 1.7 | 3.0 | 76.1 |
| ChCL-5 | 5.0 | 7.6 | 7.6 | 1.7 | 2.9 | 75.1 |
| ChCL-9.6 | 9.6 | 3.0 | 7.6 | 1.7 | 3.0 | 75.2 |
The cells were filled with the formulations listed in Tables 1 and 2 by capillary action at about 70 °C, slightly above the isotropic transition temperature of the mixtures. Cells were rapidly cooled and shear aligned to minimize defects. After recording a pre-polymerization transmission spectra, all samples were polymerized with 1.8 mW cm−2 UV light for 30 minutes (Exfo, 365 nm). Transmission spectra were recorded after polymerization to confirm the retention of the reflection notch (position and bandwidth). The cells were then drained of the liquid crystal by solvent exchange. The cells were placed horizontally in cyclohexane for periods of three days to two weeks. The cells were removed from the cyclohexane and dried under vacuum for about two hours. The polymer network adhered to the bottom substrate during solvent exchange. Subsequently, the cells were refilled with the achiral nematic liquid crystal E7 (Merck).
Reflection wavelength tuning was monitored by simultaneously collecting transmission spectra with a pair of fiber optic spectrometers (Ocean Optics USB 2000+, NIR 512) as a function of temperature. The samples were attached with thermal grease to a thermoelectric cooler (TEC) with a hole in the center. The temperature of the TEC was measured with a thermistor mounted onto the surface of the TEC with thermal epoxy. The temperature and heating/cooling rate was controlled with a 2510 TEC controller (Keithley). Transmission spectra and temperature control was synchronized and automated through the use of a custom LabView program. The optical tuning results presented within this work were measured after the cell was drained and reswollen with E7.
Thickness measurements were performed on open faced samples mounted on a temperature controlled (Keithley) TEC with a Dektak 6 M (Veeco) profilometer. The thickness measurements were obtained with a 12.5 μm radius probe with 9.8 μN (1 mg) of applied force. Appropriate control experiments validated the assumption that the gel compression from the profilometer was negligible in comparison to the temperature induced thickness changes.
Results and discussion
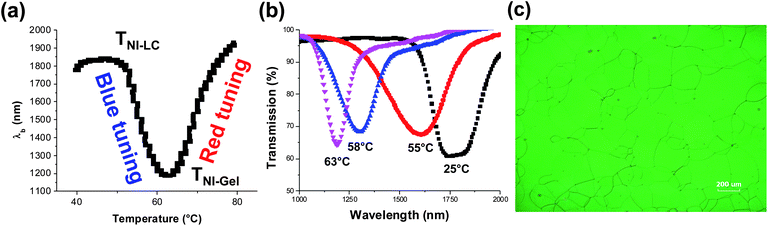
Scheme 1 illustrates the geometry of the structurally chiral nematic gels (SCNGs) as well as the underlying mechanism of the reflection wavelength tuning reported here. Fig. 2 illustrates the optical response of a representative sample (CL-1.5, Table 1) to heating. The CL-1.5 sample exhibits a reflection notch centered (λb) at approximately 1850 nm (Fig. 2a and b). Fig. 2a plots λb as a function of temperature. Upon heating, the sample exhibits temperature induced blue shifting and red shifting tuning as previously reported. At 63 °C, the pitch of the swollen chiral nematic gel compresses and the reflection wavelength shifts to nearly 1185 nm (665 nm tuning range). The notch shape and depth are maintained during the blue tuning (Fig. 2b). The data presented in Fig. 2a and b are representative of the optical response observed in all the material systems examined in this work. A representative polarized optical microscopy image is shown in Fig. 2c.We systematically varied the ratio of the chiral crosslinking monomer SL04151 to the achiral pendant monomer RM23 (maintaining a constant total monomer concentration of 20 wt%) in the samples named CL-XX (where XX is the weight percentage of SL04151, Table 1). To account for differences in the initial pitch length (λb0), we introduce the term relative tuning fraction (RTF) which is calculated from eqn (2):
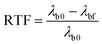 | (3) |
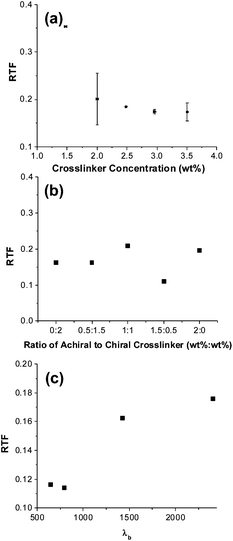
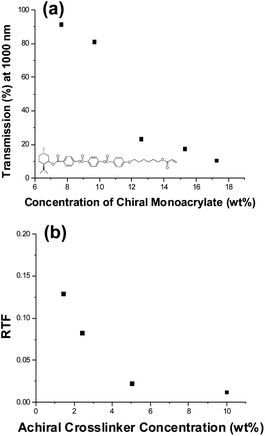
The effect of the chemical chirality of the monomer (polymer) on the resulting optical response of the SCNGs has not been examined in prior examinations. Fig. 3b examines the significance of the utilization of chiral monomer by examining the optical response of samples composed with various ratios of chiral and achiral crosslinker. These samples maintain a constant total monomer concentration of 20 wt% as well as a constant crosslinker concentration of 2 wt% (referred to as CXX-AYY where XX is the weight fraction of SL04151 and YY is the weight percentage of RM257 in Table 1). The ratio of chiral to achiral crosslinking monomer was varied from 0:2 to 2:0. The data summarized in Fig. 3b confirms that the presence and concentration of chiral crosslinker in the polymer network is not critical to the observation of thermally induced reflection notch tuning. This is most plainly evident by comparing the 0 wt% and 2 wt% data points in Fig. 3b which both exhibit a fractional tuning range of approximately 0.2. This is yet further evidence that the observed reflection upon refilling with an achiral nematic liquid crystal is attributable to translation of structural chirality.
The third structure/property examination presented in Fig. 3 examines the tuning range as a function of the initial reflection notch in cells prepared with the identical monomer concentration (in this case 2 wt% SL04151 and 18 wt% RM23). The initial reflection notch position was varied by adjusting the concentration of R1011 in the prepolymer mixture (labeled as N-XXX where XXX refers to the initial starting wavelength, Table 1). Accordingly, as R1011 concentration increased (per eqn (1)), the reflection notch was blue shifted from 2400 to 645 nm. The results summarized in Fig. 3c indicate that increasing the initial pitch length of the SCNGs can increase the overall RTF observed in a sample by over 50%.
The results presented in Fig. 3 confirm that the composition of the polymer network can influence the resulting thermally induced optical tuning of the SCNGs. The compositions examined in Fig. 3 were composed of a large percentage of the achiral pendant monoacrylate monomer RM23 mixed with either a chiral or achiral crosslinker. In Fig. 4, we examine SCNGs prepared from ternary monomer mixtures composed of the achiral crosslinking monomer RM257, the chiral pendant monoacrylate monomer SL04180, and the achiral pendant monoacrylate RM23. Employing SL04180 allows examination of samples composed with a polymer network maintaining a low concentration of crosslinking monomer as well as a large concentration of chiral centers. As shown in Fig. 4a, samples (labeled Ch-XX where XX refers to the wt% of SL04180, Table 2) composed with concentrations exceeding 12 wt% SL04180 have poor baseline transmission upon polymerization as well as after draining/refilling/reswelling with the achiral nematic liquid crystal mixture E7. The extensive scatter did not allow for direct observation of the reflection notch. This indicates poor compatibility caused by the large concentration of pendant chiral pendant mesogens in the swollen polymer network. Thus, the role of crosslinking was examined in samples with moderate chiral monoacrylate monomer concentration (7 wt%). Fig. 4b plots the RTF as a function of achiral crosslinker concentration from 1.5–10 wt% (labeled ChCL-XX where XX refers to the wt% of RM257, Table 2). The results summarized in Fig. 4b are further confirmation that increasing crosslink density by including larger concentrations of crosslinking monomer decrease the RTF. Comparison of the results presented in Fig. 4b to the results presented in Fig. 3a and b indicates that increased concentrations of chiral centers on the swollen polymer network can reduce the RTF by as much as 50% at a given crosslinker concentration.
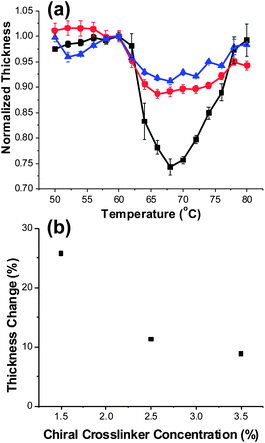
To correlate the optical tuning data presented in Fig. 2, 3, and 4 to the underlying mechanism illustrated in Scheme 1 – we characterized the thermally induced changes in thickness of open faced samples of CL-1.5, CL-2.5, and CL-3.5 (Table 1). The optical tuning of these samples was presented in Fig. 3a. From Fig. 3a, it would be expected that the thermally induced contraction (thickness change) of the swollen polymer network would decrease with increasing crosslinking monomer concentration attributable to reduction of the elasticity of the polymer network. Indeed, the results in Fig. 5a confirm that as expected – increasing the crosslinking density of the polymer networks serves to reduce the ability of the polymer network to contract. As evident in Fig. 5b, adding 2 wt% SL04151 (from 1.5% to 3.5%) serves to reduce the percent change in thickness from 26% to 9%. Notably, the fractional thickness changes are approximately 30% less than the RTF values reported in Fig. 3a. This discrepancy is most likely due to a decrease in the average refractive index at elevated temperatures.
The robustness and reliability of the gels was examined by subjecting the samples to repeated temperature cycles. As observed in Fig. 6 for the representative sample CL-1.5, the measured changes in the reflection bandgap are repeatable for at least five cycles. Importantly, the sample maintains the association of temperature and reflection wavelength – a requisite property for the employment of this color changeable material as a temperature sensor or tunable bandgap in mirrorless lasing.
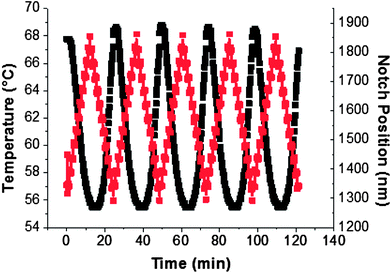 | ||
Fig. 6 Thermal cycling of the reflection color ( ) of swollen, structurally chiral nematic gel CL-1.5 from 56–68 C ( ) of swollen, structurally chiral nematic gel CL-1.5 from 56–68 C ( ). ). | ||
Conclusions
The fundamental structure/property relationships between the composition of the polymer network and the resulting optical properties for swollen, structurally chiral nematic gels are examined. These relationships were developed through systematic formulation of a number of mixtures based on monofunctional and difunctional chiral and achiral liquid crystal monomers. Of the variables examined here, crosslink density of the polymer network is shown to have a substantial impact on the overall tuning range of the samples. In samples that maintain optimum crosslink density (in the range of 1.5–3 wt%), it is shown that the employment of chiral monomer is not critical to facilitate the thermally induced reflection notch tuning. Supporting experiments examining relative thickness changes are in agreement with the relative tuning fraction observed in spectroscopic examination. Importantly, for applications in tunable CW mirrorless lasing, sensing, optics, and photonics – the samples are robust and able to undergo repeated temperature cycling.Acknowledgements
This work was supported by the Air Force Office of Scientific Research and the Materials and Manufacturing Directorate of the Air Force Research Laboratory. We thank Ludmilla Sukhomlinova, Tamas Kosa, and Bahman Taheri from Alpha Micron Inc. for providing the chiral liquid crystal diacrylate and monoacrylate monomers.Notes and references
- R. Eelkema and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 3729–3745 CAS.
- S. Pieraccini, S. Masiero, A. Ferrarini and G. P. Spada, Chem. Soc. Rev., 2011, 40, 258 RSC.
- W. Haas, J. Adams and J. Wysocki, Mol. Cryst. Liq. Cryst., 1969, 7, 371–379 CrossRef CAS.
- T. J. White, M. E. McConney and T. J. Bunning, J. Mater. Chem., 2010, 20, 9832–9847 RSC.
- R. A. Soref, J. Appl. Phys., 1970, 41, 3022–3026 CrossRef CAS.
- R. S. Pindak, C.-C. Huang and J. T. Ho, Phys. Rev. Lett., 1974, 32, 43–46 CrossRef CAS.
- F. Ania and H. Stegemeyer, Mol. Cryst. Liq. Cryst., Lett. Sect., 1985, 2, 67–76 CAS.
- F. Zhang and D. K. Yang, Liq. Cryst., 2002, 29, 1497–1501 CrossRef CAS.
- M. E. McConney, M. M. Duning, L. V. Natarajan, A. A. Voevodin, V. P. Tondiglia, T. J. White and T. J. Bunning, Mol. Cryst. Liq. Cryst., 2012, 559, 115–126 CrossRef CAS.
- M. E. McConney, T. J. White, V. P. Tondiglia, L. V. Natarajan, D.-k. Yang and T. J. Bunning, Soft Matter, 2011, 8, 318–323 RSC.
- K. Robbie, D. J. Broer and M. J. Brett, Nature, 1999, 399, 764 CrossRef CAS PubMed.
- M. Mitov and N. Dessaud, Nat. Mater., 2006, 5, 361–364 CrossRef CAS PubMed.
- J. Guo, H. Cao, J. Wei, D. Zhang, F. Liu, G. Pan, D. Zhao, W. He and H. Yang, Appl. Phys. Lett., 2008, 93, 201901 CrossRef.
- J. Guo, F. Liu, F. Chen, J. Wei and H. Yang, Liq. Cryst., 2010, 37, 171–178 CrossRef CAS.
- J. Guo, H. Wu, F. Chen, L. Zhang, W. He, H. Yang and J. Wei, J. Mater. Chem., 2010, 20, 4094–4102 RSC.
- M. E. McConney, V. P. Tondiglia, J. M. Hurtubise, L. V. Natarajan, T. J. White and T. J. Bunning, Adv. Mater., 2011, 23, 1453–1457 CrossRef CAS PubMed.
- M. E. McConney, V. P. Tondiglia, J. M. Hurtubise, T. J. White and T. J. Bunning, Chem. Commun., 2011, 47, 505–507 RSC.
- F. Castles, F. V. Day, S. M. Morris, D. H. Ko, D. J. Gardiner, M. M. Qasim, S. Nosheen, P. J. W. Hands, S. S. Choi, R. H. Friend and H. J. Coles, Nat. Mater., 2012, 11, 599–603 CrossRef CAS PubMed.
- K. Urayama, Y. Okuno, T. Kawamura and S. Kohjiya, Macromolecules, 2002, 35, 4567–4569 CrossRef CAS.
- K. Urayama, Macromol. Symp., 2010, 291–292, 89–94 CrossRef CAS.
- Y. Okuno, K. Urayama and S. Kohjiya, J. Chem. Phys., 2003, 118, 9854–9860 CrossRef CAS.
- Y. O. Arai, K. Urayama and S. Kohjiya, Polymer, 2004, 45, 5127–5135 CrossRef CAS PubMed.
- M. E. McConney, T. J. White, V. P. Tondiglia, L. V. Natarajan, D.-k. Yang and T. J. Bunning, Soft Matter, 2012, 8, 318–323 RSC.
- A. Munoz, M. E. McConney, T. Kosa, P. Luchette, L. Sukhomlinova, T. J. White, T. J. Bunning and B. Taheri, Opt. Lett., 2012, 37, 2904–2906 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |