Annealing of sulfide stabilized colloidal semiconductor nanocrystals†
Ruben
Dierick‡
a,
Boris
Capon‡
b,
Hanne
Damm‡
e,
Stijn
Flamee
ac,
Pieter
Arickx‡
a,
Els
Bruneel
d,
Dirk
Van Genechten
c,
Marlies
Van Bael
e,
An
Hardy
e,
Christophe
Detavernier
b and
Zeger
Hens
*a
aPhysics and Chemistry of Nanostructures, Ghent University, Krijgslaan 281-S3, B-9000 Ghent, Belgium. E-mail: ruben.dierick@ugent.be; zeger.hens@ugent.be
bSolid State Sciences, Ghent University, Krijgslaan 281-S1, B-9000 Ghent, Belgium
cUmicore Group Research & Development, Kasteelstraat 7, B 2250 Olen, Belgium
dPhysical and Inorganic Chemistry, Ghent University, Krijgslaan 281-S3, B-9000 Ghent, Belgium
eInorganic and Physical Chemistry, Hasselt University and imec, division imomec, Agoralaan-D, B-3590 Diepenbeek, Belgium
First published on 22nd October 2013
Abstract
The effect of thermal annealing on layers of CuInS2 nanocrystals (NCs) stabilized with (NH4)2S was investigated using in situ transmission electron microscopy (TEM), in situ X-ray diffraction (XRD), thermogravimetric analysis combined with mass spectrometry (TGA-MS) and X-ray photoelectron spectroscopy (XPS). It is shown that these inorganic, chalcogen containing ligands inhibit NC sintering up to 450 °C in an inert atmosphere. On the other hand, sintering can be promoted by annealing in hydrogen gas. A similar behavior is found with Cu2ZnSnSe4 and CdSe NCs. We attribute the inhibited sintering to the oxidation of the S2− originally stabilizing the NCs to sulfite or sulfate moieties, where oxidation is possible either by exposure of the films to air or by thermal decomposition of residual solvent molecules present in the film under inert conditions.
Introduction
Colloidal semiconductor nanocrystals (NCs) are widely studied as building blocks for optical and electronic devices. This research is driven by the high precision nanocrystal synthesis methods developed over the last 20 years.1–5 These typically result in inorganic–organic hybrid materials where the nanocrystal core – with controlled size, shape and composition6–9 – is stabilized by capping of long chain organic ligands.10 The combination of well-established synthetic methods with size-tunable optical properties and suitability for solution-based processing has stimulated the development of very diverse applications in, e.g., energy conversion, opto-electronics and lighting.These applications very often make use of thin films of NCs. These films can be either one of the active parts in the final device – used for light absorption or charge carrier transport – or they can be an intermediate step in the formation of a dense, semiconductor thin film. In the former case, the long chain organic ligands make up insulating barriers that separate adjacent NCs and hinder the transport of charge carriers. Typical approaches to enhance charge carrier mobilities in NC thin films or hybrid composite devices make use of a post-synthesis ligand exchange step to decrease interparticle spacing. By using short chain ligands11–14 or bridging ligands,12–19 one is able to improve the performance of NC based solar cells, photodetectors or transistors. After ligand exchange, an annealing step is meant to remove unwanted compounds. However, since the NC properties must be preserved during annealing, this requires the use of thermally labile ligands that decompose at relatively low temperatures, where NC sintering does not yet occur.
When the final goal is the formation of a dense thin film on the other hand, annealing is indispensible to induce sintering. Typical examples here involve the formation of absorber layers for thin-film photovoltaics from colloidal nanocrystals, an approach that has been used in the case of Cu(In,Ga)(S,Se)2 (CIGS),20,21 Cu2ZnSn(S,Se)4 (CZT(S,Se))22,23 and CdTe.24 Here, the long chain organic ligands hamper the transformation of a thin film of semiconductor NCs into a dense semiconductor thin film since they induce a significant weight loss upon annealing resulting in cracks, possibly combined with undesired carbon deposits.25 To avoid these issues, a possible alternative is the use of colloidal NCs stabilized with a short chain or thermally labile ligands by means of a post-synthesis ligand exchange step. In the case of metal sulfide or metal selenide semiconductors, a more straightforward approach seems the use of NCs stabilized by chalcogenide ligands, such as S2− or Se2−, as recently introduced by Nag et al.26 These ligands occupy a smaller volume and should prevent any contamination with elements foreign to the final semiconductor compound. In the case of CIGS and CZT(S,Se), NC stabilization with chalcogenide ligands would have the additional advantage of creating an excess of chalcogen species in a NC layer prior to annealing. This could possibly be exploited to avoid the cumbersome gas-phase sulfurization or selenization step that is typically required for grain growth and thus device integration in a highly efficient CIGS-CZTSSe solar cell.27 However, the annealing and sintering behavior of chalcogenide stabilized NC layers has not been studied yet.
In this paper, we report on the effect of different thermal treatments on sintering of thin films of CuInS2 (CIS) NCs stabilized with (NH4)2S using in situ transmission electron microscopy (TEM), in situ X-ray diffraction (XRD), thermogravimetric analysis (TGA) combined with mass spectrometry (MS) and X-ray photoelectron spectroscopy (XPS). Our results indicate that the sintering of CIS NCs is inhibited during annealing under an inert atmosphere, while it is promoted when using a hydrogen containing atmosphere. Moreover, we show that similar results are obtained for other semiconductor NCs stabilized by S2−, such as Cu2ZnSnSe4 (CZTSe) and CdSe. We show that the onset of sintering under a hydrogen containing atmosphere coincides with the release of H2S from the film. Under inert conditions, the S2− species are retained in the film, where they oxidize to form sulfite or sulfate moieties, yielding a layer in which sintering is almost absent. Using XPS, the oxidation of S2− is readily demonstrated by exposing NC films to air, yet it also occurs during annealing under an inert atmosphere. Clearly, this limited sintering complicates the formation of dense films by heating films of NCs with chalcogenide ligands. On the other hand, considering devices that make use of NC films, it provides an opportunity to improve their performance through annealing.
Experimental section
Materials
Cu(acac)2 (Aldrich, 99.99%), In(acac)3 (Aldrich, 99.99%), sulfur (Merck, 99.999%), oleylamine (Acros, Tech. 80–90%), 1-octadecene (Alfa Aesar, Tech. 90%), (NH4)2S (Aldrich, 20 w% in H2O) and formamide (Fluka, ≥98%) were used without additional purification.Nanocrystal synthesis
For the synthesis of CIS NCs, a heating-up synthesis based on the procedure proposed by Panthani et al.28 was used. Cu(acac)2 (1 mmol) and In(acac)3 (1 mmol) are mixed together with S (2 mmol) and ODE (14 ml). This mixture is connected to a Schlenk line and flushed with nitrogen at room temperature during 1 h. Next, OLA (2 ml) is injected and the reaction mixture is heated up to 180 °C using a heating mantle, leading to a color change from brown to green–brown to finally black. The temperature is held at 180 °C over the course of 1 h. Afterwards, the reaction is cooled down using a water bath, toluene is added, and the CIS NCs are separated using a mixture of i-propanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (20
acetonitrile (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Following centrifugation, the clear brown–red supernatant is discarded and the CIS NCs are redispersed in toluene.
1). Following centrifugation, the clear brown–red supernatant is discarded and the CIS NCs are redispersed in toluene.
Nanocrystal processing
To exchange the initial oleylamine ligands for (NH4)2S ligands, we use a procedure similar to Nag et al.26 A given amount of the NC stock solution in toluene is first purified and redispersed in toluene two times. This toluene NC solution is combined with formamide creating a two-phase system. Afterwards, (NH4)2S is added and the solution is vigorously stirred at room temperature. Complete phase transfer of the black NC solution to formamide indicates the end of the reaction. The formamide phase containing the NCs is washed with toluene up to 5 times to remove possible leftover oleylamine. Excess (NH4)2S is then removed by precipitating the NCs using i-propanol, centrifugation and decantation. The NCs are now redissolved in formamide and this solution is used to deposit CIS NC thin films, by drop casting an amount corresponding to a dry thin film of approximately 1 μm onto a silicon substrate, on a heating plate at 60 °C.Characterization
To determine the mean diameter of the NCs used in this work, and the effect of heating in a vacuum, we used bright-field TEM images recorded with a JEM-2200FS transmission electron microscope. These samples were prepared by dipping a TEM grid in a dilute NC solution. XRD and in situ XRD patterns were recorded using a Bruker D8 Discover. Cu K alpha radiation was used as an X-ray source, while a linear Vantec detector monitored the crystallinity of the thin films. TGA-MS measurements were performed on NCs obtained after precipitation with i-propanol and subsequent drying of the pellet after centrifugation using vacuum. Approximately 7 mg of this powder was analyzed with a TA Instruments TGA Q5000 coupled with a Pfeiffer Vacuum ThermoStar™ MS. The thermal decomposition has been carried out in a 5% H2/Ar or a He flow. For 5% H2/Ar flow, settings of nitrogen have been used. More detailed analyses of the fragments formed during ionization of the evolving gas molecules have been done scanning specific m/z signals that were chosen after a wide m/z (5–145) scan to obtain a better signal-to-noise ratio. XPS analysis was performed using a S-probe spectrometer with an Al-source (monochromatized Al-radiation: 1486 eV). The measured surface was 250 μm by 1000 μm. The flood gun of 3 eV was used. Experimental data were processed using the software package CasaXPS. Prior to analysis the peak of adventitious carbon was calibrated towards 284.6 eV.Results and discussion
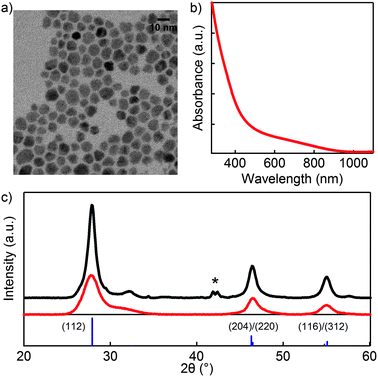
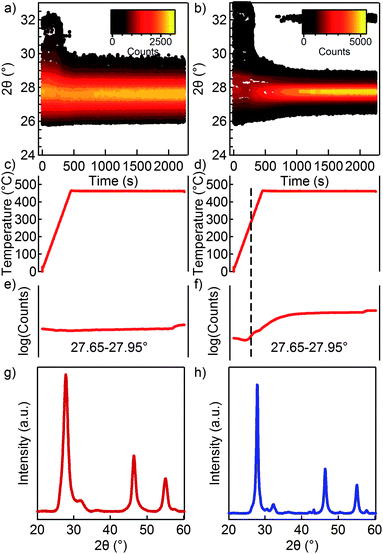
Fig. 1a shows a representative TEM micrograph of the CIS NCs used in this study. The NCs appear as quasi-spherical with a mean diameter of ≈8 nm and a size dispersion of ≈15%. According to the XRD pattern, chalcopyrite NCs are obtained with no secondary phases present, while the UV-Vis absorption spectrum (Fig. 1b) shows an absorption onset around the band gap of bulk CIS (1.54 eV). Annealing a 1 μm thin film of these as-synthesized CIS NCs to 450 °C at a rate of 1 °C s−1 in a helium atmosphere results in a sharpening of the CIS reflections as shown in the upper XRD diffractogram in Fig. 1c. According to the Scherrer equation, this sharpening of the peaks reflects crystal growth during the heat treatment. We could identify the secondary phases present in the annealed sample, indicated by (*) in Fig. 1c, as a CuxIny alloy. These likely result from a loss of sulfur upon annealing.After synthesis and purification, the original steric stabilization by oleylamine ligands can be replaced by charge stabilization using (NH4)2S in a two-phase exchange reaction.26 In Fig. S1,† we show that, apart from the nanocrystals being closer together on the TEM grid, the basic characteristics of the CIS NCs are not different from the results shown in Fig. 1. The thus obtained particles can be dispersed in formamide and deposited by drop-casting as a thin film as well. Even though CIS NCs capped by (NH4)2S are stable in formamide for up to 2 days, we deposited the thin films right after the ligand exchange to prevent further changes in surface chemistry prior to annealing. Using in situ XRD, we followed the effect of annealing on a thus formed thin film of (NH4)2S stabilized CIS NCs. Fig. 2a shows the evolution of the signal intensity in the 2θ region around the CIS (112) peak as a function of time during a thermal treatment in helium following the indicated temperature profile in Fig. 2. Most notably, no significant peak sharpening is observed, pointing towards an inhibition of sintering by (NH4)2S ligands as compared to long chain organic ligands (see Fig. 1c). On the other hand, we found that the sintering of CIS NCs with (NH4)2S ligands can be triggered using forming gas during the process (5% H2 in He). In this case (Fig. 2b), the (112) peak sharpens throughout the process, indicating growth of the CIS NCs. For comparison, XRD patterns are shown after the annealing in helium (Fig. 2c) and forming gas (Fig. 2d). Taking a closer look at the diffracted intensity in the 27.65–27.95 2θ range as a function of temperature, we find that it remains constant under an inert atmosphere, while it starts increasing from ≈300 °C under reducing conditions.
To further confirm the difference in annealing behavior between OLA capped and (NH4)2S stabilized CIS NCs, we compared their behavior using in situ TEM. Dip coated layers were heated to 550 °C in a vacuum, as shown in Fig. 3. As expected, CIS NCs capped with oleylamine sinter in the range of 450–500 °C (Fig. 3a and b), even showing the formation of large crystallites with dimensions exceeding 100 nm and interparticle adhesion. This behavior is not observed in CIS NCs with (NH4)2S ligands (Fig. 3c and d) subjected to the same treatment. In this case, the CIS NC size and shape is preserved throughout the heating process. While it is difficult to set an exact temperature during in situ TEM studies, the difference between both samples is obvious and concurs with the observations from the in situ XRD measurement in Fig. 2a.
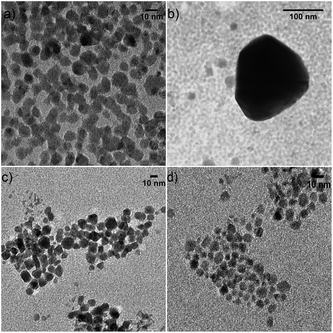 | ||
| Fig. 3 TEM images of (a and b) CIS NCs with oleylamine ligands and (c and d) (NH4)2S ligands at an estimated temperature of 450 °C. | ||
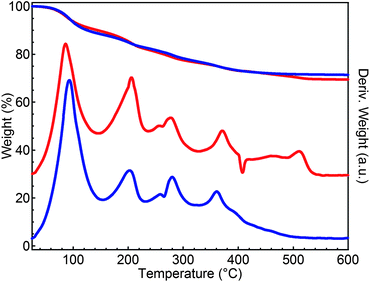
In order to identify the species leaving the nanocrystal-ligand system upon annealing, TGA-MS measurements were carried out on a powder of CIS NCs capped with (NH4)2S in both inert and reducing atmosphere. Fig. 4 shows the TGA-DTG signal when both samples were heated up to 600 °C at 10 °C min−1. In TGA, we observe an overall similar, stepwise weight loss up to a temperature of 600 °C amounting to ≈30% of the original mass. Nevertheless, the DTG signal shows a clear difference between both samples in the temperature range 400–600 °C, where the sample annealed in an inert atmosphere loses extra mass. The DTG signal also emphasizes the stepwise decomposition process.
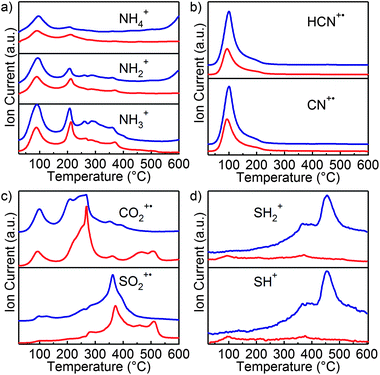
Using mass spectrometry coupled in-line with TGA, gaseous species evolving from both samples were detected in situ. As summarized in Table 1, this involves species which we suspect to originate from both (NH4)2S and formamide. While the powder used for the TGA-MS measurements appeared to be dry, fragments originating from the decomposition of the solvent used, i.e., formamide, can clearly be distinguished. The response for all the fragments in Table 1 is shown as a function of temperature in Fig. 5. At 100 °C, the largest mass loss originates from HCN/CN and CO2 fragments, and this is similar for both annealing conditions. We identify species with m/z values of 16, 17 and 18 as NH2+, NH3+ NH4+ species, originating from either (NH4)2S or formamide. Under a H2/N2 atmosphere, the corresponding signals (Fig. 5a) show an increase at high temperatures, which we attribute to the formation of H2O – which leads to fragments with the same m/z – by the reaction between H2 and residual O2, an interpretation corroborated by the reduction of the residual O2 signal at m/z = 32 (data not shown).
| m/z | Species | Proposed source |
|---|---|---|
| 16 | NH2+ | (NH4)2S/HCONH2 |
| 17 | NH3+ | (NH4)2S/HCONH2 |
| 18 | NH4+ | (NH4)2S/HCONH2 |
| 26 | CN+ | HCONH2 |
| 27 | HCN+ | HCONH2 |
| 33 | SH+ | (NH4)2S |
| 34 | SH2+ | (NH4)2S |
| 44 | CO2+ | HCONH2 |
| 64 | SO2+ | (NH4)2S |
Next, at 250 °C, another set of CO2 fragments is released, followed by the release of SO2. Importantly, fragments with m/z ratios of 33 and 34, corresponding to SH+ and SH2+, respectively, are detected from 300 °C onwards in the case of a reducing atmosphere, and are absent under inert conditions. These fragments are typical of H2S, indicating its formation only in the case of H2/Ar and their appearance coincides with the onset of sintering in the CIS nanocrystal film. Interestingly, CO2 and SO2 are detected in both conditions. We attribute CO2 to the decomposition of formamide, and we suspect that this coincides with the oxidation of S2− from (NH4)2S – the species stabilizing the NCs in formamide – to SO2 (cf. infra). In Fig. 5c, we can also observe the species responsible for the difference in DTG signals (Fig. 4). At 500 °C, compared to annealing in a reducing atmosphere, an excess amount of CO2 and SO2 leaves the sample. Since we base our annealing results in in situ XRD on thermal treatments that do not exceed 450 °C, it appears that the presence of this species is related to the inhibition of the sintering of NCs. In the case of a hydrogen containing atmosphere, this oxidized compound can be reduced to H2S, leaving the surface.
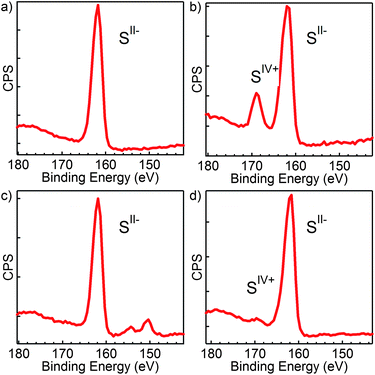
To confirm the presence of oxidized sulfur in films of CIS NCs originally stabilized by (NH4)2S, we analyzed various films using XPS. Fig. 6a shows an XPS spectrum recorded on a layer of as-deposited NCs stabilized by (NH4)2S. The spectrum shows a single peak related to S at 161 eV, which is typically attributed to a relatively weak bonding 2p state of S2−. We stress that no oxidized sulfur species are found prior to annealing, which is in any case carried out as soon as possible after deposition. After keeping this layer under ambient conditions for 3 weeks, a second feature emerges at higher binding energy (Fig. 6b), which can be related to S species in a higher oxidation state29 (i.e., S4+ or higher). We thus conclude that all sulfur in the system is initially present as S2− (CuInS2 and (NH4)2S), while the sulfide at the CIS surface progressively oxidizes to form SO2, SO32− and/or SO42−. Since we found that NC sintering is inhibited under an inert atmosphere for layers composed of solely S2− as well, we measured an XPS spectrum of these fresh layers after annealing in H2/Ar (Fig. 6c) and N2 (Fig. 6d). Only in the latter case, a slight contribution of oxidized sulfur is discernible. This shows that the oxidation of sulfide species occurs during annealing under inert conditions, probably related to the decomposition of the leftover solvent.
In conclusion, we find that observed sintering under reducing conditions is related to the release of sulfide as H2S, whereas under inert conditions, the sulfide remains in the film, being oxidized to SO2, SO32− and/or SO42− and thereby inhibiting sintering. The observation that sintering of NCs stabilized by (NH4)2S is inhibited up to relatively high temperatures was further tested for two different NC systems. In Fig. 7, we compare the annealing behavior of CZTSe and CdSe NCs processed in the same way as the CIS NCs. By following the (112) peak of kesterite CZTSe during heating in helium (Fig. 7a) and H2/Ar (Fig. 7b), we can conclude that a similar difference in sintering behavior is present. Moreover, in the case of CdSe NCs, the same difference in degree of sintering between helium (Fig. 7c) and H2/Ar (Fig. 7d) atmosphere can be found. Further characterization of these NC systems can be found in the ESI.† We suspect that the oxidation of (NH4)2S is again responsible for the difference in annealing behavior of these NCs systems, meaning that the effects we measured for CIS NCs apply to other semiconductor NCs as well.
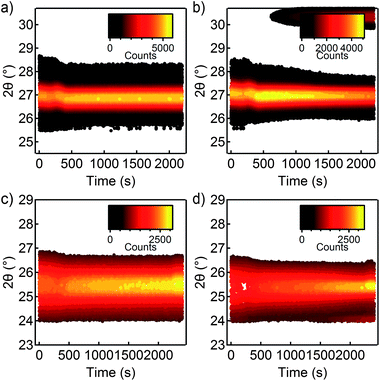 | ||
| Fig. 7 Comparison of annealing behavior of (a and b) CZTSe NCs and (c and d) CdSe NCs capped by (NH4)2S. (a and c) Annealing in helium. (b and d) Annealing in H2/Ar. | ||
Conclusions
In summary, annealing treatments of layers of CIS NCs stabilized by inorganic (NH4)2S moieties were investigated. Under inert conditions, the NCs show little sintering at temperatures up to 450 °C. This contrasts with the clear NC grain growth observed upon annealing in the presence of gaseous hydrogen. We attribute the difference between both to the behavior of the S2− species. Under a reducing atmosphere, the onset of sintering concurs with the release of H2S, while under an inert atmosphere, sulfide is retained in the film and oxidized, thereby inhibiting sintering. Importantly, the oxidized sulfur compounds can originate either from exposure to air or from the decomposition of solvent molecules during the annealing process itself. Similar findings apply to CZTSe and CdSe NCs, where nanocrystal sintering was equally inhibited under an inert atmosphere. While the NC stabilization by (NH4)2S moieties provides a way to stabilize CIS NCs in polar solvents while decreasing the carbon content and the interparticle distance in a NC thin film to a minimum, this lack of sintering is a considerable drawback for applications such as thin film photovoltaics that require dense thin films made from large crystallites. Annealing in a reducing atmosphere solves this problem only partially, since the crystal sizes obtained can compete with NCs stabilized by long, organic ligands. On the other hand, the inhibition of sintering by surface oxidation provides the opportunity to preserve nanocrystal properties throughout a high temperature annealing step. This can be of use for devices that rely on thin films of closely packed nanocrystals, such as quantum dot-based solar cells and photodetectors.Acknowledgements
The authors acknowledge Ghent University (ZH and CDT; GOA project), the Belgian Science Policy Office (ZH; BelSPO, IAP 7.35), the FWO-Vlaanderen (ZH; project nr. G.0760.12) and the IWT-Vlaanderen (SF; Baekeland grant) for funding this research.References
- C. Murray, D. Norris and M. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- C. Murray, S. Sun and W. Gaschler, IBM J. Res. Dev., 2001, 45, 47 CrossRef CAS.
- E. M. Chan, C. Xu, A. W. Mao, G. Han, J. S. Owen, B. E. Cohen and D. J. Milliron, Nano Lett., 2010, 10, 1874 CrossRef CAS PubMed.
- M. Protière, N. Nerambourg, O. Renard and P. Reiss, Nanoscale Res. Lett., 2011, 6, 472 CrossRef PubMed.
- S. Abe, R. K. Čapek, B. De Geyter and Z. Hens, ACS Nano, 2012, 6, 42 CrossRef CAS PubMed.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389 CrossRef CAS PubMed.
- X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. Alivisatos, Nature, 2000, 404, 59 CrossRef CAS PubMed.
- I. Moreels, K. Lambert, D. De Muynck, F. Vanhaecke, D. Poelman, J. C. Martins, G. Allan and Z. Hens, Chem. Mater., 2007, 19, 6101 CrossRef CAS.
- R. Costi, A. E. Saunders and U. Banin, Angew. Chem., 2010, 49, 4878 CAS.
- Z. Hens, I. Moreels, B. Fritzinger and J. C. Martins, in Comprehensive Nanoscience and Technology, ed. D. Andrews, G. D. Scholes and G. P. Wiederrecht, Academic Press, Oxford, 2011, vol. 5, pp. 21–49 Search PubMed.
- K. W. Johnston, A. G. Pattantyus-Abraham, J. P. Clifford, S. H. Myrskog, D. D. MacNeil, L. Levina and E. H. Sargent, Appl. Phys. Lett., 2008, 92, 151115 CrossRef.
- J. Seo, S. J. Kim, W. J. Kim, R. Singh, M. Samoc, A. N. Cartwright and P. N. Prasad, Nanotechnology, 2009, 20, 095202 CrossRef PubMed.
- C.-Y. Kuo, M.-S. Su, C.-S. Ku, S.-M. Wang, H.-Y. Lee and K.-H. Wei, J. Mater. Chem., 2011, 21, 11605 RSC.
- M. H. Zarghami, Y. Liu, M. Gibbs, E. Gebremichael, C. Webster and M. Law, ACS Nano, 2010, 4, 2475 CrossRef CAS PubMed.
- C.-Y. Kuo, M.-S. Su, C.-S. Ku, S.-M. Wang, H.-Y. Lee and K.-H. Wei, J. Mater. Chem., 2011, 21, 11605 RSC.
- J. M. Luther, M. Law, Q. Song, C. L. Perkins, M. C. Beard and A. J. Nozik, ACS Nano, 2008, 2, 271 CrossRef CAS PubMed.
- E. J. D. Klem, H. Shukla, S. Hinds, D. D. MacNeil, L. Levina and E. H. Sargent, Appl. Phys. Lett., 2008, 92, 212105 CrossRef.
- V. Porter and S. Geyer, J. Phys. Chem. C, 2008, 112, 2308 CAS.
- D. H. Webber and R. L. Brutchey, J. Am. Chem. Soc., 2012, 134, 1085 CrossRef CAS PubMed.
- Q. Guo, G. M. Ford, H. W. Hillhouse and R. Agrawal, Nano Lett., 2009, 9, 3060 CrossRef CAS PubMed.
- C. Jiang, J.-S. Lee and D. V. Talapin, J. Am. Chem. Soc., 2012, 134, 5010 CrossRef CAS PubMed.
- Q. Guo, G. M. Ford, W.-C. Yang, B. C. Walker, E. A. Stach, H. W. Hillhouse and R. Agrawal, J. Am. Chem. Soc., 2010, 132, 17384 CrossRef CAS PubMed.
- Y. Cao, M. S. Denny, J. V. Caspar, W. E. Farneth, Q. Guo, A. S. Ionkin, L. K. Johnson, M. Lu, I. Malajovich, D. Radu, H. D. Rosenfeld, K. R. Choudhury and W. Wu, J. Am. Chem. Soc., 2012, 134, 15644 CrossRef CAS PubMed.
- J. Jasieniak, B. I. MacDonald, S. E. Watkins and P. Mulvaney, Nano Lett., 2011, 11, 2856 CrossRef CAS PubMed.
- M. Drndić, M. V. Jarosz, N. Y. Morgan, M. A. Kastner and M. G. Bawendi, J. Appl. Phys., 2002, 92, 7498 CrossRef.
- A. Nag, M. V. Kovalenko, J.-S. Lee, W. Liu, B. Spokoyny and D. V. Talapin, J. Am. Chem. Soc., 2011, 133, 10612 CrossRef CAS PubMed.
- C. J. Hibberd, E. Chassaing, W. Liu, D. B. Mitzi, D. Lincot and A. N. Tiwari, Prog. Photovoltaics, 2010, 18, 434 CAS.
- M. G. Panthani, V. Akhavan, B. Goodfellow, J. P. Schmidtke, L. Dunn, A. Dodabalapur, P. F. Barbara and B. A. Korgel, J. Am. Chem. Soc., 2008, 2, 16770 CrossRef PubMed.
- NIST Standard Reference Database 20, Version 4.1 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3tc31393j |
| ‡ SIM SoPPoM project. |
| This journal is © The Royal Society of Chemistry 2014 |