Dichotomous adsorption behaviour of dyes on an amino-functionalised metal–organic framework, amino-MIL-101(Al)†
Enamul
Haque
,
Victor
Lo
,
Andrew I.
Minett
,
Andrew T.
Harris
and
Tamara L.
Church
*
Laboratory of Sustainable Technology, School of Chemical & Biomolecular Engineering, The University of Sydney, NSW 2006, Australia. E-mail: tamara.church@sydney.edu.au; Fax: +61-2-9351-2854; Tel: +61-2-93512926
First published on 18th November 2013
Abstract
An amino-functionalised metal–organic framework (MOF), aluminium aminoterephthalate (amino-MIL-101(Al)), has been applied to the adsorptive removal of dyes (cationic methylene blue, MB, and anionic methyl orange, MO) from aqueous solutions in order to examine the effect of the amino group on sorption behaviour. Adsorption isotherms and thermodynamic studies indicated the spontaneous adsorption of MB with a maximum adsorption capacity at 30 °C (762 ± 12 mg gMOF−1) higher than those observed for MB on other MOFs and most other materials. In contrast, lower adsorption capacities were observed in the adsorption of the same dye on the analogous non-amino-functionalised framework (MIL-101(Al), 195 mg g−1) and in the adsorption of MO by amino-MIL-101(Al) (188 ± 9 mg g−1), suggesting that an electrostatic interaction between the amino groups of the MOF and the cationic dye MB may have contributed to the high adsorption capacity. The adsorptions of both dyes on amino-MIL-101(Al) were spontaneous, endothermic, and entropy-driven, as is common for dye adsorptions. However, the ΔS value obtained for the adsorption of MB (346 J mol−1 K−1) was extreme. Further analysis demonstrated that after exposure to MB, the ordered amino-MIL-101(Al) structure was absent, ∼30% of the Al3+ was lost to solution, and significant changes occurred in the X-ray photoelectron spectrum of the MOF. On the other hand, the MOF structure was intact following the adsorption of MO. Several groups have exploited electrostatic interactions to improve dye adsorption; however, these proved excessive in the case of MB (but not MO) adsorption on amino-MIL-101(Al).
1. Introduction
Metal–organic frameworks (MOFs)1 are crystalline porous materials constructed from multifunctional ligands and metal ions. Recently, MOFs have become popular for their diverse, porous structures, high surface areas and potential applications in the fields of catalysis,2 gas adsorption/storage,3 biomedicine and drug delivery,4 separation,2c,5 adsorption of organic molecules,6 luminescence,7 electrode materials,8 and magnetism,9 and as carriers for nanomaterials.10 In the particular field of adsorption, the removal of dyes,11 alkylaromatics and phenols,12 pharmaceuticals (furosemide and sulfasalazine),13 and sulfur compounds6c,14 from the liquid phase have been reported.We became interested in the use of MOFs to remove dyes from contaminated water because considerable amounts of coloured wastewater are generated from industries such as the textile, leather, paper, printing, dyestuff, and plastic industries.15 Dyes are generally difficult to degrade because they are very stable to light and oxidation;16 however, water quality is highly influenced by colour,15 and even a small amount of dye is highly visible and undesirable. Moreover, many dyes are considered to be toxic and even carcinogenic.15–17 Physical, chemical and biological methods have been investigated for the removal of dyes from contaminated water,15–17 and adsorption is considered competitive among these because it is highly efficient, economically feasible, and requires only simple design.16,18 MOFs have some advantages as adsorbents in that their structures and pore sizes are tunable.1 Further, they contain polar and polarisable bonds, and can even bear open (or, in aqueous solution, water-coordinated) coordination sites on metal atoms. Thus electrostatic or coordinative interactions can increase11c,19 (or decrease19a) the dye-sorption capacity of a structure. Electronic interactions have also been invoked in the sorption of dyes on MOF-235, a cationic MOF with intercalated anions.11b
In addition to as-synthesised MOFs,11 some tailored materials have been tested in dye adsorption. Inspired by Ma and co-workers, who reported the adsorption of anionic dyes on ammonium-functionalised MCM-41,20 Jhung and co-workers grafted ethylenediamine onto MIL-101 (MIL = Materials of Institute Lavoisier) to produce sorbents that adsorbed the anionic dye methyl orange (MO, Fig. S1†).11c The best adsorption was obtained when MIL-101 was functionalised with the diamine and then protonated, presumably forming dangling ammonium groups; however, even the diamine-functionalised framework was a more effective sorbent than bare MIL-101. Studies on the impact of pH suggested that electrostatic interactions assisted the adsorption of the anionic dye even on the diamine-functionalised framework. More recently, Li et al. formed a MOF–graphite oxide composite that adsorbed more methylene blue (MB, Fig. S1†) than the MOF alone.19b
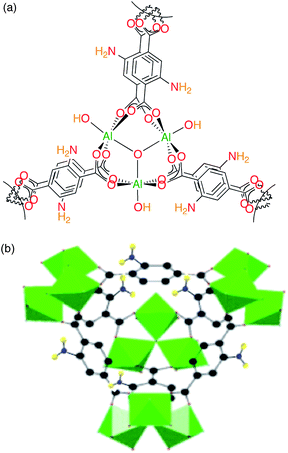
Though an amino-functionalised MOF has been tested in the adsorption of anionic MO, we reasoned that a functional group bearing a lone pair of electrons should also improve the sorption of cationic dyes such as MB. Rather than use the post-synthesis grafting method that was demonstrated by Jhung and co-workers,11c we chose to study the adsorption of both MB and MO from aqueous solution onto amino-functionalised MIL-101(Al) (amino-MIL-101(Al)). Amino-MIL-101(Al) is built from supertetrahedral building units formed by aminoterephthalate ligands and trimeric Al(III) octahedral clusters,21 and thus bears an amino group on every linker. Whereas these supertetrahedral enclose tetrahedral micropores, the extended structure of amino-MIL-101(Al) is composed of two types of quasi-spherical mesoporous cages formed by 12 pentagonal and 16 hexagonal faces (Fig. 1) with windows 1.2 and 1.6 nm, respectively, in diameter.22 It is an active catalyst for the condensation of benzaldehyde and ethyl cyanoacetate,22 and its iron analogue, amino-MIL-101(Fe), has been used in drug delivery23 and medical imaging.24 Unfunctionalised MIL-101(Cr) has been used as a sorbent for the cationic dye malachite green; however, its positive surface charge was detrimental to adsorption capacity.19a
2. Experimental
Amino-MIL-101(Al) was synthesised using a reported solvothermal method.21 2-Aminoterephthalic acid (0.56 g, HO2C–C6H3NH2–CO2H, Sigma–Aldrich, 99%) and aluminum chloride hexahydrate (0.51 g, AlCl3·6H2O, Sigma–Aldrich, 99%) were mixed with 30 mL of N,N-dimethylformamide ((CH3)2NCHO, Sigma–Aldrich, 99.8%) and stirred until a clear solution was formed. The resulting mixture was placed in a Teflon-lined reactor and heated for 6 h at 130 °C in a microwave (MARS-5, CEM, 300 W). The resulting yellow powder was filtered under vacuum, washed with acetone, and dried at 100 °C under vacuum. To remove organic species trapped within the pores, the product was activated in methanol at 80 °C overnight and then dried at 100 °C under vacuum.The products were analysed by X-ray diffraction (Siemens D5000, CuKα radiation) over 2θ = 2–20°. The crystallite morphologies were examined using a field emission scanning electron microscope (FESEM, Zeiss ultra Plus) operated at 5 kV. FTIR spectra were obtained with a Bruker IFS66V spectrometer. Nitrogen physisorption was carried out using an Autosorb-iQ (Quantachrome Instruments, USA) adsorption unit at liquid nitrogen temperature (−196 °C). Samples were evacuated at 150 °C for 12 h prior to analysis. Surface areas were calculated using the Brunauer–Emmett–Teller (BET)25 model from the nitrogen adsorption isotherms over P/P0 = 0.05–0.15. The Barrett–Joyner–Halenda (BJH)26 method was used to calculate the pore size distribution from the adsorption branch. Thermal stability was studied by heating the sample in a thermogravimetric analyser (TGA, TA Instruments, Q500) at 5 °C min−1 under an air flow of 60 mL min−1. X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi, Thermo scientific, UK; X-ray source: monochromated Al Kα, Power: 164 W (10.8 mA and 15.2 kV); binding energy reference: C1s = 285.0 eV for adventitious hydrocarbon) was used to examine the chemical states of different elements in amino-MIL-101(Al) before and after MB adsorption. 27Al spectra were recorded at UNSW on a Bruker Avance III 700 operating at 182.49 MHz, using a 2.5 mm triple resonance CPMAS probe. Samples (∼10 mg) were packed into 2.5-mm o.d. rotors and spun at the magic angle at 20 kHz and at ambient temperature. Data was acquired using a single pulse (∼14°, π/12 pulse) with a 1-s recycle delay. Finally, ICP-OES measurements were performed on a Varian 720-ES spectrometer. The wavelength monitored was 396.152 nm, and the calibration curve was prepared from dilutions of a 1000-ppm Al3+ solution in 2% HNO3(aq.) (Choice Analytical).
Stock solutions of MB and MO (1000 ppm each, assuming that ρsolution = 1.0 mg L−1) were prepared by dissolving solid MB (C16H18ClN3S·3H2O, MW: 373.9, Sigma–Aldrich, ≥82% dye content) and MO (C14H14N3NaO3S, MW: 327.33, Sigma–Aldrich, ≥85% dye content) separately in ultrapure water (18.2 MΩ cm, Sartorius Arium 611D). The dye contents were considered when calculating concentrations; thus, for 1.0 g MB, a mass of (1.0/0.82) g was used. Working solutions of MB and MO were prepared by sequential dilution of the stock solution with ultrapure water. Stock solutions of MB and MO ([dye] = 1–30 ppm]) were used to construct calibration curves (Fig. S2†) for absorbance at 665 and 464 nm respectively, using a Varian Cary 50UV UV-visible spectrophotometer.
Before adsorption, the adsorbent was dried overnight under vacuum at 100 °C and kept in a desiccator. In a typical adsorption experiment, the adsorbent (∼5 mg) was weighed precisely and added to an aqueous dye solution (100 mL) with a known dye concentration between 20 and 200 ppm. The dye solutions containing the adsorbents were mixed well with magnetic stirring and maintained for a fixed time (5 min to 12 h) at 30 °C in a thermal control chamber (Thermoline Scientific). After a pre-determined time, the solution was separated from the adsorbent with a syringe filter (0.45 μm, Millex, Millipore). The final dye concentration was calculated by comparing the UV-vis absorbance to the appropriate calibration curve; samples with [dye]0 > 30 ppm were diluted before analysis. The adsorption quantity was measured from the calibration curve using eqn (1):
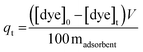 | (1) |
Adsorption rates were fit to both pseudo-second- and pseudo-first-order models.27 Maximum adsorption capacities were calculated using Langmuir adsorption isotherms obtained after adsorption for 12 h. In order to determine ΔGads, ΔHads and ΔSads, the adsorptions were repeated at 40 and 50 °C, and van't Hoff plots were prepared.
3. Results and discussion
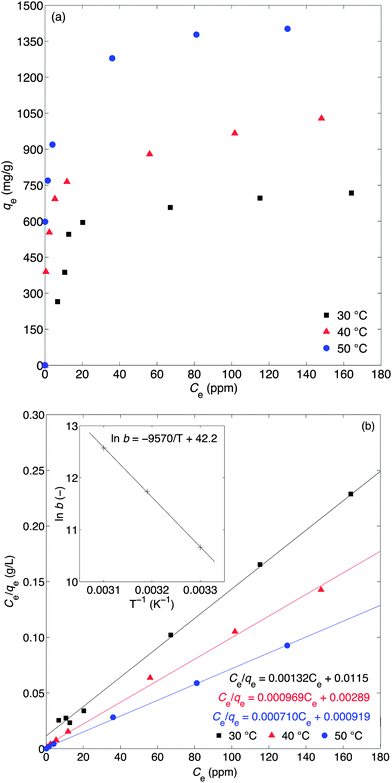
Amino-MIL-101(Al) was synthesised in the microwave at 130 °C. Its XRD pattern is shown in Fig. 2a, and is similar to the calculated pattern for MIL-101(Cr)28 and the experimental patterns for amino-MIL-101(Al),21 amino-MIL-101(Fe),29 and MIL-101(Cr).28,30 This evinced the formation of the MIL-101 phase (Fig. S3†). A scanning electron microscopy (SEM) image of our amino-MIL-101(Al) (Fig. 2b) showed a homogeneous surface morphology with an average particle diameter of approximately 800 nm. The FT-IR spectrum of amino-MIL-101(Al) (Fig. 2c) displayed the two characteristic bands of the amino group: the δ(N–H) (scissoring) vibration at 1624 cm−1 and the ν(C–N) absorption distinctive of aromatic amines at 1336 cm−1.22,31 The nitrogen adsorption–desorption isotherm (Fig. 2d) measured at −196 °C showed the characteristic steps and type VI isotherm observed for MIL-101 structures.28 The Brunauer–Emmett–Teller (BET) surface area,25 calculated from the adsorption isotherm over P/P0 = 0.05–0.15, was 1980 m2 g−1, similar to the reported BET surface area of amino-MIL-101(Al) (2100 m2 g−1).22 The pore size distribution (Fig. S4†) of amino-MIL-101(Al), calculated from the adsorption branch of the isotherm using the Barrett–Joyner–Halenda (BJH) equation,26 was bimodal, with narrow peaks at pore sizes of 1.10 and 1.52 nm. The purified amino-MIL-101(Al) was stable in air up to 492 °C (Fig. S5†). The adsorption ability of amino-MIL-101(Al) was first tested by exposing 10 mg of the solid to 50 mL of a 50-ppm aqueous MB solution for 6 h, after which the supernatant appeared colourless (Fig. S6†). Thus we examined the kinetics and thermodynamics of the adsorption. The saturation adsorption was investigated by combining 5 mg of amino-MIL-101(Al) with 100 mL of aqueous MB ([MB]0 = 20–40 ppm) for 12 h. The quantity of MB adsorbed over this time is displayed in Fig. 3a, and the source UV-vis spectra are shown in Fig. S7.† Saturation occurred after approximately 2 h, and the total amount of MB adsorbed increased with [MB]0, demonstrating favourable adsorption characteristics at high MB concentrations. The same has been observed for other dye adsorptions on MOFs.11b,c,19a The reaction with [MB]0 = 40 ppm was performed three times, and the maximum adsorption was reproducible within 2% (see Table 1 and Fig. 4). The rate of MB adsorption onto amino-MIL-101(Al) was measured over a 12-h period. Like many dye-adsorption reactions, this adsorption could not be described rigorously by first- or second-order kinetics, so true kinetic constants were not extracted. However, in order to compare the rate of MB adsorption on amino-MIL-101(Al) to those on reported sorbents, the change in MB concentration with time was treated with two common kinetic models. Fig. S8a† shows the adsorption of MB from solutions with initial MB concentrations of 20, 30 and 40 ppm, fit to a pseudo-second-order kinetic model (see ESI† for details).27 The calculated values of k2, along with the relevant correlation coefficients (R2), are shown in Tables 1 and S2.† The value of k2 for the adsorption of MB on amino-MIL-101(Al) increased slightly with increased initial dye concentration, similar to previous observations,11b,c,19a,32 but contrary to the case of malachite green on MIL-100(Fe).19a The adsorption data were also fit to a pseudo-first-order kinetic model (see ESI and Fig. S8b†), and the extracted values of k1 and R2 are displayed in Tables 1 and S3.† The rate of adsorption was clearly better described by pseudo-second-order than pseudo-first-order kinetics. Nevertheless, the values of both k2 and k1 determined for the adsorption of MB on amino-MIL-101(Al) were much higher than those reported for MOF-235,11b activated carbon,33 chitosan-g-poly(acrylic acid)–vermiculite hydrogel composite,34 magnetic modified beer yeast,35 alginate–hydrolysed oak sawdust composite,36 papaya seed,37 grass waste,38 and graphene,39 as shown in Table 2. Not only did amino-MIL-101(Al) adsorb MB more quickly than many adsorbents, but it also took up large amounts of the dye. Adsorption isotherms were measured after 12 h, and the maximum adsorption capacity was calculated from the plot in Fig. 4b according to the Langmuir equation (eqn (2)).40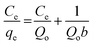 | (2) |
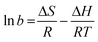 | (3) |
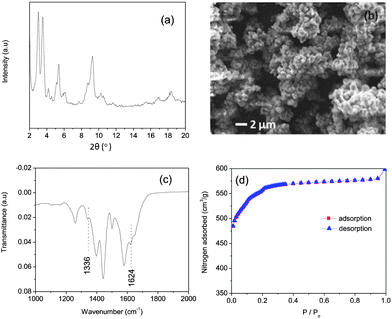 | ||
| Fig. 2 Characterisation data for amino-functionalised MIL-101(Al): (a) XRD pattern; (b) SEM image; (c) FT-IR spectrum; (d) nitrogen adsorption–desorption isotherm recorded at −196 °C. | ||
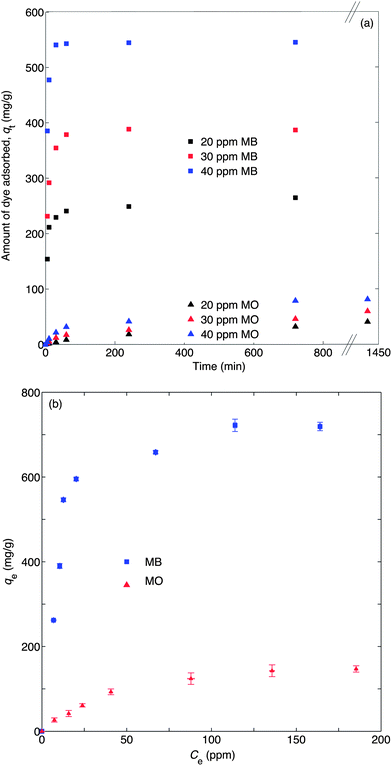 | ||
| Fig. 3 (a) Effect of contact time on the adsorption of MB or MO by 5 mg amino-MIL-101(Al) at 30 °C with [dye]0 = 20, 30 or 40 ppm; (b) isotherms for MB and MO adsorption after 12 h of adsorption. | ||
| Adsorbent | Pore size (nm) | BET Surface areaa (m2 g−1) | Total pore volumeb (cm3 g−1) | Dye | Kinetic data for [dye]0 = 40 ppmc | Adsorption capacity, Qod (mg g−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| Pseudo-second-order kinetic constant (g mg−1 min−1) | Pseudo-first-order kinetic constant (min−1) | ||||||||
| k 2 | R 2 | k 1 | R 2 | ||||||
| a Calculated over P/P0 = 0.05–0.15. b Calculated for P/P0 = 0.99. c The data were not completely described by either first- or second-order models, so the rate ‘constants’ depend on [dye]0. Constants and R2 values were determined by fitting data to standard kinetic models; see Fig. S8 and S14 for the relevant plots and Tables S2 and S3 for data collected at [dye]0 = 20 or 30 ppm. d Measured after a 12 h adsorption at 30 °C using Langmuir equation; see Fig. 4 and S13. | |||||||||
| Amino-MIL-101 | 1.10 | 1980 | 1.02 | MB | (2.6 ± 1.3) × 10−3 | 0.999 | (7 ± 3) × 10−2 | 0.9 | 762 ± 12 |
| 1.52 | MO | (1.3 ± 0.3) × 10−4 | 0.993 | (8.7 ± 0.9) × 10−3 | 0.98 | 188 ± 9 | |||
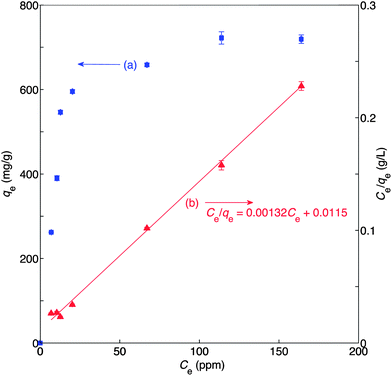 | ||
| Fig. 4 (a) Isotherm for MB adsorption over 5 mg of amino-MIL-101(Al) after 12 h at 30 °C; and (b) Langmuir plot of the isotherm of (a). | ||
| Adsorbent | [dye]0 (ppm) | k 1 (min−1) | k 2 (g mg−1 min−1) | Ref. |
|---|---|---|---|---|
| a Not determined. | ||||
| Amino-MIL-101(Al) | 40 | 0.07 ± 0.03 | 0.0026 ± 0.0013 | This work |
| MOF-235 | 40 | —a | 2.18 × 10−4 | 11b |
| Bamboo-based activated carbon | 200 | 0.00129 | 1.27 × 10−4 | 33c |
| Activated carbon from periwinkle | 200 | 0.0152 | 1.88 × 10−4 | 33b |
| Magnetic cellulose beads entrapping activated carbon | 374 | —a | 1.41 × 10−4 | 33d |
| Chitosan-g-poly(acrylic acid)–vermiculite hydrogel composite | 1000 | 0.0370 | 5.75 × 10−4 | 34 |
| Magnetic modified beer yeast | 300 | 0.0300 | 9.00 × 10−5 | 35 |
| Alginate–hydrolysed oak sawdust composite | 200 | 0.0134 | 1.61 × 10−3 | 36 |
| Papaya seed | 300 | 0.0304 | 1.70 × 10−3 | 37 |
| Grass waste | 380 | 0.0283 | 1.70 × 10−3 | 38 |
| Graphene | 40 | 0.0096 | 1.00 × 10−4 | 39 |
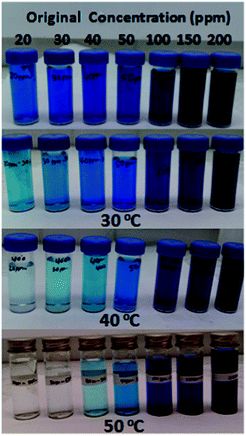 | ||
| Fig. 5 Digital images of MB solutions before and after 12 h over 5 mg of amino-MIL-101(Al) at 30, 40 and 50 °C. | ||
The enthalpy change ΔH for MB adsorption over amino-MIL-101(Al) was positive, +77.9 kJ mol−1, confirming that the adsorption was endothermic. The entropy change ΔS was +346 J mol−1 K−1. According to eqn (4), the free energies of adsorption at 30, 40 and 50 °C were −26.19 ± 0.04, −30.1 and −33.0 kJ mol−1, respectively (Table 3). These negative free energies confirmed that adsorption was spontaneous under the experimental conditions used.
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b | (4) |
| Dye | MOF | T (°C) | Q o (mg g−1)a | ΔGa (kJ mol−1) | ΔHa (kJ mol−1) | ΔSa (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| a Adsorption isotherms and van't Hoff plots for MB and MO adsorption on amino-MIL-101 are given in Fig. 6 and S15, respectively, and the adsorption isotherm and van't Hoff plot for MB on MIL-101(Al) is given in Fig. S23. | ||||||
| MB | Amino-MIL-101(Al) | 30 | 762 ± 12 | −26.19 ± 0.04 | 77.9 | 346 |
| 40 | 1032 | −30.1 | ||||
| 50 | 1409 | −33.0 | ||||
| MO | Amino-MIL-101(Al) | 30 | 188 ± 9 | −21.0 ± 0.3 | 0.489 | 74.3 |
| 40 | 192.1 | −22.8 | ||||
| 50 | 198.6 | −23.5 | ||||
| MB | MIL-101(Al) | 30 | 195 | −23.3 | 0.603 | 78.8 |
| 40 | 216 | −24.1 | ||||
| 30 | 195 | −23.3 | ||||
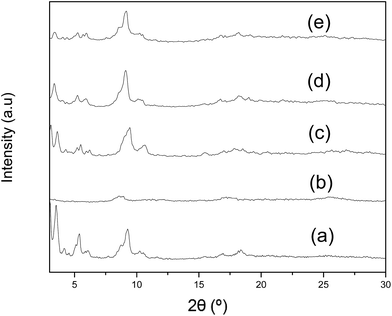
The high positive value of ΔS for the adsorption of MB by amino-MIL-101(Al) indicated a significant loss of order. Moderate increases in entropy (ΔS = 50–200 J mol−1 K−1) are common during in dye adsorption;11c,17,19,48b,49 when observed for dye adsorption on MOFs, these values have been attributed to the displacement of multiple surface-bound water molecules by a single (larger) dye molecule.11c,19a However, the magnitude of ΔS for MB adsorption on amino-MIL-101(Al) was much greater than the values measured for MO adsorption on MIL-101(Cr)11c or malachite green adsorption on MIL-100(Fe);19a only the adsorption of MO on MOF-235 has shown a larger value of ΔS.11b The value of ΔH measured here was also high compared to other dye adsorptions on MIL materials.11c,19a In an effort to understand the dramatic thermodynamic parameters measured here, we examined the MB–MOF composite. Thus, following exposure to MB in solution ([MB]0 = 40 ppm, 50 °C, 12 h), the MOF was filtered from solution and dried at 100 °C under vacuum for 12 h. X-ray photoelectron spectroscopy (XPS; Fig. S12 and S13, Tables S4 and S5†) of the as-synthesised and recovered dyes showed that the electron binding energies associated with some of the carbon and nitrogen atoms in the MOF structure changed upon dye exposure. In particular, a new C1s peak appeared at 287.8 eV, and several other C1s peaks decreased in intensity (Fig. S13†), suggesting significant structural alteration of the MOF after the absorption of the MB dye. It was clear from the XRD patterns of the amino-MIL-101(Al) before and after adsorption (Fig. 7, cf. a and b) that the adsorption of MB interrupted its long-range order, which likely contributed to the high positive entropy change observed. This structural change could not have been the result of reaction with water, as amino-MOF-101(Al) was stable in pure water under the reaction conditions (up to 50 °C for 12 h; see Fig. 7d).
MOFs can, in some instances, change their structures upon strong interaction with an adsorbate,50 and this appears to occur in the present case, though at the expense of an ordered structure. We therefore considered the possibility that the interaction between MB and amino-MIL-101(Al) should be described as a reaction rather than an adsorption, that is, whether metal–carboxylate bonds were broken upon dye adsorption. The as-synthesised and recovered dyes were thus examined using solid-state magic-angle spinning (MAS) 27Al spectroscopy (Fig. S22†). The spectrum showed a peak at δ = −10.0 ppm, which was similar to the 27Al NMR chemical shift measured for the as-synthesised material (δ = −7.3 ppm, Fig. S22†). Both were indicative of octahedral coordination at Al; however, the sample that was exposed to MB showed a signal that was less symmetric, and could have represented more than one type of Al nucleus. Further, as would be expected for both pore-filling by an adsorbate or structural collapse, the MB–MOF composite had a much lower surface area (154 m2 g−1) than the as-synthesised amino-MIL-101(Al) (1980 m2 g−1, see above). However, the MB–MOF composite showed a clear hysteresis loop over P/P0 = 0.45–0.80, suggesting the formation of mesopores. Most telling, though, was the aluminium content of the solution after MB adsorption. An aluminium concentration of 1.8 ppm was measured, indicating that ∼30% of the metal was lost from the amino-MIL-101(Al). This, combined with the change in the XRD pattern of the solid, indicated that MB reacted with some, but not necessarily all, of the amino-MIL-101(Al).
The disruption of the amino-MIL-101(Al) structure upon MB exposure explains the significantly unfavourable enthalpy upon adsorption, as at least some of the metal–carboxylate bonds that held the structure together were broken and weakened. This loss was apparently greater than the combined favourable enthalpy change from the electrostatic interactions and any van der Waals forces between the dye and the MOF. Moreover, based upon the values of ΔH and ΔS for the reaction, the destruction of the ordered MOF structure, and the dissolution of Al3+ ions, appears to be the driving force for the interaction. This is in contrast to the case of MB adsorption on a MOF–graphite oxide material, which occurred with more moderate thermodynamic parameters (ΔH = 39.26 kJ mol−1, ΔS = 167.5 J mol−1 K−1), as the XRD pattern of that material upon recycling still reflected an ordered crystalline phase.19b It is not clear whether a loss of crystallinity was responsible for the high ΔS values observed in the adsorptions of MB and MO on MOF-235, as that material was not characterised after adsorption.11b
In order to determine whether the amino groups in the amino-MIL-101(Al) material were involved in its interaction with MB, we tested an analogous adsorption. Thus, the amino-free parent framework, MIL-101(Al), was exposed to aqueous solutions of MB at 30, 40, and 50 °C. The adsorption curves, Langmuir isotherms and van't Hoff plots are shown in Fig. S23† and summarised in Table 3. MIL-101(Al) took up significantly less MB than its amino-functionalised analogue, adsorbing a maximum of 195 mg g−1 at 30 °C. This was approximately one-fourth of the capacity of amino-MIL-101(Al) for MB, and only slightly higher than the capacity of MOF-235 for the same dye.11b Contrary to the adsorption of MB on amino-MIL-101(Al), that on the unfunctionalised MIL-101(Al) was only weakly temperature-dependent. This was borne out in the thermodynamic parameters of the reaction (Table 3); the reaction was nearly thermoneutral, and incurred a modest entropy gain that was well within the range common for dye adsorptions.11c,17,19,48b,49 The lower adsorption capacity of MIL-101(Al) for MB, as well as the unexceptional ΔH and ΔS values observed for the adsorption reaction, suggested that this adsorption did not induce a significant change in the crystal structure of the MOF, and this was confirmed by the XRD pattern of MIL-101(Al) after MB adsorption (Fig. 7e), which still showed the MIL-101 phase.
Given the severe impact of dye adsorption on the MOF structure, we also investigated the structure of the adsorbed dye. Thus, samples of the dye–MOF composites that were produced by adsorption at 30, 40, and 50 °C were sonicated in deionised H2O for 12 h, and the UV-vis spectrum of the resulting solutions were measured (Fig. S10†). In all cases, these displayed the UV spectrum of MB (λmax = 666 nm), indicating that at least some of the MB was adsorbed without reacting further with the MOF. However, complete recovery of the dye was not obtained, so we cannot exclude the possibility that some of the dye was altered upon reaction with the MOF.
The regeneration of an adsorbent is important for its commercial feasibility and, although amino-MIL-101(Al) adsorbed more MB than any other MOF tested to date, the changes to its structure upon adsorption were inauspicious for its reuse. We attempted to regenerate the used adsorbent (amino-MOF–MB) by filtering it from the solution, washing several times with water, and drying at 100 °C overnight. It was then activated with deionised water under ultrasound for 60 min11b,c and tested as an adsorbent over 12 h at 30 °C (Fig. S11†). Though the maximum adsorption capacity of fresh amino-MIL-101(Al) for MB was 762 ± 12 mg g−1, the value for the ‘regenerated’ material was only 14.8 mg g−1. Thus the degradation of the MOF structure that occurred upon MB adsorption had a strong negative impact on its reusability.
We had originally chosen an amino-functionalised MOF as a sorbent with the expectation that the Lewis basic amine group would interact with the cationic dye. To further study the importance of these electrostatic interactions, we compared the adsorption of MB by amino-MIL-101(Al) to that of an anionic dye, methyl orange (MO). The quantity of MO adsorbed increased, though not dramatically, at higher initial dye concentrations (Fig. 3b). Fig. S14† shows the adsorption isotherms and Langmuir plots for MO adsorption over amino-MIL-101(Al). The maximum adsorption capacity (Qo) of amino-MIL-101(Al) for MO adsorption, calculated from eqn (2) using data from Fig. S14b,† was 188 ± 9 mg g−1 (average of three experiments), which is four times lower than that for MB. The dyes have similar molecular weights, so the comparison is similar on a molar basis. Significantly though, the adsorption capacity of amino-MIL-101(Al) for MO is higher than those of other reported adsorbents, including diatomite,46Spirodela polyrrhiza biomass,51 MCM-22,49a magnetic maghemite–chitosan nanocomposite,52 MOF-23511b and fly ash,53 as shown in Table S6.†
The adsorption of MO on amino-MIL-101(Al) was also evaluated as a function of time, and fit to pseudo-first-order and pseudo-second-order models to calculate values for k1 and k2 (Fig. S15† and Table 1). Both increased slightly upon increasing the initial concentration of MO. Whereas the adsorption of MB on amino-MIL-101(Al) had been much better represented mathematically by pseudo-second-order kinetics, the case for MO adsorption was more ambiguous, with the models giving similar quality descriptions of the data (see Table 1). This indicated that the rates of MB and MO adsorption were not controlled by the same factors. Fig. 3c and S18† compare the adsorptions of MO and MB on amino-MIL-101(Al); the latter was adsorbed approximately an order of magnitude faster (Fig. S19 and S20†).
The thermodynamics of the interaction between MO and amino-MIL-101(Al) were also examined (Fig. S16†). The reaction was favourable, but both ΔGads and the maximum adsorption capacity were relatively insensitive to temperature (Table 3). Unlike the adsorption of MB, which was highly endothermic, that of MO had ΔH ∼ 0. This implied that, unlike MB adsorption, MO adsorption had little impact on the structure of amino-MIL-101(Al); any disruption of the MOF structure upon MO adsorption was almost completely compensated by the van der Waals forces between the dye and the high-surface-area MOF structure. The entropy change upon MO adsorption was also modest (ΔS = 74.3 J mol−1 K−1, falling in the range generally observed for dye adsorption,11c,17,19,48b,49), consistent with the suggestion that it had little impact on the MOF structure. This was also supported by the XRD pattern of amino-MIL-101(Al) after MO adsorption (Fig. 7c), which was similar to that of the MOF alone (Fig. 7a). Nevertheless, the surface area of amino-MIL-101(Al) dropped dramatically, to 123 m2 g−1, upon exposure to MO ([MO]0 = 40 ppm, T = 50 °C, t = 12 h), indicating that significant dye adsorption occurred within the pores of the material. On the other hand, no significant hysteresis loop was noted in the N2 physisorption isotherm of the MO–MOF composite (Fig. S21†). Like the 27Al NMR spectrum of the MB–MOF composite, that of the MO–MOF composite showed a peak near 0 ppm (at δ = −9.5 ppm; Fig. S22†) that was less symmetrical than the peak seen for amino-MIL-101(Al). Given the complex NMR peak shapes observed, and the significant differences between the thermodynamic and X-ray diffraction data obtained for the interaction of amino-MIL-101(Al) and each of the dyes, we do not interpret this as evidence that the same reactions that occur between amino-MIL-101(Al) and MB occur between the MOF and MO. Rather, we can say that the Al atoms in amino-MOF-101(Al) remain octahedrally coordinated after exposure to either dye; further investigations will be necessary before conclusions can be drawn from the line shapes observed in the 27Al NMR studies. Further evidence that the interaction between MO and amino-MIL-101(Al) was an adsorption could be gleaned from analysis of the solution following adsorption; only 0.07 ppm Al was detected (cf. 1.8 ppm following exposure to aqueous MB, see above).
The lack of drastic structural change in amino-MIL-101(Al) upon MO adsorption suggested that it might be a reusable adsorbent for this substrate. Thus, the used adsorbent was regenerated using the procedure described above and applied to a fresh solution of MO. The procedure was then repeated. In these second and third uses of amino-MIL-101(Al), its maximum adsorption capacities for MO were 99.3 and 19.5 mg g−1 (Fig. S17†). Thus, the MOF lost approximately half of its adsorption capacity upon reuse, and 80% of its remaining capacity in an additional use. Though significant, this change was small compared to the case for MB, where Q0 dropped to <2% of its initial value upon a single reuse.
The kinetic, thermodynamic, and structural data all indicated that amino-MIL-101(Al) adsorbed cationic MB and anionic MO via different mechanisms. Although this was, to some degree, by design, in that an amino-bearing MOF was chosen in order to favour the adsorption of cationic dyes, the effect proved much larger than anticipated, and the adsorption of MB occurred at the expense of the MOF structure. To our knowledge, this effect has not been reported previously; however, it should clearly be considered in the ongoing design of tailored adsorbents for dyes.
4. Conclusions
Harmful dyes (cationic MB and anionic MO) can be efficiently removed from contaminated water by the amino-functionalised MOF, amino-MIL-101(Al). This MOF showed a superior adsorption capacity (up to 762 mg g−1) and high adsorption rate for MB adsorption, but only one-fourth as much of the anionic dye MO. Moreover, MIL-101(Al) absorbed significantly less MB than its amine-functionalised counterpart. Thus the electrostatic interaction between the cationic MB and the electron lone pairs on the amino groups in the MOF led to a remarkable material for MB adsorption. However, the large entropy increase that drove the reaction between amino-MIL-101(Al) and the cationic dye reflected a concomitant disruption of the MOF structure and dissolution of aluminium ions; this was not observed upon adsorption of the anionic dye. Attempts to reuse the sorbent demonstrated that, although fresh amino-MIL-101(Al) quickly adsorbed significant amounts of MB, it was a very poor sorbent upon reuse. On the other hand, though as-synthesised amino-MIL-101(Al) adsorbed only one fourth as much MO, its structure was better conserved, and it could be reused more effectively, following that interaction. Thus, although the flexible synthesis of MOFs allowed us to construct a material tailored to the removal of a cationic dye from solution, its affinity for the dye proved greater than its stability. Nevertheless, the high surface area of the MOF meant that it was still a good sorbent for an anionic dye, and it was more stable in that reaction.Acknowledgements
E. Haque is grateful to the University of Sydney for an International Scholarship. A. T. Harris acknowledges ongoing support from the Australian Research Council (ARC) through a Future Fellowship. The authors are grateful to Dr Bill Gong of the University of New South Wales for performing the X-ray photoelectron spectroscopy, and to Drs Aditya Rawal & James Hook of the NMR Facility, Mark Wainwright Analytical Centre, University of NSW, Australia, for performing the 27Al NMR spectroscopy, as well as to Dr Shuranjan Sarkar, Kyungpook National University, South Korea, for assistance with manuscript graphics.Notes and references
- (a) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC; (b) S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; (c) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed.
- (a) L. Alaerts, E. Séguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. De Vos, Chem.–Eur. J., 2006, 12, 7353 CrossRef CAS PubMed; (b) P. Forster and A. Cheetham, Top. Catal., 2003, 24, 79 CrossRef CAS; (c) P. Horcajada, S. Surblé, C. Serre, D.-Y. Hong, Y.-K. Seo, J.-S. Chang, J.-M. Grenèche, I. Margiolaki and G. Férey, Chem. Commun., 2007, 2820 RSC; (d) Y. K. Hwang, D.-Y. Hong, J.-S. Chang, S. H. Jhung, Y.-K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef CAS PubMed; (e) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (f) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (g) L. G. Qiu, A. J. Xie and L. D. Zhang, Adv. Mater., 2005, 17, 689 CrossRef CAS; (h) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS PubMed; (i) C.-D. Wu, A. Hu, L. Zhang and W. Lin, J. Am. Chem. Soc., 2005, 127, 8940 CrossRef CAS PubMed; (j) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed; (k) R.-Q. Zou, H. Sakurai and Q. Xu, Angew. Chem., Int. Ed., 2006, 45, 2542 CrossRef CAS PubMed.
- (a) B. Chen, N. W. Ockwig, A. R. Millward, D. S. Contreras and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4745 CrossRef CAS PubMed; (b) H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem.–Eur. J., 2005, 11, 3521 CrossRef CAS PubMed; (c) M. Dincǎ and J. R. Long, J. Am. Chem. Soc., 2005, 127, 9376 CrossRef PubMed; (d) D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033 CrossRef CAS PubMed; (e) L. J. Murray, M. Dinč and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (f) F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833 CrossRef CAS PubMed; (g) J. L. C. Rowsell, E. C. Spencer, J. Eckert, J. A. K. Howard and O. M. Yaghi, Science, 2005, 309, 1350 CrossRef CAS PubMed; (h) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS PubMed; (i) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (j) S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef CAS PubMed; (k) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed; (l) B. Kesanli, Y. Cui, M. R. Smith, E. W. Bittner, B. C. Bockrath and W. Lin, Angew. Chem., Int. Ed., 2005, 44, 72 CrossRef CAS PubMed; (m) Y. Li and R. T. Yang, J. Am. Chem. Soc., 2005, 128, 726 CrossRef PubMed; (n) J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308 RSC; (o) Y. Liu, J. F. Eubank, A. J. Cairns, J. Eckert, V. C. Kravtsov, R. Luebke and M. Eddaoudi, Angew. Chem., Int. Ed., 2007, 46, 3278 CrossRef CAS PubMed; (p) A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998 CrossRef CAS PubMed; (q) R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966 CrossRef CAS PubMed; (r) L. Pan, M. B. Sander, X. Huang, J. Li, M. Smith, E. Bittner, B. Bockrath and J. K. Johnson, J. Am. Chem. Soc., 2004, 126, 1308 CrossRef CAS PubMed; (s) X. Zhao, B. Xiao, A. J. Fletcher, K. M. Thomas, D. Bradshaw and M. J. Rosseinsky, Science, 2004, 306, 1012 CrossRef CAS PubMed.
- (a) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef CAS PubMed; (b) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774 CrossRef CAS PubMed; (c) P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974 CrossRef CAS PubMed.
- (a) R. Kitaura, K. Seki, G. Akiyama and S. Kitagawa, Angew. Chem., Int. Ed., 2003, 42, 428 CrossRef CAS PubMed; (b) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (c) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed; (d) S. Ma, D. Sun, X.-S. Wang and H.-C. Zhou, Angew. Chem., Int. Ed., 2007, 46, 2458 CrossRef CAS PubMed.
- (a) B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390 CrossRef CAS PubMed; (b) C. Chen, M. Zhang, Q. Guan and W. Li, Chem. Eng. J., 2012, 183, 60 CrossRef CAS PubMed; (c) K. A. Cychosz, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 6938 CAS; (d) X.-X. Huang, L.-G. Qiu, W. Zhang, Y.-P. Yuan, X. Jiang, A.-J. Xie, Y.-H. Shen and J.-F. Zhu, CrystEngComm, 2012, 14, 1613 RSC; (e) L. Pan, D. H. Olson, L. R. Ciemnolonski, R. Heddy and J. Li, Angew. Chem., Int. Ed., 2006, 45, 616 CrossRef CAS PubMed; (f) T. K. Trung, P. Trens, N. Tanchoux, S. Bourrelly, P. L. Llewellyn, S. Loera-Serna, C. Serre, T. Loiseau, F. Fajula and G. Férey, J. Am. Chem. Soc., 2008, 130, 16926 CrossRef CAS PubMed; (g) X. Wang, L. Liu and A. J. Jacobson, Angew. Chem., Int. Ed., 2006, 45, 6499 CrossRef CAS PubMed.
- (a) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; (b) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (c) D. T. de Lill, N. S. Gunning and C. L. Cahill, Inorg. Chem., 2004, 44, 258 CrossRef PubMed; (d) B. V. Harbuzaru, A. Corma, F. Rey, P. Atienzar, J. L. Jordá, H. García, D. Ananias, L. D. Carlos and J. Rocha, Angew. Chem., Int. Ed., 2008, 47, 1080 CrossRef CAS PubMed; (e) Z. Li, G. Zhu, X. Guo, X. Zhao, Z. Jin and S. Qiu, Inorg. Chem., 2007, 46, 5174 CrossRef CAS PubMed.
- G. Férey, F. Millange, M. Morcrette, C. Serre, M.-L. Doublet, J.-M. Grenèche and J.-M. Tarascon, Angew. Chem., Int. Ed., 2007, 46, 3259 CrossRef PubMed.
- (a) N. Guillou, C. Livage, M. Drillon and G. Férey, Angew. Chem., Int. Ed., 2003, 42, 5314 CrossRef CAS PubMed; (b) S. M. Humphrey, T. J. P. Angliss, M. Aransay, D. Cave, L. A. Gerrard, G. F. Weldon and P. T. Wood, Z. Anorg. Allg. Chem., 2007, 633, 2342 CrossRef CAS; (c) D. Maspoch, D. Ruiz-Molina and J. Veciana, J. Mater. Chem., 2004, 14, 2713 RSC.
- (a) S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2005, 127, 13744 CrossRef CAS PubMed; (b) H. R. Moon, J. H. Kim and M. P. Suh, Angew. Chem., Int. Ed., 2005, 44, 1261 CrossRef CAS PubMed.
- (a) A. A. Adeyemo, I. O. Adeoye and O. S. Bello, Toxicol. Environ. Chem., 2012, 94, 1846 CrossRef CAS; (b) E. Haque, J. W. Jun and S. H. Jhung, J. Hazard. Mater., 2011, 185, 507 CrossRef CAS PubMed; (c) E. Haque, J. E. Lee, I. T. Jang, Y. K. Hwang, J.-S. Chang, J. Jegal and S. H. Jhung, J. Hazard. Mater., 2010, 181, 535 CrossRef CAS PubMed.
- (a) E. Haque, J. W. Jun, S. N. Talapaneni, A. Vinu and S. H. Jhung, J. Mater. Chem., 2010, 20, 10801 RSC; (b) M. Maes, S. Schouteden, L. Alaerts, D. Depla and D. E. De Vos, Phys. Chem. Chem. Phys., 2011, 13, 5587 RSC; (c) M. Maes, F. Vermoortele, L. Alaerts, J. F. M. Denayer and D. E. De Vos, J. Phys. Chem. C, 2010, 115, 1051 CrossRef.
- K. A. Cychosz and A. J. Matzger, Langmuir, 2010, 26, 17198 CrossRef CAS PubMed.
- (a) S. Achmann, G. Hagen, M. Hämmerle, I. M. Malkowsky, C. Kiener and R. Moos, Chem. Eng. Technol., 2010, 33, 275 CrossRef CAS; (b) G. Blanco-Brieva, J. M. Campos-Martin, S. M. Al-Zahrani and J. L. G. Fierro, Fuel, 2011, 90, 190 CrossRef CAS PubMed; (c) K. A. Cychosz, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2009, 131, 14538 CrossRef CAS PubMed.
- G. Crini, Bioresour. Technol., 2006, 97, 1061 CrossRef CAS PubMed.
- S. Chen, J. Zhang, C. Zhang, Q. Yue, Y. Li and C. Li, Desalination, 2010, 252, 149 CrossRef CAS PubMed.
- A. Mittal, A. Malviya, D. Kaur, J. Mittal and L. Kurup, J. Hazard. Mater., 2007, 148, 229 CrossRef CAS PubMed.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70 CrossRef CAS PubMed.
- (a) S.-H. Huo and X.-P. Yan, J. Mater. Chem., 2012, 22, 7449 RSC; (b) L. Li, X. L. Liu, H. Y. Geng, B. Hu, G. W. Song and Z. S. Xu, J. Mater. Chem. A, 2013, 1, 10292 RSC.
- Q. Qin, J. Ma and K. Liu, J. Hazard. Mater., 2009, 162, 133 CrossRef CAS PubMed.
- E. Stavitski, M. Goesten, J. Juan-Alcañiz, A. Martinez-Joaristi, P. Serra-Crespo, A. V. Petukhov, J. Gascon and F. Kapteijn, Angew. Chem., Int. Ed., 2011, 50, 9624 CrossRef CAS PubMed.
- P. Serra-Crespo, E. V. Ramos-Fernandez, J. Gascon and F. Kapteijn, Chem. Mater., 2011, 23, 2565 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS PubMed.
- K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- (a) G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed; (b) D.-Y. Hong, Y. K. Hwang, C. Serre, G. Férey and J.-S. Chang, Adv. Funct. Mater., 2009, 19, 1537 CrossRef CAS.
- S. Bauer, C. Serre, T. Devic, P. Horcajada, J. Marrot, G. Férey and N. Stock, Inorg. Chem., 2008, 47, 7568 CrossRef CAS PubMed.
- S. H. Jhung, J.-H. Lee, J. W. Yoon, C. Serre, G. Férey and J.-S. Chang, Adv. Mater., 2007, 19, 121 CrossRef CAS.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- B. H. Hameed and A. A. Rahman, J. Hazard. Mater., 2008, 160, 576 CrossRef CAS PubMed.
- (a) S. Altenor, B. Carene, E. Emmanuel, J. Lambert, J.-J. Ehrhardt and S. Gaspard, J. Hazard. Mater., 2009, 165, 1029 CrossRef CAS PubMed; (b) O. S. Bello, I. A. Adeogun, J. C. Ajaelu and E. O. Fehintola, Chem. Ecol., 2008, 24, 285 CrossRef CAS; (c) B. H. Hameed, A. T. M. Din and A. L. Ahmad, J. Hazard. Mater., 2007, 141, 819 CrossRef CAS PubMed; (d) X. Luo and L. Zhang, J. Hazard. Mater., 2009, 171, 340 CrossRef CAS PubMed.
- Y. Liu, Y. Zheng and A. Wang, J. Environ. Sci., 2010, 22, 486 CrossRef CAS.
- J.-X. Yu, L.-Y. Wang, R.-A. Chi, Y.-F. Zhang, Z.-G. Xu and J. Guo, Environ. Sci. Pollut. Res., 2013, 20, 543 CrossRef CAS PubMed.
- M. M. A. El-Latif, A. M. Ibrahim and M. F. El-Kady, J. Am. Sci., 2010, 6, 267 Search PubMed.
- B. H. Hameed, J. Hazard. Mater., 2009, 162, 939 CrossRef CAS PubMed.
- B. H. Hameed, J. Hazard. Mater., 2009, 166, 233 CrossRef CAS PubMed.
- T. Liu, Y. Li, Q. Du, J. Sun, Y. Jiao, G. Yang, Z. Wang, Y. Xia, W. Zhang, K. Wang, H. Zhu and D. Wu, Colloids Surf., B, 2012, 90, 197 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361 CrossRef CAS.
- (a) E. N. El Qada, S. J. Allen and G. M. Walker, Chem. Eng. J., 2008, 135, 174 CrossRef CAS PubMed; (b) G. Duman, Y. Onal, C. Okutucu, S. Onenc and J. Yanik, Energy Fuels, 2009, 23, 2197 CrossRef CAS; (c) S. Timur, E. Ikizoglu and J. Yanik, Energy Fuels, 2006, 20, 2636 CrossRef CAS; (d) J. Yamashita, M. Shioya, T. Kikutani and T. Hashimoto, Carbon, 2001, 39, 207 CrossRef CAS.
- S. Senthilkumaar, P. R. Varadarajan, K. Porkodi and C. V. Subbhuraam, J. Colloid Interface Sci., 2005, 284, 78 CrossRef CAS PubMed.
- J.-x. Yu, B.-h. Li, X.-m. Sun, J. Yuan and R.-a. Chi, Appl. Biochem. Biotechnol., 2010, 160, 1394 CrossRef CAS PubMed.
- F. Raposo, M. A. De La Rubia and R. Borja, J. Hazard. Mater., 2009, 165, 291 CrossRef CAS PubMed.
- G. McKay, J. F. Porter and G. R. Prasad, Water, Air, Soil Pollut., 1999, 114, 423 CrossRef CAS.
- M. A. Al-Ghouti, M. A. M. Khraisheh, S. J. Allen and M. N. Ahmad, J. Environ. Manage., 2003, 69, 229 CrossRef CAS PubMed.
- S. Eftekhari, A. Habibi-Yangjeh and S. Sohrabnezhad, J. Hazard. Mater., 2010, 178, 349 CrossRef CAS PubMed.
- (a) S. Wang, L. Li, H. Wu and Z. H. Zhu, J. Colloid Interface Sci., 2005, 292, 336 CrossRef CAS PubMed; (b) K. P. Singh, D. Mohan, S. Sinha, G. S. Tondon and D. Gosh, Ind. Eng. Chem. Res., 2003, 42, 1965 CrossRef CAS.
- (a) S. Wang, H. Li and L. Xu, J. Colloid Interface Sci., 2006, 295, 71 CrossRef CAS PubMed; (b) Z.-M. Ni, S.-J. Xia, L.-G. Wang, F.-F. Xing and G.-X. Pan, J. Colloid Interface Sci., 2007, 316, 284 CrossRef CAS PubMed; (c) A. Bhatnagar, E. Kumar, A. K. Minocha, B.-H. Jeon, H. Song and Y.-C. Seo, Sep. Sci. Technol., 2009, 44, 316 CrossRef CAS.
- (a) C. Serre, C. Mellot-Draznieks, S. Surblé, N. Audebrand, Y. Filinchuk and G. Férey, Science, 2007, 315, 1828 CrossRef CAS PubMed; (b) D. Tanaka, K. Nakagawa, M. Higuchi, S. Horike, Y. Kubota, T. C. Kobayashi, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2008, 47, 3914 CrossRef CAS PubMed.
- P. Waranusantigul, P. Pokethitiyook, M. Kruatrachue and E. S. Upatham, Environ. Pollut., 2003, 125, 385 CrossRef CAS.
- R. Jiang, Y.-Q. Fu, H.-Y. Zhu, J. Yao and L. Xiao, J. Appl. Polym. Sci., 2012, 125, E540 CrossRef CAS.
- P. Janoš, H. Buchtová and M. Rýznarová, Water Res., 2003, 37, 4938 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Structures and calibration curves for dyes, characterisation of amino-MIL-101(Al) before and after dye exposure, UV-vis spectra and adsorption data, rate data and treatment, adsorption of MB on MIL-101(Al). See DOI: 10.1039/c3ta13589f |
| This journal is © The Royal Society of Chemistry 2014 |