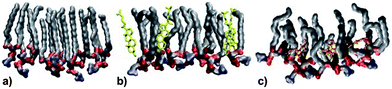Melatonin directly interacts with cholesterol and alleviates cholesterol effects in dipalmitoylphosphatidylcholine monolayers
Youngjik
Choi
ab,
Simon J.
Attwood
c,
Matthew I.
Hoopes
d,
Elizabeth
Drolle
ab,
Mikko
Karttunen
bd and
Zoya
Leonenko
*abc
aDepartment of Biology, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada N2L 3G1. E-mail: zleonenk@uwaterloo.ca; Tel: +1-519-888-4567 ext. 38273
bWaterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada N2L 3G1
cDepartment of Physics and Astronomy, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada N2L 3G1
dDepartment of Chemistry, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada N2L 3G1
First published on 7th November 2013
Abstract
Melatonin is a pineal hormone that has been shown to have protective effects in several diseases that are associated with cholesterol dysregulation, including cardiovascular disease, Alzheimer's disease, and certain types of cancers. Cholesterol is a major membrane constituent with both a structural and functional influence. It is also known that melatonin readily partitions into cellular membranes. We investigated the effects of melatonin and cholesterol on the structure and physical properties of a 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayer as a simple membrane model using the Langmuir–Blodgett (L–B) monolayer technique and molecular dynamics (MD) simulations. We report that melatonin increases the area per lipid and elastic compressibility of the DPPC monolayer in a concentration dependent manner, while cholesterol has the opposite effect. When both melatonin and cholesterol were present in the monolayer, the compression isotherms showed normalization of the area per molecule towards that of the pure DPPC monolayer, thus indicating that melatonin counteracts and alleviates cholesterol's effects. Atomistic MD simulations of melatonin enriched DPPC systems correlate with our experimental findings and illustrate the structural effects of both cholesterol and melatonin. Our results suggest that melatonin is able to lessen the influence of cholesterol through two different mechanisms. Firstly, we have shown that melatonin has a fluidizing effect on monolayers comprising only lipid molecules. Secondly, we also observe that melatonin interacts directly with cholesterol. Our findings suggest a direct nonspecific interaction of melatonin may be a mechanism involved in reducing cholesterol associated membrane effects, thus suggesting the existence of a new mechanism of melatonin's action. This may have important biological relevance in addition to the well-known anti-oxidative and receptor binding effects.
Introduction
Melatonin is a pineal hormone produced in the brain.1 It is a small molecule (the structure is shown in Fig. 1) that can easily partition into the lipid membrane and change its biophysical properties.2 Melatonin has been shown to have protective effects in several diseases, including cardiovascular disease3 Alzheimer's disease,4 and certain types of cancers.5 In many cases the protective effects involved were non-specific and receptor-independent.5,6 Understanding the non-specific interaction of melatonin with lipid membranes may have important potential therapeutic applications, since the membrane action of melatonin may play a role in its cytoprotective effects.7 Although a detailed understanding of the interaction of melatonin with cellular membranes is currently unknown, it may be of crucial importance for the development of new therapeutic applications.One of the questions of particular importance is related to cholesterol. It has been proposed that melatonin may directly compete with cholesterol for binding with the PC group in lecithin reverse micelles,8 and this relationship may be important in cholesterol implicated diseases, such as cardiovascular diseases3 Alzheimer's disease9 and certain types of cancers.10 Previous publications on melatonin and cholesterol show that serum total cholesterol and LDL cholesterol levels have been negatively correlated with melatonin levels in pre- and post-menopausal women.11 Several in vitro studies of atherosclerosis development, in relation to cardiovascular diseases, have reported that melatonin reduced plasma levels of total cholesterol, very low-density lipoprotein (VLDL)-cholesterol and low-density lipoprotein (LDL) cholesterol in hypercholesterolemic rats.3 Although the exact mechanism is unknown, it is thought that melatonin may possibly augment endogenous cholesterol clearance.3
Cholesterol is an ubiquitous constituent of lipid membranes in general and is particularly rich in membrane raft regions. It has recently attracted a lot of attention in relation to several diseases such as Alzheimer's4,9 and cancer.12 Against amyloid-β peptide toxicity involved in Alzheimer's disease, melatonin has been shown to have cytoprotective effects that are independent of its membrane receptors. Furthermore, regarding amyloid toxicity, it has been shown that melatonin is distinctly advantageous over other antioxidants, such as vitamins C and E.4 Melatonin's anticancer effect on prostate cancer cells has also been found to be receptor independent.12 Given the general non-specificity of melatonin's therapeutic effects, and the indications that melatonin directly influences the membrane through non-specific interactions, we focus our attention on the non-specific effects of melatonin in model membranes in comparison to cholesterol.
Previous studies have demonstrated melatonin's ability to non-specifically bind to and interact with the lipid membrane, and to alter its biophysical properties. It has been postulated that melatonin may lie between the phosphocholine head groups and change the structure and fluidity of the lipid membrane.2,13 Various techniques have been used, including Fourier transform infrared spectroscopy, differential scanning calorimetry, spectrophotometry, atomic force microscopy, Langmuir monolayer, fluorescence and ESR spectroscopies, viscosimetry, nuclear magnetic resonance (NMR), neutron scattering, and MD simulations.2,7,8,13–17 It was shown that melatonin decreases the main phase transition temperature in a variety of different lipid systems. This implies that it induces higher fluidity in membrane lipids.2,13,15,18 On the other hand, decreased membrane dynamics have also been reported in DPPC,17 DPPG,16 rat brain homogenate,19 and in DMPC.13,20 Our recent work utilizing neutron scattering and MD simulations showed that melatonin induced thinning of the membrane in DOPC and DPPC, which indicates higher membrane fluidity.2 One of the previous studies focused on melatonin and cholesterol in lecithin reversed micelles using NMR and FTIR spectroscopy.8 They indicated that melatonin and cholesterol compete for binding to the lipid head groups. However, the precise effects on the biophysical properties of cholesterol-containing lipid assembly were not assessed.
We investigated how melatonin changes the structural and mechano-elastic properties of model phospholipid monolayers. We compared these effects with those induced by cholesterol in order to further the understanding of the molecular interactions of melatonin with phosphatidylcholine assemblies. Langmuir–Blodgett (L–B) isotherms and atomistic MD simulations were used to study the effects of melatonin on the lateral compression and structure of a DPPC monolayer. We tested the effect of melatonin on model DPPC and DPPC/cholesterol systems, which are commonly used lipid models for lipid membrane and membrane rafts.21
Materials and methods
Chemicals and sample preparation
Melatonin, cholesterol, and DPPC were all purchased from Sigma-Aldrich in powder form and were used without further purification. Melatonin was directly dissolved in Millipore water (resistivity > 18.2 MΩ) by gentle agitation to yield the final solutions used in the monolayer subphase.20Lyophilized, powdered 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was dissolved in chloroform (1.0 mg mL−1). 10 μL of the DPPC solution was deposited using a micropipette on to a Langmuir–Blodgett microtrough (NIMA, UK) with a subphase volume of approximately 50 mL. Melatonin solutions in water were used as the subphase at 0.2 mM, and 1.0 mM concentrations, to allow melatonin partitioning from water subphase into the lipid monolayer. Such high pharmacological concentrations of melatonin have been used in previous melatonin and membrane studies before.14,15,20,22 We chose such concentrations in order to observe its effects most clearly. Cholesterol enriched monolayer samples were prepared by dissolving cholesterol and DPPC in chloroform and spreading the chloroform solution on to the trough. Cholesterol to lipid percentages were: 5%, 20%, 33% and 100% by mol%. 10 μL of the sample solution was deposited for each isotherm experiment. The compression rate for the isotherms was 80 cm2 min−1 or 9.8 Å2/molecule × min. The subphase was kept at room temperature at 24 °C. The isotherms were repeated in at least triplicate in order to obtain an accurate average for each concentration and averaged isotherms are shown in the results.
Each pressure versus area isotherm represents an average of between three and six separate experiments, with the error bars calculated as standard errors of the mean.
The elastic compressibility (C) was calculated from the pressure versus area isotherm using eqn (1). Cs−1 is the compression modulus, A is the area in the trough, π is the pressure.
| Cs−1 = −A(dπ/dA) | (1) |
In general, a lower Cs−1 value means an increase in the monolayer compressibility.23
Atomistic MD simulations
We performed MD simulations of pure DPPC, DPPC with 20% cholesterol and DPPC with 12% melatonin at the temperature of 320 K and a pressure of 1 bar. The simulation box had 512 lipids fully hydrated with about 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 water molecules. The simulations were run with the GROMACS (version 4) software package24 and the GROMOS 53a6 force-field.25 The protocol we applied is the same that has been successfully used in our previous studies.2,26,27 The model for melatonin is the same that was used in our previous study.2 To summarize the protocol and the rest of the models: for water, we used the simple point charge (SPC) model,28 the SETTLE29 algorithm was used to constrain bond lengths for water and LINCS30 was used for the lipids. The Lennard–Jones interactions were smoothly switched off and a cutoff of 0.9 nm was used. The electrostatic interactions were computed using the particle-mesh Ewald method31,32 with a real space cutoff of 1.4 nm, beta spline interpolation (of order 4), and a direct sum tolerance of 10−6. Full periodic boundary conditions were applied and a time step of 2 fs was used. For initial relaxation, the steepest decent algorithm was used. About 120 ns NpT simulation was run as the final step of equilibration before the production runs. The Parrinello–Rahman33 and the Parrinello–Donadio–Bussi v-rescale34 algorithms were used for baro- and thermostats with relaxation times of 0.5 ps and 0.1 ps respectively.
000 water molecules. The simulations were run with the GROMACS (version 4) software package24 and the GROMOS 53a6 force-field.25 The protocol we applied is the same that has been successfully used in our previous studies.2,26,27 The model for melatonin is the same that was used in our previous study.2 To summarize the protocol and the rest of the models: for water, we used the simple point charge (SPC) model,28 the SETTLE29 algorithm was used to constrain bond lengths for water and LINCS30 was used for the lipids. The Lennard–Jones interactions were smoothly switched off and a cutoff of 0.9 nm was used. The electrostatic interactions were computed using the particle-mesh Ewald method31,32 with a real space cutoff of 1.4 nm, beta spline interpolation (of order 4), and a direct sum tolerance of 10−6. Full periodic boundary conditions were applied and a time step of 2 fs was used. For initial relaxation, the steepest decent algorithm was used. About 120 ns NpT simulation was run as the final step of equilibration before the production runs. The Parrinello–Rahman33 and the Parrinello–Donadio–Bussi v-rescale34 algorithms were used for baro- and thermostats with relaxation times of 0.5 ps and 0.1 ps respectively.
Results
L–B monolayer compression isotherms
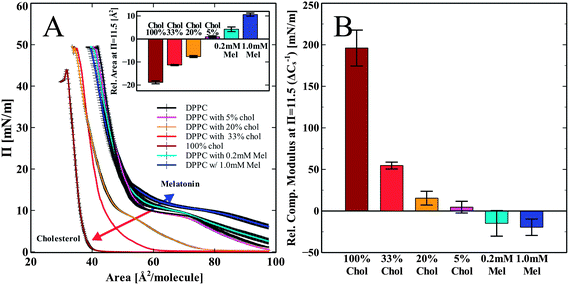
DPPC monolayer pressure versus area isotherms were recorded using the L–B trough. Fig. 2 shows the independent effects of both melatonin and cholesterol on DPPC monolayer isotherms and elastic compressibility. We chose to compare the changes in the monolayer area per molecule as well as in the compressibility modulus of all the groups at the pressure 11.5 mN m−1. The shift in the isotherms area per molecule was less obvious with error bars overlapping at the high pressure regions (pressures > 20 mN m−1). The increased error in the isotherms in the higher pressure regions may be indicative of a solubilizing effect on the lipid molecules into the subphase,35,36 but also may be due to increased molecular order and tightened molecular packing by increasing head group hydration.37 At a pressure of 11.5 mN m−1, differences induced by melatonin were most noticeable. We chose the lower pressure of 11.5 mN m−1 to demonstrate the differences in the isotherms in terms of the area per molecule. This pressure corresponds closely to the mean molecular area of 60–64 Å2, which is equivalent to what is found for DPPC bilayers.38,39 Hsu et al. considered 60 Å2 to be the most appropriate bilayer equivalent molecular area40Fig. 2A shows the compression isotherms for pure DPPC monolayers spread on a water subphase and DPPC with cholesterol increasing from 5 to 33 mol% and a pure cholesterol monolayer (100% cholesterol). Increasing amounts of cholesterol shift the isotherms down and to the left as compared to the pure DPPC control (Fig. 2A). This is in good agreement with previously reported data.41 Melatonin is known to dissolve in water and is also able to partition into the monolayer from the water subphase.13 When the lipid monolayer was spread onto a subphase containing melatonin, we observed a shift of the isotherm up and to the right. This is opposite to the shift induced by cholesterol. This indicates a concentration dependent increase in the area per molecule as compared to the pure DPPC control due to incorporation of melatonin into the monolayer, as shown in the inset (Fig. 2A). The isotherms for the 5% cholesterol-enriched monolayer also showed a very slight increase in the area per molecule. Higher concentrations of cholesterol (>5%) showed decreased area per molecule for all concentrations, indicative of increased lipid packing density (condensing effect). The overall trend of the concentration dependent effect of cholesterol is in good agreement with previous reports.41,42The calculated elastic compressibility modulus (Cs−1) decreased in the presence of melatonin (Fig. 2B). At a pressure of 11.5 mN m−1 the compressibility modulus at 1 mM melatonin decreased by 20 ± 10 mN m−1 relative to the pure DPPC isotherm. The decrease in the Cs−1 values shows that melatonin enhances the elastic compressibility, thus reducing the stiffness of the monolayer. In contrast to melatonin, cholesterol increased the Cs−1 values compared to those observed for the DPPC monolayer in a concentration dependent manner from 4 ± 7 mN m−1 at 5% cholesterol to 196 ± 22 mN m−1 at 100% cholesterol, thus making the monolayer stiffer and less compressible.
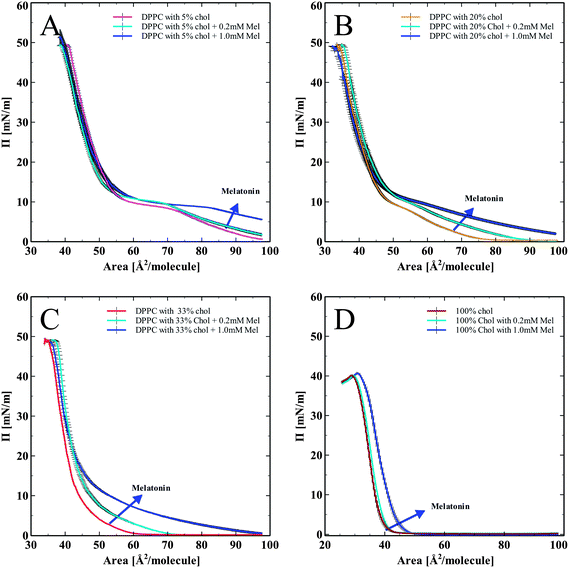
In order to assess the effects of cholesterol and melatonin together, we spread DPPC monolayers containing cholesterol (5, 20, and 33 mol%), and a pure cholesterol monolayer (100%), onto the subphase of melatonin solutions (0.2 mM and 1.0 mM). The corresponding pressure versus area isotherms are shown in Fig. 3. Fig. 3A–D show isotherms for DPPC monolayer with progressively increasing concentrations of cholesterol from 5 to 20, 33 and 100 mol% of cholesterol. All isotherms show a normalization and recovery toward the DPPC only isotherm, in terms of lipid area per molecule. In Fig. 3A, DPPC with 5 mol% cholesterol and melatonin showed an area per molecule increase compared to DPPC with cholesterol-only. Monolayers with 20% cholesterol spread on a melatonin subphase are shown in Fig. 3B. The LE to LE/LC (Liquid Expanded/Liquid Condensed) phase transition (the characteristic kink in the DPPC isotherm just below 10 mN m−1) diminishes due to the effects of cholesterol. Melatonin's effect of increasing the area per molecule is also seen. The lack of clear LE to LE/LC phase transition in the isotherms with melatonin indicates that cholesterol effects on the phase transition of the monolayer persisted even with the highest concentration of melatonin tested.
Monolayers with high (33 mol%) cholesterol and with melatonin in the subphase in Fig. 3C showed a similar trend as with the previous hybrid isotherms (shown in Fig. 3A and B). The influence of cholesterol has completely diminished the LE to LE/LC phase transition kink (indicated by circles in Fig. 3A–C). Melatonin increased the area per molecule, but the influence of cholesterol on the shape of the isotherm was persistent. Fig. 3D shows pure cholesterol isotherms with melatonin in the subphase. Without any phospholipids present, melatonin increased the area per molecule of cholesterol, indicating its capacity to partition into the cholesterol monolayer and modify the molecular arrangement of cholesterol in monolayers.
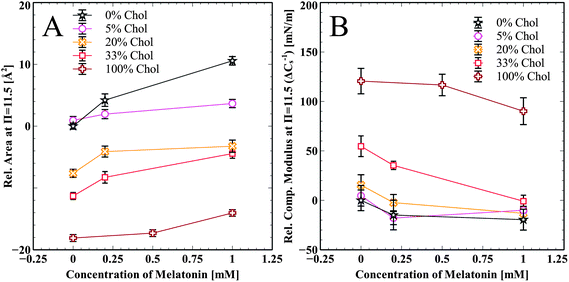
Summarized effects of melatonin on cholesterol enriched DPPC and pure cholesterol monolayers are shown in Fig. 4A. The change in area per molecule relative to pure DPPC at π = 11.5 mN m−1 is shown for all cholesterol samples and at all melatonin concentrations. Melatonin increases the area per molecule in all of the isotherms in a concentration dependent manner. Fig. 4B shows the relative change in the compressibility modulus, compared to pure DPPC at π = 11.5 mN m−1, for all cholesterol containing samples and at all melatonin concentrations. Melatonin decreased the compressibility modulus for all samples, except the 5 mol% cholesterol, in a concentration dependent manner. The compressibility change for the 5 mol% sample is very close to that of the pure DPPC sample and the slight increase for the 1.0 mM sample is within error.
MD simulations
MD simulations were performed at 320 K. This is above the main transition temperature for DPPC bilayers. This temperature was chosen to be able to compare with the monolayer results: at 297 K, DPPC monolayers are in the liquid expanded phase (corresponds to the liquid disordered or Lα phase for a bilayer) up to a surface pressure of about 15 mN m−1.43 Thus, the results from the bilayer simulations should provide a very good qualitative picture of the molecules' behaviors in the monolayer system.Fig. 5 shows a snapshot from the MD simulation of melatonin inside a DPPC monolayer (melatonin molecules shown in orange). Melatonin locates just below the DPPC headgroups within the acyl chain regions with the indole group facing away from the polar headgroups. Unlike cholesterol, which is able to align its ring system with the lipid hydrocarbon chains, melatonin's smaller ring system and hydrophobicity of the indole group do not allow such alignment. Instead, melatonin rather pushes the chains apart and causes lipid disorder. Fig. 6 shows a detailed comparison between pure DPPC, DPPC + cholesterol, and DPPC + melatonin systems, resolving structures of individual lipid, cholesterol and melatonin molecules. The location of melatonin molecules within the DPPC monolayer is below the DPPC headgroups well within the acyl chain region. Detailed MD simulations of DPPC and DPPC with cholesterol systems were reported before and not shown here.27 Here, we concentrate on the comparisons between the effects of cholesterol and melatonin. We have zoomed into a small region to demonstrate the effects of cholesterol and melatonin. The effects shown in the snapshots are general and observed throughout the systems. The addition of cholesterol leads to a 15% decrease in the area per lipid as compared to a pure DPPC system. This is also apparent when comparing Fig. 6A and B. Melatonin has a stronger effect in the opposite direction as shown in Fig. 6C. The DPPC acyl chains become more disordered and the area per lipid increases by ∼4%. As a comparison of the figures shows, unlike cholesterol, which tends to hydrogen bond with the lipids, the smaller melatonin molecules are located well below the phosphocholine (PC) headgroups penetrating deeper in the acyl chain region. Cholesterols' hydroxyl groups readily formed hydrogen bonds mostly with the carbonyl groups of the lipids. This is in full agreement with previous studies, see ref. 44 for a review. Melatonin behaved differently: the interactions of melatonin were largely dependent on the hydrophobic interactions of the indole groups and the acyl chains. MD simulations also indicated increased presence of water molecules inside the monolayer with melatonin present (data not shown).
Discussion
Overall, the resulting changes in the lipid biophysical properties due to melatonin were an increased area per molecule and a decreased compressibility modulus of the monolayers. These effects were consistently observed for the full range of monolayer compositions tested: from pure lipid monolayer to pure cholesterol monolayer and the various mixtures. The results suggest that melatonin has both a spreading effect on the DPPC/cholesterol molecules, and also a softening effect on the monolayers. The findings are in agreement with previous reports that showed increased molecular area and decreased thickness in a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) monolayer with melatonin13 and in both 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and DPPC bilayers.2An especially notable result of this study is that we have evidence for direct interaction between cholesterol and melatonin (shown in Fig. 3D as well as in Fig. 4B). The pure cholesterol monolayer showed an increase in the area per molecule and a decrease in compressibility modulus, when melatonin was present in the subphase and partitioned into the monolayer. This suggests that melatonin is able to incorporate between both the phospholipids and also in between cholesterol molecules. Previously, it was known that melatonin prevented membrane fluidity decrease due lipid peroxidation, for which its anti-oxidative properties have been attributed.14,15,22,45 Our results show that melatonin interacts directly with the lipids and the cholesterol molecules, thus increasing membrane fluidity and alleviating cholesterol's condensing effect in the membrane.
A summarizing schematic of our results is shown in Fig. 7. We hypothesize that melatonin and cholesterol, when mixed in the lipid monolayer, will counteract each other's effects, resulting in a monolayer similar to the original DPPC monolayer with the characteristic molecular area for DPPC. The resulting hybrid L–B isotherms, when both melatonin and cholesterol were present in the monolayer, were close but did not fully recover to match the normal DPPC control isotherm (Fig. 3B and C). Thus, attributes of cholesterol in the isotherms remained in conjunction with the increased molecular area due to melatonin. In Fig. 7D, we depicted melatonin's influence on the monolayer as not disturbing the cholesterol rich domains. However, it is likely that both the cholesterol rich domains as well as the lipids are affected as melatonin had similar effects in the pure cholesterol monolayer as it did in the pure lipid monolayers.
Our MD simulation data shows that both cholesterol and the lipid molecules form hydrogen bonds, however, melatonin's interaction was mainly driven by hydrophobicity. Thus, it is possible that melatonin is able to interact with both cholesterol and the phospholipids through hydrophobic interactions without necessarily strongly influencing the hydrogen bonding among the cholesterol and DPPC molecules. However, Bongiorno et al.8 reported that melatonin's ability to hydrogen bond through its –NH groups is similar to the ability of cholesterol's –OH groups in lecithin reverse micelles. Thus, both the hydrophobic interaction as well as through hydrogen bonding both might be at play. In an analogous manner, hydrophobicity can limit or allow small molecules, such as ethanol and methanol, to form hydrogen bonds with lipids.39,46,47 Such effects may also depend on concentration.39 Our data on mixed monolayers containing cholesterol, DPPC and melatonin showed that the attributes of cholesterol in diminishing the LE to LE/LC transition, as well as melatonin's influence in increasing the area per molecule were both present in the mixed isotherms (Fig. 3). This may indicate that melatonin influences the monolayer without necessarily specifically targeting and disturbing the cholesterol–lipid interactions, which are mainly driven by hydrogen bonding. Therefore, the hydrophobic interaction may have a greater influence on the overall monolayer biophysical properties than its ability to disturb the hydrogen bonding.
Our MD simulations show that both cholesterol and melatonin reside below the DPPC headgroups. Cholesterol displayed its well-known condensing and ordering effects,27,44 but melatonin induced disorder along the acyl chains (Fig. 6) of the DPPC molecules and an increase in the lipid area per molecule as already seen in our previous bilayer study.2 Previous works on the orientation of melatonin in the lipids have postulated melatonin to locate between the headgroups of DPPC.8,18 In contrast, our present MD data and recent work2 show that it resides below the lipid heads, penetrating deeper into the acyl chain regions than what was previously hypothesized. This is due to the hydrophobicity of the indole group and a competition between hydrogen bonding, steric and hydrophobic effects. Such competition is difficult to characterize quantitatively, but we illustrate this by comparing the behaviors of cholesterol and its analogues.48–50 For example, the difference in behavior between melatonin and cholesterol resembles the difference between cholesterol and demethylated cholesterol.49 Despite the fact that demethylated cholesterol has the ability to hydrogen bond via its OH-group, demethylation (removal of the methyl groups from cholesterol's rough side) leads to larger fluctuations in its orientation despite enhanced van der Waals interactions. On the other hand, the effect of hydrogen bonding can be seen by comparing cholesterol to ketosterone. In ketosterone, cholesterol's OH-group is replaced by a ketone group which is not able to hydrogen bong like the OH group. This allows the molecule to move around and even undergo flip-flops inside the bilayer.51 Being a smaller molecule, the fluctuations in melatonin's orientation are large and the competition between hydrogen bonding, hydrophobicity and van der Waals interactions leads to behavior that resembles both of the above examples. The importance of steric effects for cholesterol and one of its analogues has been shown in detail by Martinez-Seara et al.27 Their results demonstrate the importance of close interactions with the sterol ring, its methyl groups and the acyl chains. In the case of melatonin, the small indole ring is not able to interact strongly with the acyl chain, allowing it to move more freely under the head group region close to the glycerol backbone. This gives rise to an increased membrane area and, hence, disorder along the acyl chains. Based on our observation that the monolayers with both cholesterol and melatonin yield similarly shaped isotherms as that of cholesterol enriched monolayers, we hypothesized that melatonin may incorporate into the cholesterol enriched monolayer without disturbing the condensed microdomains created by the presence of cholesterol (Fig. 7D). However, further studies are needed to verify the exact molecular level arrangement of cholesterol and melatonin in a lipid monolayer, and it is also possible that melatonin affects the molecular spacing within the cholesterol-enriched domains as well.
Melatonin's influence in decreasing the elastic compressibility modulus, as well as the increase in the area per molecule may involve increased water interaction with the head groups, for several reasons. It has been reported by other groups that melatonin can augment hydration of the head groups.19 The increase in the hydration of the lipid head groups may reduce the prevalence of highly dense liquid condensed areas within the monolayer, since it increases the area per lipid that promotes the acyl chain dynamic motional freedom (i.e. fluidity) due to entropy.52 Increased lipid molecular area and acyl chain fluidity due to hydration would naturally lead to lowering the thickness of the bilayer,53–55 which was also reported for melatonin.2,13
Conclusion
Our results show that melatonin has a significant influence on the compressibility of DPPC monolayers in an opposing manner to that of cholesterol. With melatonin present, both an increase in the area per molecule, as well as a decrease in the compressibility modulus of the monolayer were observed in a concentration dependent manner. The results indicate that both cholesterol and melatonin have a sustained influence on the monolayer, which supports the co-existence of the two molecules rather than a competitive exclusion. Significantly, pure cholesterol monolayers without any phospholipids also showed increased area per molecule and decreased elastic compressibility modulus with melatonin. Our MD results are in a good agreement with experiments and indicate an increase in DPPC lipid tail disorder and molecular area due to the presence of melatonin, which is opposite to the condensing effect of cholesterol.Our results suggest that melatonin has a direct fluidizing effect in the membrane and counteracts the condensing effect of cholesterol. The results suggest that melatonin may be actively involved in counteracting cholesterol-induced changes of membrane structure and properties. High cholesterol induced membrane changes have been associated with various diseases such as cardiovascular diseases,3 cancer,5 and Alzheimer's disease.4 The results of our study demonstrate that melatonin's ability to provide protection against such diseases might involve, at least in part, its receptor independent interaction with the cell membrane.
Acknowledgements
This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grants to Z Leonenko and to M Karttunen. Y Choi acknowledges Waterloo Institute for Nanotechnology (WIN) Fellowship, and E Drolle acknowledges the NSERC PhD Scholarship and WIN Fellowship. The authors also thank University of Waterloo and Ontario Ministry of Training, Colleges and Universities for their support.References
- A. Brzezinski, N. Engl. J. Med., 1997, 336, 186–195 CrossRef CAS PubMed.
- E. Drolle, N. Kucerka, M. I. Hoopes, Y. Choi, J. Katsaras, M. Karttunen and Z. Leonenko, Biochim. Biophys. Acta, Biomembr., 2013, 1828, 2247–2254 CrossRef CAS PubMed.
- S. Tengattini, R. J. Reiter, D. Tan, M. P. Terron, L. F. Rodella and R. Rezzani, J. Pineal Res., 2008, 44, 16–25 CAS.
- S. A. Rosales-Corral, D. Acuña-Castroviejo, A. Coto-Montes, J. A. Boga, L. C. Manchester, L. Fuentes-Broto, A. Korkmaz, S. Ma, D. Tan and R. J. Reiter, J. Pineal Res., 2012, 52, 167–202 CrossRef CAS PubMed.
- Vijayalaxmi, C. R. Thomas Jr, R. J. Reiter and T. S. Herman, J. Clin. Oncol., 2002, 20, 2575–2601 CrossRef CAS PubMed.
- M. A. Pappolla, M. J. Simovich, T. Bryant-Thomas, Y. Chyan, B. Poeggeler, M. Dubocovich, R. Bick, G. Perry, F. Cruz-Sanchez and M. A. Smith, J. Pineal Res., 2002, 32, 135–142 CrossRef CAS.
- A. Saija, A. Tomaino, D. Trombetta, M. L. Pellegrino, B. Tita, S. Caruso and F. Castelli, Eur. J. Pharm. Biopharm., 2002, 53, 209–215 CrossRef CAS.
- D. Bongiorno, L. Ceraulo, M. Ferrugia, F. Filizzola, A. Ruggirello and V. T. Liveri, J. Pineal Res., 2005, 38, 292–298 CrossRef CAS PubMed.
- G. Di Paolo and T. Kim, Nat. Rev. Neurosci., 2011, 12, 284–296 CrossRef CAS PubMed.
- M. Demierre, P. D. R. Higgins, S. B. Gruber, E. Hawk and S. M. Lippman, Nat. Rev. Cancer, 2005, 5, 930–942 CrossRef CAS PubMed.
- H. Tamura, Y. Nakamura, A. Narimatsu, Y. Yamagata, A. Takasaki, R. J. Reiter and N. Sugino, J. Pineal Res., 2008, 45, 101–105 CrossRef CAS PubMed.
- R. M. Sainz, J. C. Mayo, D. Tan, J. León, L. Manchester and R. J. Reiter, Prostate, 2005, 63, 29–43 CrossRef CAS PubMed.
- V. R. De Lima, M. S. B. Caro, M. L. Munford, B. Desbat, E. Dufourc, A. A. Pasa and T. B. Creczynski-Pasa, J. Pineal Res., 2010, 49, 169–175 CAS.
- J. J. García, R. J. Reiter, J. M. Guerrero, G. Escames, B. P. Yu, C. S. Oh and A. Muñoz-Hoyos, FEBS Lett., 1997, 408, 297–300 CrossRef.
- J. J. García, R. J. Reiter, J. Pié, G. G. Ortiz, J. Cabrera, R. M. Sáinz and D. Acuña-Castroviejo, J. Bioenerg. Biomembr., 1999, 31, 609–616 CrossRef.
- I. Sahin, F. Severcan and N. Kazanci, J. Mol. Struct., 2007, 834–836, 195–201 CrossRef CAS PubMed.
- F. Severcan, I. Sahin and N. Kazanci, Biochim. Biophys. Acta, Biomembr., 2005, 1668, 215–222 CrossRef CAS PubMed.
- V. R. De Lima, M. S. B. Caro, M. I. B. Tavares and T. B. Creczynski-Pasa, J. Pineal Res., 2007, 43, 276–282 CrossRef PubMed.
- S. B. Akkas, S. Inci, F. Zorlu and F. Severcan, J. Mol. Struct., 2007, 834–836, 207–215 CrossRef CAS PubMed.
- E. J. X. Costa, C. S. Shida, M. H. Biaggi, A. S. Ito and M. T. Lamy-Freund, FEBS Lett., 1997, 416, 103–106 CrossRef CAS.
- M. Edidin, Annu. Rev. Biophys. Biomol. Struct., 2003, 32, 257–283 CrossRef CAS PubMed.
- J. J. García, R. J. Reiter, G. G. Ortiz, C. S. Oh, L. Tang, B. P. Yu and G. Escames, J. Membr. Biol., 1998, 162, 59–65 CrossRef.
- Z. Wang and S. Yang, ChemPhysChem, 2009, 10, 2284–2289 CrossRef CAS PubMed.
- B. Hess, C. Kutzner, D. Van Der Spoel and E. Lindahl, J. Chem. Theory Comput., 2008, 4, 435–447 CrossRef CAS.
- C. Oostenbrink, T. A. Soares, N. F. A. Van Der Vegt and W. F. Van Gunsteren, Eur. Biophys. J., 2005, 34, 273–284 CrossRef CAS PubMed.
- J. Wong-Ekkabut and M. Karttunen, J. Chem. Theory Comput., 2012, 8, 2905–2911 CrossRef CAS.
- H. Martinez-Seara, R. Tomasz, M. Karttunen, I. Vattulainen and R. Reigada, PLoS One, 2010, 5, e11162, DOI:10.1371/journal.pone.0011162.
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, Intermol. Forces, 1981, 331–342 CrossRef CAS.
- S. Miyamoto and P. A. Kollman, J. Comput. Chem., 1992, 13, 952–962, DOI:10.1002/jcc.540130805.
- B. Hess, H. Bekker, H. J. C. Berendsen and J. G. E. M. Fraaije, J. Comput. Chem., 1997, 18, 1463–1472, DOI:10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-H.
- U. Essmann, L. Perera, M. L. Berkowitz, T. Darden, H. Lee and L. G. Pedersen, J. Chem. Phys., 1995, 103, 8577–8593 CrossRef CAS.
- M. Karttunen, J. Rottler, I. Vattulainen and C. Sagui, Curr. Top. Membr., 2008, 60, 49–89 CrossRef CAS.
- M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182–7190 CrossRef CAS.
- G. Bussi, D. Donadio and M. Parrinello, J. Chem. Phys., 2007, 126, 014101 CrossRef PubMed.
- T. F. Schmidt, L. Caseli, T. M. Nobre, M. E. D. Zaniquelli and O. N. Oliveira Jr, Colloids Surf., A, 2008, 321, 206–210 CrossRef CAS PubMed.
- M. Subirade, C. Salesse, D. Marion and M. Pezolet, Biophys. J., 1995, 69, 974–988 CrossRef CAS.
- C. Nunes, G. Brezesinski, C. Pereira-Leite, J. L. F. C. Lima, S. Reis and M. Lúcio, Langmuir, 2011, 27, 10847–10858 CrossRef CAS PubMed.
- J. F. Nagle and S. Tristram-Nagle, Biochim. Biophys. Acta, Biomembr., 2000, 1469, 159–195 CrossRef CAS.
- A. N. Dickey and R. Faller, Biophys. J., 2007, 92, 2366–2376 CrossRef CAS PubMed.
- T. T. Hsu, D. L. Leiske, L. Rosenfeld, J. M. Sonner and G. G. Fuller, Langmuir, 2013, 29, 1948–1955 CrossRef CAS PubMed.
- P. Wydro and K. Hąc-Wydro, J. Phys. Chem. B, 2007, 111, 2495–2502 CrossRef CAS PubMed.
- E. E. Berring, K. Borrenpohl, S. J. Fliesler and A. B. Serfis, Chem. Phys. Lipids, 2005, 136, 1–12 CrossRef CAS PubMed.
- G. Ma and H. C. Allen, Langmuir, 2006, 22, 5341–5349 CrossRef CAS PubMed.
- T. Róg, M. Pasenkiewicz-Gierula, I. Vattulainen and M. Karttunen, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 97–121 CrossRef PubMed.
- M. Karbownik, R. J. Reiter, W. Qi, J. J. Garcia, D. Tan, L. C. Manchester and Vijayalaxmi, Mol. Cell. Biochem., 2000, 211, 137–144 CrossRef CAS.
- B. W. Lee, R. Faller, A. K. Sum, I. Vattulainen, M. Patra and M. Karttunen, Fluid Phase Equilib., 2005, 228–229, 135–140 CrossRef CAS PubMed.
- M. Patra, E. Salonen, E. Terama, I. Vattulainen, R. Faller, B. W. Lee, J. Holopainen and M. Karttunen, Biophys. J., 2006, 90, 1121–1135 CrossRef CAS PubMed.
- A. M. Smondyrev and M. L. Berkowitz, Biophys. J., 2001, 80, 1649–1658 CrossRef CAS.
- T. Rog, M. Pasenkiewicz-Gierula, I. Vattulainen and M. Karttunen, Biophys. J., 2007, 92, 3346–3357, DOI:10.1529/biophysj.106.095497.
- T. Róg, I. Vattulainen, M. Jansen, E. Ikonen and M. Karttunen, J. Chem. Phys., 2008, 129, 154508 CrossRef PubMed.
- T. Róg, L. M. Stimson, M. Pasenkiewicz-Gierula, I. Vattulainen and M. Karttunen, J. Phys. Chem. B, 2008, 112, 1946–1952 CrossRef PubMed.
- A. Borba, F. Lairion, A. Disalvo and R. Fausto, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 2553–2562 CrossRef CAS PubMed.
- R. Jay Mashl, H. Larry Scott, S. Subramaniam and E. Jakobsson, Biophys. J., 2001, 81, 3005–3015 CrossRef.
- E. A. Disalvo, F. Lairion, F. Martini, E. Tymczyszyn, M. Frías, H. Almaleck and G. J. Gordillo, Biochim. Biophys. Acta, Biomembr., 2008, 1778, 2655–2670 CrossRef CAS PubMed.
- E. R. Pinnick, S. Erramilli and F. Wang, Mol. Phys., 2010, 108, 2027–2036 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |