Homochiral Self-Sorting of BINOL Macrocycles†
Scott W. Sisco and Jeffrey S. Moore*
Department of Chemistry, University of Illinois at Urbana-Champaign, 600 S. Mathews Ave., Urbana, IL 61801, USA. E-mail: jsmoore@illinois.edu; Tel: +1 217-244-1646
First published on 2nd September 2013
Abstract
Chiral arylene–ethynylene macrocycles (AEMs) were synthesized via alkyne metathesis-mediated depolymerization of BINOL-based polymers. Homochiral dimers are selectively obtained from metathesis of heterochiral polymers. Thermodynamic analysis and computational modeling suggests the homochiral self-sorting to be entropy-driven due to the greater symmetry of the homochiral dimers over the heterochiral dimer. This symmetry-controlled reaction is a novel approach to achieving high selectivity in dynamic covalent macrocycle synthesis. Importantly, the result describes a new paradigm in dynamic covalent chemistry that will enable efficient synthesis of new chiral architectures.
Introduction
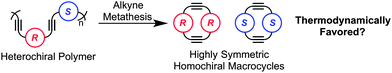
Homochiral self-sorting of a racemic mixture is a known phenomenon most commonly observed in the solid state.1 Spontaneous resolution of enantiomers during crystallization or conglomerate crystallization is well documented ever since Pasteur's classic experiment.2 In recent years, several successful efforts to translate this self-sorting concept to chiral mixtures in solution have been demonstrated.3 However, the majority of these experiments exploited self-sorting via non-covalent interactions such as metal–ligand bonding,3a hydrogen-bonding,3b–d π–π stacking,3a and steric hindrance.3a Dynamic covalent chemistry (DCC) could enable homochiral self-sorting via covalent bond formation due to its reversibility and thermodynamic control.4 In recent years, DCC has been increasingly used in the synthesis of chiral macrocyclic architectures.5–7 Thermodynamic control of the product distribution can lead to high yields and efficiency. However, in most cases the use of racemic starting materials generates large product distributions of both homochiral and heterochiral macrocycles due to similar reactivity of diastereomeric building blocks under DCC conditions.5 Moreover, templates5 or precipitation7 are generally needed to amplify a chiral macrocycle product to a reasonable yield. Tilley et al. has shown the only example of macrocyclization via DCC with inherent selectivity for homochiral products in solution using reversible zirconocene coupling.8 However, no rationalization was provided to explain the observed homochiral selectivity. A more in-depth energy analysis of homochiral self-sorting via DCC would be advantageous in the development of more efficient chiral macrocyclizations, dynamic chiral resolutions, and chiral dynamic libraries.Since DCC is reversible, understanding a system's thermodynamics will provide higher efficiency and greater control over the product distribution. In recent work, we have investigated how shape and geometry influence thermodynamically controlled synthesis of arylene–ethynylene macrocycles (AEMs)9viaalkyne metathesis.10 Extension of this analysis to chiral architectures may bring new insight to factors that affect homochiral self-sorting via DCC. To achieve high selectivity and efficiency, a thorough comparative analysis must be completed on the potential chiral products. The primary differences between homochiral and heterochiral species are stereochemistry and symmetry. On the other hand, the structural connectivity between species is often very similar due to the diastereomeric relationship, which explains why large product distributions are often observed in the absence of library perturbation.5,7 We hypothesize the inherent symmetry differences between homochiral and heterochiral products offers a useful attribute to achieve homochiral self-sorting. Herein, we describe the homochiral self-sorting of macrocyclesviaalkyne metathesis and the impact of symmetry and entropy on the chiral energy landscape (Fig. 1).
Results and discussion
Our studies began with the 1,1′-binaphthyl-2,2′-diol (BINOL) motif, a C2-symmetric atropisomer capable of generating macrocycles with high symmetry. Although the incorporation of BINOL in AEMs has been previously explored,11 these syntheses were under kinetic control and chiral self-sorting was not investigated. First, to demonstrate a thermodynamically controlled synthesis of chiral AEMs two homochiral polymers were prepared and subjected to alkyne metathesis to establish the feasibility and efficiency of macrocyclization. To accomplish this, two BINOL-based poly(arylene–ethynylene)s of opposite chirality were synthesized viapolymerization of the corresponding enantiopure R-BINOL and S-BINOL monomers. Each monomer type contains a different solubilizing chain, tetradecyloxy for R-BINOL and triethyleneglycol for S-BINOL, to enable facile analysis of self-sorting experiments. The monomers were synthesized from methyl 5-iodosalicylate (Scheme 1).12 After etherification, Sonogashira coupling of R1 and S1 with trimethylsilylacetylene followed by desilylation yielded alkynesR3 and S3. Hydrolysis of R1, R3, S1, and S3 followed by carbodiimide coupling with R-BINOL and S-BINOL provided the monomers R2, R4, S2, and S4, respectively. Sonogashira polymerization of monomers R2 and R4 provided homochiral polymerR-P1 with molecular weight of Mn = 25 kDa. Polymerization of S2 and S4 provided polymerS-P2 of the opposite chirality with molecular weight of Mn = 14 kDa. The molecular weights (Mn) of R-P1 and S-P2 were determined by gel permeation chromatography (GPC) with polystyrene standards.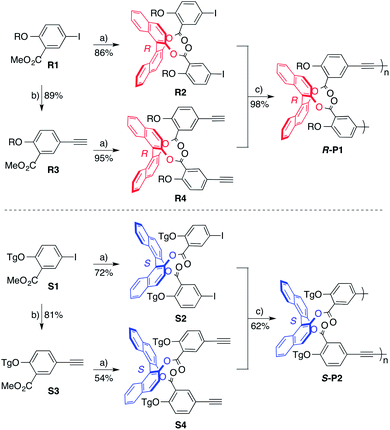 | ||
| Scheme 1 Synthesis of BINOL polymers. Reaction conditions: (a) LiOH, THF/H2O, reflux, 18 h; R-BINOL or S-BINOL (0.5 eq.), DMAP (1.5 eq.), EDC (1.5 eq.), DCM, rt, 24 h. (b) Trimethylsilylacetylene (5 eq.), CuI (0.05 eq.), PdCl2(PPh3)2 (0.05 eq.), THF/diisopropylamine, rt, 18 h; TBAF (1.2 eq.), acetic acid (1.2 eq.), THF, rt, 10 min. (c) CuI (0.05 eq.), Pd(PPh3)4 (0.05 eq.), THF/diisopropylamine, 60 °C, 48 h. R = C14H29, Tg = (C2H4O)3CH3. | ||
Next, each polymer was subjected to depolymerization–macrocyclization conditions viaalkyne metathesis using the trisamidomolybdenum(VI) propylidyne complex MoCEt[NAr(tBu)]3 and triphenylsilanol as the ligand (Scheme 2).13 Efficient depolymerization of R-P1viaalkyne metathesis provided a large decrease in molecular weight to a single sharp peak as seen by GPC (Fig. 2 top). After removal of 1,2,4-trichlorobenzene, purification was achieved by preparative-thin layer chromatography (prep-TLC) using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hexane–dichloromethane to provide RRRRRR-2mer in 51% yield. Depolymerization of the polymerS-P2viaalkyne metathesis yielded a similar shift in molecular weight to a single peak as shown by the GPC trace (Fig. 2 bottom). After removal of 1,2,4-trichlorobenzene, purification was achieved by prep-TLC using 4
3 hexane–dichloromethane to provide RRRRRR-2mer in 51% yield. Depolymerization of the polymerS-P2viaalkyne metathesis yielded a similar shift in molecular weight to a single peak as shown by the GPC trace (Fig. 2 bottom). After removal of 1,2,4-trichlorobenzene, purification was achieved by prep-TLC using 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 methanol–acetone–dichloromethane to provide SS-SS-2mer in 55% yield.
70 methanol–acetone–dichloromethane to provide SS-SS-2mer in 55% yield.
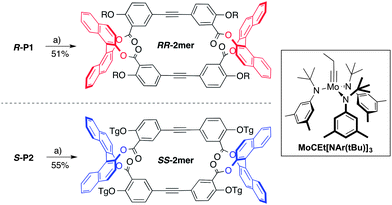 | ||
| Scheme 2 Synthesis of RRRR/SSSS-2mersviaalkyne metathesis. Reaction conditions: (a) MoCEt[NAr(tBu)]3 (10 wt%), Ph3SiOH (15 wt%), 1,2,4-trichlorobenzene, 25 °C, 24 h. Ar = 3,5-dimethylbenzene. R = C14H29, Tg = (C2H4O)3CH3. | ||
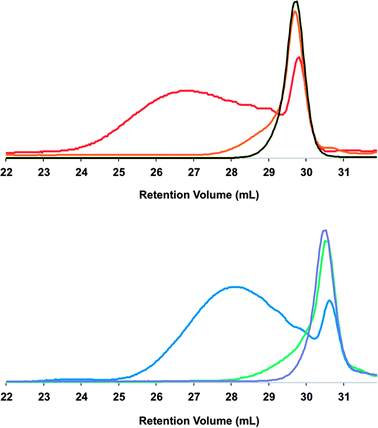 | ||
| Fig. 2 GPC traces of polymers and macrocycles. Top: R-P1 (red), crude R-P1depolymerization (orange), and pure RRRRRR-2mer (black). Bottom: S-P2 (blue), crude S-P2depolymerization (green), and pure SS-2mer (purple). | ||
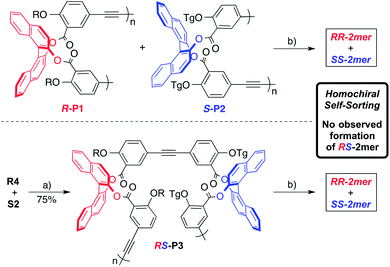
Since alkyne metathesis is reversible, the product distribution is under thermodynamic control.9b Dynamic libraries comprised of building blocks of a single chirality can only form homochiral compounds as seen in Scheme 2. However, introducing building blocks of the opposite chirality greatly increases the complexity of the dynamic library because formation of heterochiral compounds is possible. In addition, symmetry will now play a larger role since more macrocycles are accessible with varying stereochemistry. To probe the effect of chirality and symmetry on the product distribution, several mixing experiments were performed (Scheme 3). The extent of homochiral self-sorting was monitored by mass spectrometry taking advantage of the fact that both R-BINOL and S-BINOL monomers contain side chains of different masses. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of R-P1 and S-P2 were subjected to alkyne metathesis to probe the reaction selectivity. Field desorption (FD) mass spectra analysis of the crude mixture indicates sole formation of the homochiral RR-2mer and SS-2mer products establishing the thermodynamic preference for these homochiral dimers (Fig. 3b).14 Additionally, GPC analysis of the crude mixture shows a distinct peak for each macrocycle respectively (Fig. 3a). To further confirm our hypothesis, an alternating heterochiral polymerRS-P3 with a molecular weight of Mn = 19 kDa (Scheme 3) was prepared and subjected to alkyne metathesis to probe the selectivity. After depolymerizationviaalkyne metathesis, FDmass spectra analysis again displayed only peaks related of homochiral macrocycles.14 No peaks for heterochiral products are observed. The crude RS-P3depolymerization mixture displayed a GPC trace similar to the mixed homochiral polymermetathesis experiment (Fig. 3a). These results are in agreement with a homochiral self-sorting process where the homochiral products are more stable than the mixed, heterochiral species.
1 mixture of R-P1 and S-P2 were subjected to alkyne metathesis to probe the reaction selectivity. Field desorption (FD) mass spectra analysis of the crude mixture indicates sole formation of the homochiral RR-2mer and SS-2mer products establishing the thermodynamic preference for these homochiral dimers (Fig. 3b).14 Additionally, GPC analysis of the crude mixture shows a distinct peak for each macrocycle respectively (Fig. 3a). To further confirm our hypothesis, an alternating heterochiral polymerRS-P3 with a molecular weight of Mn = 19 kDa (Scheme 3) was prepared and subjected to alkyne metathesis to probe the selectivity. After depolymerizationviaalkyne metathesis, FDmass spectra analysis again displayed only peaks related of homochiral macrocycles.14 No peaks for heterochiral products are observed. The crude RS-P3depolymerization mixture displayed a GPC trace similar to the mixed homochiral polymermetathesis experiment (Fig. 3a). These results are in agreement with a homochiral self-sorting process where the homochiral products are more stable than the mixed, heterochiral species.
 | ||
| Scheme 3 Self-sorting mixing experiments. Top: mixed homochiral depolymerization. Bottom: heterochiral depolymerization. Reaction conditions: (a) CuI (0.05 eq.), Pd(PPh3)4 (0.05 eq.), THF/diisopropylamine, 60 °C, 48 h. (b) MoCEt[NAr(tBu)]3 (10 wt%), Ph3SiOH (15 wt%), 1,2,4-trichlorobenzene, 25 °C, 24 h. Ar = 3,5-dimethylbenzene. R = C14H29, Tg = (C2H4O)3CH3. | ||
 | ||
| Fig. 3 (a) GPC traces of RS-P3 and mixing experiments. Crude R-P1 + S-P2depolymerization (cyan), RS-P3 (dark green), and crude RS-P3depolymerization (maroon). (b) FD-MSspectra of self-sorting. | ||
As stated previously, the use of racemic or heterochiral starting materials in DCC often provides complex mixtures of both heterochiral and homochiral macrocycles5 rather than selective formation of homochiral macrocycles as shown here. Thus, it would be advantageous to take this opportunity to understand the observed homochiral self-sorting in order to learn the necessary conditions to develop other self-sorting chiral systems and materials. In DCC, the major products are the most thermodynamically stable or those with the lowest Gibbs free energy, in which both enthalpy (H) and entropy (S) have a role. Elucidating the relative contribution of both these terms will enable greater understanding of the factors that affect homochiral self-sorting of macrocyclesvia DCC.
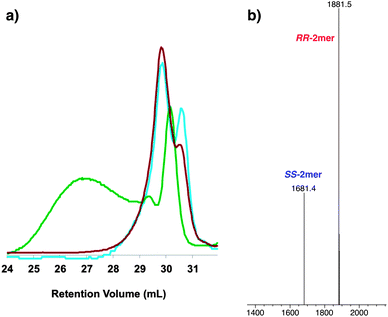
In macrocyclizations under thermodynamic control, molecular strain is often the dominant factor that determines the major product.9b The most stable macrocycles will have minimal ring strain, which corresponds to a lower enthalpy value. The enthalpy was estimated using computational modeling at the HF/3-21G* level (Fig. 4). Methoxy groups were used as substituents to shorten the calculation time. One of the homochiral RRRR/SSSS-2mers was modeled along with the heterochiral RS-2mer. The ΔH between the homochiral and heterochiral dimers is only 1.23 kcal mol−1, which is not surprising considering the structural similarity between the two macrocycles. However, it is interesting to note that the small calculated enthalpy difference is not enough to provide the observed homochiral selectivity.
 | ||
Fig. 4 Energy minimized structures of SS-2mer and RS-2mer. Enthalpy values estimated at HF/3-21G* level. Symmetry number (σ) determined from point group. TSσ = TR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(σ) at T = 298 K. ln(σ) at T = 298 K. | ||
Entropy differences are typically associated with higher disorder.15 This is especially true with regard to DCC, macrocyclizations and self-assembly phenomena where it is postulated that maximizing the number of species in solution increases entropy due to greater disorder. However, both the homochiral and heterochiral dimers are the same size macrocycle, therefore the same number of species would be produced for a selective or non-selective macrocyclization. Alternatively, entropy can be interpreted in terms of changes in information.16 Specifically, an increase in entropy is associated with loss of information (indistinguishability) or increased symmetry.17 We hypothesized that from an informational entropy viewpoint, the more symmetrical homochiral RRRR/SSSS-2mers are entropically favored over the heterochiral RS-2mer.18 A system with homochiral products will display higher symmetry than one containing heterochiral products. The homochiral macrocycles are each composed of two homochiral building blocks, whereas the heterochiral macrocycle is composed of two stereochemically distinct building blocks. Symmetry analysis of the homochiral and heterochiral dimers indicates a point group of D2 and C1, respectively. These differences in symmetry will have an impact on the rotational entropy, since structures with higher symmetry have a larger number of indistinguishable orientations reached by rotation. The symmetry number (σ) is related to the number of indistinguishable orientations and the symmetry point group. The increase in rotational entropy due to the differences in symmetry corresponds to a TΔSσ of 0.82 kcal mol−1 at 298 K (Fig. 4).19 It is expected that the translational and conformational entropy of the homochiral and heterochiral dimers will both be positive but the differences to be small due to the similar mass and conformational volume of each macrocycle.20 Factoring this with the estimated ΔH will produce a ΔG of at least −2.05 kcal mol−1. This ΔG value corresponds to a product distribution containing >97% homochiral dimers.21 The significant entropic contribution in this dynamic macrocyclization is unique due to the higher symmetry of the homochiral products. This symmetry-driven DCC reaction is a novel approach for driving the equilibrium in a dynamic covalent chiral library.
To further validate this hypothesis, the heterochiral polymerRS-P3 was subjected to alkyne metathesis at low temperature (Fig. 5a). At lower temperatures the entropic factor will be smaller so formation of heterochiral RS-2mer should be more competitive. Toluene was used as the solvent since 1,2,4-trichlorobenzene freezes at 16 °C.22 A cold room kept at 5 °C was used to maintain the temperature for 24 h. After depolymerization, FDmass spectra analysis revealed peaks for the homochiral macrocyclesRRRRRR-2mer and SS-2mer as well as the heterochiral macrocycleRS-2mer (Fig. 5b). The result of this experiment is consistent with entropy being a significant driving force for homochiral macrocycle formation.
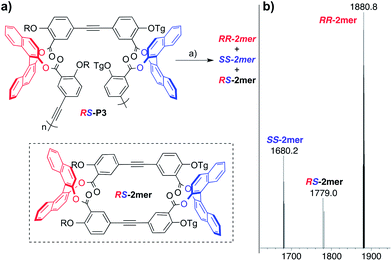 | ||
| Fig. 5 Metathesis at low temperature. (a) RS-P3depolymerization. Reaction conditions: (a) MoCEt[NAr(tBu)]3 (10 wt%), Ph3SiOH (15 wt%), toluene, 5 °C, 24 h. Ar = 3,5-dimethylbenzene. R = C14H29, Tg = (C2H4O)3CH3. (b) FD-MSspectra after metathesis. | ||
Conclusions
Covalent homochiral self-sorting in solution was achieved by alkyne metathesis-mediated depolymerization of a heterochiral BINOL-based polymer. The extension of alkyne metathesis to a chiral system established the feasibility of a thermodynamically controlled synthesis of chiral AEMs. Energy analysis of the possible dimers and low temperature metathesis revealed a novel entropy-driven macrocyclization due to increased symmetry of the homochiral products. A greater understanding of homochiral self-sorting via DCC will enable the creation of more efficient chiral dynamic combinatorial libraries and macrocycle synthesis. In addition, the concept of symmetry-driven thermodynamic macrocyclizations could have implications beyond homochiral self-sorting. Potential applications include origin of life research, asymmetric catalyst design, and dynamic thermodynamic chiral resolutions.Acknowledgements
This work was supported by the National Science Foundation (CHE-1010680). Additional financial support was provided by the James R. Beck Fellowship (S.W.S.).Notes and references
- (a) D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975 CAS; (b) C. P. Brock, W. B. Schweizer and J. D. Dunitz, J. Am. Chem. Soc., 1991, 113, 9811 CrossRef CAS; (c) H. D. Flack, Helv. Chim. Acta, 2003, 86, 905 CrossRef CAS; (d) C. Viedma, Phys. Rev. Lett., 2004, 94, 065504 CrossRef.
- L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed.
- (a) M. M. Safont-Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784 CrossRef CAS PubMed; (b) E. Murguly, R. McDonald and N. R. Branda, Org. Lett., 2000, 2, 3169 CrossRef CAS PubMed; (c) A. T. Cate, P. Y. W. Dankers, H. Kooijman, A. L. Spek, R. P. Sijbesma and E. W. Meijer, J. Am. Chem. Soc., 2003, 125, 6860 CrossRef PubMed; (d) D. M. Chung and J. S. Nowick, J. Am. Chem. Soc., 2004, 126, 3062 CrossRef CAS PubMed.
- (a) S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef; (b) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652 CrossRef CAS PubMed.
- (a) P. T. Corbett, H. L. Tong, J. K. M. Sanders and S. Otto, J. Am. Chem. Soc., 2005, 127, 8902 CrossRef CAS PubMed; (b) A. González-Álvarez, I. Alfonso and V. Gotor, Chem. Commun., 2006, 2224 RSC; (c) F. Bulos, S. L. Robert, R. L. E. Furlan and J. K. M. Sanders, Chem. Commun., 2007, 3092 RSC; (d) M.-K. Chung, C. M. Hebling, J. W. Jorgenson, K. Severin, S. J. Lee and M. R. Gagné, J. Am. Chem. Soc., 2008, 130, 11819 CrossRef CAS PubMed; (e) M.-K. Chung, K. Severin, S. J. Lee, M. L. Waters and M. R. Gagné, Chem. Sci., 2011, 2, 744 RSC.
- (a) H. Brunner and H. Schiessling, Angew. Chem., Int. Ed., 1994, 33, 125 CrossRef; (b) X. X. Zhang, J. S. Bradshaw and R. M. Izatt, Chem. Rev., 1997, 97, 3313 CrossRef CAS PubMed; (c) Z. Gross and S. Ini, Org. Lett., 1999, 1, 2077 CrossRef CAS; (d) J. Gawronski, H. Kolbon, M. Kwit and A. Katrusiak, J. Org. Chem., 2000, 65, 5768 CrossRef CAS PubMed; (e) J. Gregolinski, J. Lisowski and T. Lis, Org. Biomol. Chem., 2005, 3, 3161 RSC; (f) D. Xu and R. Warmuth, J. Am. Chem. Soc., 2008, 130, 7520 CrossRef CAS PubMed; (g) J. Sun, J. L. Bennett, T. J. Emge and R. Warmuth, J. Am. Chem. Soc., 2011, 133, 3268 CrossRef CAS PubMed; (h) S. R. Beeren and J. K. M. Sanders, Chem. Sci., 2011, 2, 1560 RSC.
- (a) N. Iwasawa and H. Takahagi, J. Am. Chem. Soc., 2007, 129, 7754 CrossRef CAS PubMed; (b) S. Ito, K. Ono and N. Iwasawa, J. Am. Chem. Soc., 2012, 134, 13962 CrossRef CAS PubMed.
- L. L. Schafer and T. D. Tilley, J. Am. Chem. Soc., 2001, 123, 2683 CrossRef CAS PubMed.
- (a) D. Zhao and J. S. Moore, Chem. Commun., 2003, 7, 807 RSC; (b) W. Zhang and J. S. Moore, Angew. Chem., Int. Ed., 2006, 45, 4416 CrossRef CAS PubMed; (c) M. Iyoda, J. Yamakawa and M. J. Rahman, Angew. Chem., Int. Ed., 2011, 50, 10522 CrossRef CAS PubMed.
- (a) W. Zhang and J. S. Moore, J. Am. Chem. Soc., 2004, 126, 12796 CrossRef CAS PubMed; (b) W. Zhang and J. S. Moore, J. Am. Chem. Soc., 2005, 127, 11863 CrossRef CAS PubMed; (c) S. W. Sisco and J. S. Moore, J. Am. Chem. Soc., 2012, 134, 9114 CrossRef CAS PubMed.
- (a) S. Anderson, U. Neidlein, V. Gramlich and F. Diederich, Angew. Chem., Int. Ed., 1995, 34, 1596 CrossRef CAS; (b) D. L. An, T. Nakano, A. Orita and J. Otera, Angew. Chem., Int. Ed., 2002, 41, 171 CrossRef CAS.
- See ESI† for monomer and polymer synthesis.
- J. Heppekausen, R. Stade, R. Goddard and A. Fürstner, J. Am. Chem. Soc., 2010, 132, 11045 CrossRef CAS PubMed.
- See ESI† for FDmass spectra.
- (a) K. G. Denbigh, Principles of Chemical Equilibrium, Cambridge University Press, London, 1966 Search PubMed; (b) H. B. Callen, Thermodynamics and an Introduction to Thermostatics, John Wiley and Sons, New York, 2nd edn, 1985 Search PubMed.
- (a) C. E. Shannon, Bell Syst. Tech. J., 1948, 27, 379 CrossRef; (b) A. Ben-Naim, J. Chem. Educ., 2011, 88, 594 CrossRef CAS.
- S.-K. Lin, J. Chem. Inf. Comput. Sci., 1996, 36, 367 CrossRef CAS.
- A similar idea was proposed to explain the formation of more symmetric imine cages over lower symmetry cages, but the significance is unclear since the more symmetric cage (Oh) contains the more stable syn, antiimine conformation compared with the less symmetric cage (Td) containing less favorable syn, syn conformation (4.9 kcal mol−1 per salicyldiimine unit): P. Skowronek, B. Warzajtis, U. Rychlewska and J. Gawroński, Chem. Commun., 2013, 49, 2524 RSC.
- ΔSσ = R
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(σSS-2mer/σRS-2mer) = R
ln(σSS-2mer/σRS-2mer) = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(4/1).
(a) S. W. Benson, Thermochemical Kinetics, Wiley-Interscience, New York, 2nd edn, 1976 Search PubMed;
(b) E. Estrada and D. Avnir, J. Am. Chem. Soc., 2003, 125, 4368 CrossRef CAS PubMed.
ln(4/1).
(a) S. W. Benson, Thermochemical Kinetics, Wiley-Interscience, New York, 2nd edn, 1976 Search PubMed;
(b) E. Estrada and D. Avnir, J. Am. Chem. Soc., 2003, 125, 4368 CrossRef CAS PubMed. - M. Mammen, E. I. Shakhnovich, J. M. Deutch and G. M. Whitesides, J. Org. Chem., 1998, 63, 3821 CrossRef CAS . ΔSvibrational is expected to be a negligible component.
- Keq = 10−ΔG/1.36. For a ΔG < −2.05 kcal mol−1 the Keq > 32, corresponding to >97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of homochiral RR/SS-2mer to heterochiral RS-2mer.
3 ratio of homochiral RR/SS-2mer to heterochiral RS-2mer. - Homochiral self-sorting is observed in toluene at 25 °C. See ESI† for FDspectra.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, NMR spectra, GPCspectra of polymers and macrocycles, and MSspectra. See DOI: 10.1039/c3sc52018h |
| This journal is © The Royal Society of Chemistry 2014 |