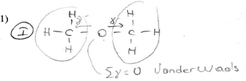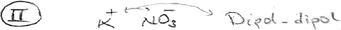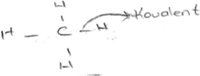Are creative comparisons developed by prospective chemistry teachers evidence of their conceptual understanding? The case of inter- and intramolecular forces
Gulten
Sendur
Dokuz Eylul University, Chemistry education, Izmir, Turkey. E-mail: gulten.sendur@deu.edu.tr
First published on 14th July 2014
Abstract
The aim of this study is to determine prospective chemistry teachers' creative comparisons about the basic concepts of inter- and intramolecular forces, and to uncover the relationship between these creative comparisons and prospective teachers' conceptual understanding. Based on a phenomenological research method, this study was conducted with 101 prospective chemistry teachers studying in the Chemistry Education Department at a state university in Turkey in the academic year 2011–2012. The research made use of two data collection instruments, a creative comparison questionnaire and semi-structured interviews. The concepts of “non-polar covalent bonds, dipole–dipole force, hydrogen bonds, ionic bonds, covalent bonds, polar covalent bonds, and van der Waals force” were set out in the creative comparison questionnaire and the prospective teachers were asked to complete the sentences about these concepts (example: ionic bond is like .......... because ...........). Content analysis techniques were employed in the analysis of the creative comparisons set out in the questionnaire. The analysis of the data revealed that the prospective teachers generally developed their creative comparisons based on the formation and strength of the different bonds and that they also had alternative conceptions, particularly regarding the formation of covalent bonds, hydrogen bonds, and the van der Waals force. Semi-structured interviews were conducted in order to learn more about this and obtain detailed information about the level of understanding of the prospective teachers. Twelve prospective teachers were selected for the interviews on the basis of the creative comparisons they had developed in the creative comparison questionnaire. The analysis of the interviews showed that the level of conceptual understanding of the prospective teachers was not tightly linked to the complexity of their creative comparisons. However, it was seen that the creative comparisons submitted by the prospective teachers could be used to infer their conceptual understanding. Also, these creative comparisons could be helpful in determining what the prospective teachers' alternative conceptions were.
Introduction
The main aim of teaching chemistry is to help students understand natural phenomena, scientific principles and theories (Rompayom et al., 2011). Sometimes, this aim can be difficult to achieve in the learning process as many phenomena are difficult to understand, and the scientific principles and concepts are abstract and complex (Collette and Chiappetta, 1994). Thus, some studies show that when students are faced with a difficult concept, they try to understand it by relating ideas to existing knowledge and their own world experiences (Ünal et al., 2006). In this process, metaphors, analogies, and similes play an important role as they bridge the known to the unknown, and alter the conceptual system of existing knowledge by modifying and strengthening its associations (Kanthan and Mills, 2006).Metaphors are cognitive devices to conceptualize or think about the world (Erdoğan and Erdoğan, 2013). Also, metaphors are considered the strongest device that an individual can use to understand and explain an abstract, complex fact at a high level (Yob, 2003; Saban, 2004; Aubusson et al., 2006) According to Lakoff and Johnson (1980), our conceptual structures are built on metaphors, which help us to understand the world in terms of what is familiar. In this context, analyzing metaphors enables us to discover how people think about something (Saban et al., 2006; Zheng and Song, 2010). At the same time, Thomas (2006) stated that students might be able to express the essence of their understanding in science classrooms through the use of metaphors. In another study, Amin (2009) argued that abstract concepts like energy did not contain elements related to direct experience, however, metaphors about these concepts based on experiential knowledge reveal understanding. He also claimed that developing an understanding of an abstract concept may rely extensively on metaphors pervasive in both everyday and scientific language. As a result of this, Amin stated that metaphorical representations of concepts can be accepted as additional sources of conceptual change. Furthermore, Amin argued that metaphors could be used to identify potential obstacles to conceptual change.
At the same time, analogies are effective tools since they allow students to relate their scientific ideas to ideas that students find familiar, and as a result of this, the unfamiliar is made familiar (Coll, 2006). Analogies can serve as a conceptual bridge between existing and targeted knowledge (Glynn, 1995). In this context, analogies are made up of two parts, a source (familiar to someone) and a target (unknown to someone) (Justi and Gilbert, 2006). Thus, analogy allows an explicit comparison between two systems that share some level of structural similarity (Taber, 2013). In addition, the use of analogies evokes emotion, interest, and creative insight (Gilbert, 1989; Duit, 1991). Apart from analogy, a comparison between two concepts can be expressed as a simile (Chiappe et al., 2003). Similes also differ from analogies in that similes map attributes; while analogies map structural similarities; similes are intended as analogies in a narrower sense (Miller, 1993; Israel et al., 2004). A simile is defined as “a figure of speech in which two essentially unlike things are compared, often in a phrase introduced by ‘like’ or ‘as’ (Kanthan and Mills, 2006).
There is much discussion in the science education literature about the definition of “metaphor”, “analogy” and “simile” (Lancor, 2014a, 2014b). For example, Nakipoğlu and Taber (2013) stated that when using a metaphor, “A is said to be B”, however, in an analogy, “A is said to be like B.” They said that the aim of using metaphors and analogies is often the same, but the comparison in a metaphor is implicit. On the other hand, some researchers have stated that in metaphors, “A is like B”, and they use metaphors to make connections between two domains like other researchers (Gentner, 1988; Christidou et al., 1997; Cameron, 2002; Saban et al., 2006; Seung et al., 2011). Also, Gentner and Jeziorski (1993) have explained that analogies map relational structures independently of object descriptions, but that metaphors have a broad category, and they include analogies, object descriptions and other kinds of matches.
In this study, the term “creative comparisons” was adopted as an inclusive term for metaphors, analogies, and similes. On the other hand, because the prospective teachers in the study, though asked to generate comparisons in the format “A is like B”, formed comparisons that fell into the categories of both analogy and simile, the term “creative comparisons” in our research encompasses only analogies, and similes. In other words, some prospective teacher-generated comparisons based on structural similarity between two systems, a type of comparison that is known as an analogy. However, some comparisons were simpler than analogies, and based on common attributes between two systems, such comparisons being known as similes. At the same time, one of the reasons the term “creative comparisons” was used in the study was that by asking students to generate comparisons about science concepts, they were given the opportunity to express their ideas in a unique way, and as a result, to be creative in the context of learning science (Lancor, 2014b; Taber, in press).
Student-generated creative comparisons are very important for both students and teachers. While student-generated creative comparisons can help students to develop a deeper understanding of the content, they also provide a tool that teachers can use to evaluate how students are making the connection (Lancor, 2014a, 2014b). In this context, Lancor (2014b) stated that allowing students to creatively express their ideas gives the teachers a different perspective than can be acquired from traditional assessment techniques. In addition, Taber (in press) expressed that teachers legitimize and value students' creative ideas in science by considering student-generated comparisons.
Although the importance of student-generated creative comparisons has been accepted in the literature, the contributions of these to scientific understanding are still being discussed. For example, Vosniadou (2009) stated that metaphors are accepted as structures of perceptual, experiential knowledge both as being embodied in and as having some degree of coherence at the same time. On the other hand, the author has also stated that when multiple metaphors are used to ground the understanding of scientific concepts, a great deal of care should be exercised since many students do not have the epistemological sophistication to interpret conceptual metaphors. As a result, she argued that fragmentation and alternative conceptions could occur in the learning process. In another study, Brookes and Etkina (2007) investigated how physicists represent their ideas in language in the domain of quantum mechanics and how physics students interpret the language they read and hear. The researchers documented how physicists use many metaphors in their language to speak about quantum mechanics; however, some students over-extend and misapply the metaphors in physicists' speech and writing. In the light of these results, Amin et al. (2012) suggested that more research about the role of metaphors in the learning process could be conducted. One of these studies was conducted by Jeppsson et al. (2013) to reveal the role of metaphors in scientific problem-solving. The results of the research showed that a range of metaphors are used in problem-solving enabling flexible, experiential construals of abstract scientific concepts. There is still a need, however, for studies investigating whether or not a relationship between creative comparisons and conceptual understanding exists. The present study thus seeks to identify whether or not there is a relationship between creative comparisons and conceptual understanding about inter- and intramolecular forces.
In the last two decades, many researchers have reported that students have inadequate conceptual understanding of the basic concepts related to inter- and intramolecular forces and fail to integrate their mental models into a coherent conceptual framework (Peterson and Treagust, 1989; Griffiths and Preston, 1992; Herron, 1996; Bodner and Domin, 1998; Coll and Taylor, 2001; Coll and Treagust, 2001a). These results were not surprising since these topics included concepts that are far from students' daily experiences; e.g. students cannot see an atom, or a molecule and its interactions (Birk and Kurtz, 1999; Gabel, 1999; Griffiths and Preston, 1999; Tan and Treagust, 1999; Coll and Taylor, 2002; Taber and Coll, 2002; Ünal et al., 2006). In addition, one of the reasons for students' difficulties in understanding these topics may be that these topics are associated with some mathematical and physical concepts such as orbitals, electronegativity, and polarity (Levy Nahum et al., 2007). These factors affect the formation of alternative conceptions about inter- and intramolecular forces. Previous studies revealed four main categories of students' difficulties and alternative conceptions regarding these concepts. These are below:
• Students confuse intra- and intermolecular forces (Treagust, 1988; Peterson and Treagust, 1989; Peterson et al., 1989; Taber, 1998; Tan and Treagust, 1999; Barker and Millar, 2000; Ünal, 2007).
• Students could not discriminate ionic and covalent bonds from each other (Taber, 1997a; Boo, 1998; Coll and Treagust, 2001a; Ünal et al., 2010).
• Students have a poor understanding of the electrostatic nature of chemical bonding (Taber, 1995, 1998; De Posada, 1997; Boo, 1998, 2000, Taber et al., 2012).
• Students are unaware of differences in the strength of inter- and intramolecular forces (Treagust, 1988; Peterson and Treagust, 1989; Boo, 1998; Tan and Treagust, 1999).
In such cases, the students can use creative comparison to facilitate their understanding Thus, Nicoll (2001) found that a number of students who could not provide the scientific explanation for bonding phenomena tried to link it to a related concept that was correct in and of itself. Similarly, Harrison and Treagust (2000) stated that students use analogies consistently in their explanations. In another study, Coll and Treagust (2001b) reported that students at all academic levels, in secondary school as well as on undergraduate and graduate (PhD) levels, could use analogies to explain some concepts of chemical bonding. However, Coll and Treagust did not extensively investigate in their study the analogies used by students nor their conceptual understanding or alternative conceptions. This was due to the fact that they only used a semi-structured interview protocol to identify students' analogies. As a result, the students were not able to make an extensive use of analogies to explain the basic concepts of chemical bonding.
Consequently, it is necessary to identify the creative comparisons learners use to explain the basic concepts of inter- and intramolecular forces and to investigate the relationship between these creative comparisons and learners' conceptual understanding. The present study investigates the creative comparisons prospective chemistry teachers used to explain some basic concepts of inter- and intramolecular forces. At the same time, the prospective chemistry teachers were stratified at a high, middle, and low level according to their creative comparisons. It was aimed to find out the level of their conceptual understanding related to concepts. The objective here was to determine whether or not the creative comparisons used by the prospective chemistry teachers at the different levels were evidence of their conceptual understanding. The findings of the present study may provide evidence as to how learners' creative comparisons meet their level of conceptual understanding. Additionally, this study will help chemistry teachers, chemistry educators and curriculum developers to be aware of how learners try to understand some of the difficult chemistry concepts by relating ideas to existing knowledge and their own world experiences. These creative comparisons could also be used in pedagogical implications.
Purpose and research questions
The purpose of this research is to find out prospective chemistry teachers' creative comparisons about “the basic concepts of inter- and intramolecular forces”, and to reveal the relationship between these creative comparisons and their conceptual understanding. Based on the purpose, three research questions are investigated:(1) Which creative comparisons were used to explain “the basic concepts of inter- and intramolecular forces” by the prospective chemistry teachers?
(2) Under which categories are proposed creative comparisons by prospective chemistry teachers about “the basic concepts of inter- and intramolecular forces” classified in terms of their common features?
(3) What is the relationship between prospective chemistry teachers' creative comparisons and conceptual understanding?
Method
The phenomenographic research method was used in the study. At the foundation of phenomenographic research is defining, analyzing and understanding how individuals conceptualize phenomena occurring in the world around them (Marton, 1994; Vanderstoep and Johnston, 2009). In this method, the phenomenon is defined through the perspective of the learner (Ashworth and Lucas, 1998). Since the objective of this study is to discover creative comparisons prospective chemistry teachers used to explain the basic concepts of the subject of inter- and intramolecular forces and to uncover the relationship between these creative comparisons, and the prospective teachers' conceptual understanding, the phenomenographic method was thought to be an appropriate method to use.The study group
The study group for the research was selected by the method of typical case sampling, which is a version of the purposeful sampling method. In this sampling method, the objective is to learn enough about a typical situation in order to be able to inform others who are not familiar with the situation (Patton, 1987; Lodico et al., 2010). The study group for the research comprised of 101 prospective chemistry teachers (55 women, 46 men) who were studying in the Chemistry Education Department of a state university in Turkey during the 2011–2012 academic year. All of the prospective teachers volunteered to participate in the study. Each prospective teacher gave an informed consent form one week before the study commenced. The prospective teachers in the study group were made up of 21 first-year students and 20 students in their 2nd, 3rd, 4th and 5th years. The ages of the teacher candidates were in the range of 18–24 years and most came from middle-income families. The prospective teachers in the study group had studied the topic of inter- and intramolecular forces in their chemistry classes in middle school and in the General Chemistry-I course they had taken in the fall semester of their first year at the university. It was thought that since this was the case, the prospective teachers in the study group would be able to readily use their previous knowledge to answer the questions in the creative comparison questionnaire and in the semi-structured interviews.Data collection and instrument
The research made use of two data collection instruments, a creative comparison questionnaire and semi-structured interviews. All data were gathered in Turkish. The researcher herself translated the material to English. Also, these translations were controlled by a faculty member in chemistry education and by a native English speaker.Creative comparison questionnaire
In the data collection stage of the study, a questionnaire was prepared that was designed to discover what creative comparisons prospective teachers used to explain the subject of inter- and intramolecular forces. Similar questionnaires were developed to examine students' comparisons about some concepts such as school, computer teacher, knowledge, cell, and global warning (Saban, 2008; Yener and Özkadif, 2010; Saban, 2011; Dogru and Sarac, 2013). To establish content validity for the creative comparison questionnaire, three experts in the field were consulted to determine the concepts that would define the foundation of the topic. At the same time, a pilot study was conducted using the creative comparison questionnaire with 25 prospective teachers outside of the sample; it was determined that allowing 15 minutes for each concept would be sufficient. Before the questionnaires were filled out, the teacher candidates were given information about creative comparisons and then asked to generate creative comparisons for the concepts of “non-polar covalent bonds, dipole–dipole force, hydrogen bonds, ionic bonds, covalent bonds, polar covalent bonds, and the van der Waals force” and explain the reason they chose such a creative comparison by completing the sentences in the questionnaire (example: ionic bond is like ............ because ...........). Thus in the implementation of the study, the teacher candidates were asked to complete their sentence for each concept in 15 minutes. The researcher timed each question, telling the prospective teachers to go on to the next concept at the end of 15 minutes.Semi-structured interview
Semi-structured interviews were conducted to learn the level of understanding of the prospective teachers about the basic concepts of inter- and intramolecular forces. Through stratified sampling, twelve prospective teachers were selected for the interviews on the basis of the creative comparisons they had developed in the creative comparison questionnaire. According to the creative comparisons they created, the prospective teachers were stratified on three levels – high, middle and low – and four prospective teachers were randomly selected from each stratum. The criteria taken into consideration in forming the strata were the following:• High: this stratum comprised the prospective teachers who were able to create and clearly and accurately explain creative comparisons for 6 or more concepts and did not contain any alternative conceptions.
• Middle: this stratum comprised the prospective teachers who were able to create and clearly and accurately explain creative comparisons for 3–5 concepts and did not contain any alternative conceptions.
• Low: this stratum comprised the prospective teachers who were able to create and clearly and accurately explain creative comparisons for 1–3 concepts and could contain alternative conceptions.
The stratification of the prospective teachers according to the creative comparisons they formed was carried out by a faculty member in chemistry education. The interviews were conducted individually with each prospective teacher. Each interview was completed in 20–25 minutes. An audio recorder was used in the interviews and the entire interview was recorded. In the interview, each prospective teacher was asked four questions that had been developed by the researcher. Besides these main questions, the prospective teachers were also asked some sub-questions about the responses they gave. The opinions of three specialists in chemistry education were enlisted in developing the questions for the interviews. The interview questions took their final form after revisions and additions were made in line with the recommendations of the specialists. To facilitate a more productive interview and establish which parts of the discussion should be emphasized and which difficulties could be encountered, a pilot run was carried out with 4 prospective teachers outside of the study group. This pilot study provided the means to determine the time that was needed for the interview and the chance to test the proposed questions. At the end of the pilot application, the researchers were able to define which questions would be comprehensible and suitable for the prospective teachers' level. The questions and their content are presented in Table 1.
| Question number | Content |
|---|---|
| 1 | Defining intramolecular bonds. |
| 2 | Defining intermolecular forces. |
| 3 | Comparison of the strength of intramolecular and intermolecular forces. |
| 4 | Impact of intermolecular forces on the physical properties of molecules. |
During the course of the interviews, the prospective teachers were asked to write down the formulae of the molecules mentioned in the questions. The purpose of this was to find out how the prospective teachers represented the concepts on a symbolic level.
Data analysis
Content analysis techniques were employed in the analysis of the creative comparisons set out in the questionnaire. The analysis made use of the stages of analysis employed by other research, namely: (1) naming; (2) eliminating and refining; (3) compiling and developing categories; (4) establishing validity and reliability; (5) calculating and interpreting percentages and frequencies for creative comparisons (Saban et al., 2006; Saban, 2008, Özder, 2013).(1) Naming: in this stage, a review was made as to whether the creative comparisons created by the prospective teachers were meaningful; a mark was placed on papers that contained responses that were lacking creative comparisons or explanations. The meaningful creative comparisons created by the prospective teachers were listed in alphabetical order.
(2) Eliminating and refining: in this stage, after the creative comparisons created for each concept by the prospective teachers were reviewed again, the creative comparisons that had been marked in some prospective teachers' papers in the naming stage were dropped from the study. In total, 14 prospective teachers' papers were eliminated, and 101 prospective teachers' papers were involved in this study.
(3) Compiling and developing categories: in this stage, the common characteristics of the creative comparisons were compiled to develop categories. These categories were developed based on the prospective teachers written responses. The researcher reviewed the prospective teachers' sentences with particular consideration paid to the reasons why or how they related the creative comparisons to the concept. The reason was generally placed in the sentence after the word “because.” The researcher herself classified the creative comparisons according to the characteristics of intra- and intermolecular forces. For example, some prospective teachers created creative comparisons considering the formation of intra- and intermolecular forces. Some of the creative comparisons were created by considering the strength of intra-and intermolecular forces. In Table 2, one sample statement in relating the category to each concept is presented. More detailed examples have been given in the Results section.
| Concept | Category | Sample statement |
|---|---|---|
| Ionic bond | Formation | “Ionic bond is like the teacher–student relationship because the teacher gives the student knowledge and the student takes in that knowledge. In ionic bonds, too, metals give electrons and non-metals receive electrons.” PT-5 |
| Strength | “Ionic bond is like concrete because concrete is strong and hard to destroy. Ionic bonds are stronger than other bonds, than for instance covalent bonds.” PT-20 | |
| Covalent bonds | Formation | “Covalent bond is like public property because the electrons that make up the covalent bond are shared. Public property is used in the same way by the public.” PT-13 |
| Strength | “Covalent bond is like a chain because a chain won't break easily. Covalent bond won't break easily since covalent bond is one of the intramolecular forces. Intramolecular forces are stronger than the intermolecular forces.” PT-71 | |
| Polar covalent bond | Formation | “Polar covalent bond is like two siblings with different features because a polar covalent bond is formed by different non-metal atoms.” PT-10 |
| Sharing electrons unequally | “Polar covalent bond is like a scale with more weight on one side because one side is heavier. Non-metal atoms that form a polar covalent bond attract electrons with different forces so the electrons are more concentrated on one side.” PT-86 | |
| Non-polar covalent bond | Formation | “Non-polar covalent bond is like an asocial person because non-social people are only happy with themselves. They prefer to be with themselves rather than with other people. Non-polar covalent bond is formed with the same kind of non-metal atoms.” PT-84 |
| Sharing electrons equally | “Non-polar covalent bond is like equal rights because the atoms in non-polar covalent bond attract the electrons they share between them with equal force.” PT-23 | |
| Hydrogen bond | Formation | “Hydrogen bond is like a family because a family is made of particular people such as a mother, a father and children. A hydrogen bond is like a family because this bond forms between hydrogen and Fluorine, Oxygen and Nitrogen.” PT-25 |
| Strength | “Hydrogen bond is like thin people because since the hydrogen bond is between the molecules, it is not as strong as the inside of the molecule; it is weak, like thin people.” PT-59 | |
| Physical properties | “Hydrogen bond is like the brain because the brain can manage our body. Similarly, hydrogen bond can change or manage the boiling point, the viscosity.” PT-56 | |
| Dipole–dipole force | Formation | “Dipole–dipole force is like people who are diametrically opposite to each other because people with opposite personalities tend to attract each other. There is an attraction there. And this force appears between the opposite poles of polar molecules.” PT-4 |
| Strength | “Dipole–dipole force is like a person of medium strength because this attraction is stronger than van der Waals force but weaker than a hydrogen bond.” PT-49 | |
| Van der Waals force | Formation | “The van der Waals force is like the feeling of liking someone momentarily because a woman and a man may see each other and feel a sudden attraction between them. A similar situation exists in the van der Waals force. As noble gas or molecules get close to each other, when electrons are more on one side, momentary dipoles, in other words, + and − charges, will be produced. These momentary charges will affect the other molecules as well. The attraction between momentary charges is the van der Waals force.” PT-81 |
| Strength | “The van der Waals force is like a family having weak relations with other relatives because if we think about a family and its relatives as molecules, the weak relation between them is the van der Waals force; the force is a weak attraction between molecules.” PT-46 | |
| Molecules were affected by van der Waals force | “The van der Waals force is like a mobile phone because today, everyone has one. The van der Waals force, too, is a force is present in polar as well as non-polar molecules.” PT-84 | |
| Physical properties | “The van der Waals force is like a flying balloon because among the molecules, only molecules like F2, Cl2 that have this force are weaker than other molecules (H2O) at the boiling point. This is why only molecules that have the van der Waals force boil at a lower temperature.” PT-49 | |
(4) Testing validity and reliability: the opinion of a specialist was enlisted to ensure the reliability of the study. To this end, a faculty member in the department of chemistry education was asked to match the creative comparisons drawn from the study to the categories identified in Table 2. Similarly, the researcher matched the prospective teachers' creative comparisons to the categories. The percentage of agreement between the categories established by the specialist and the researcher was calculated as 0.92 using the formula of Miles and Huberman (1994). According to Fleiss and Levin (1981), the values above 0.75 are considered “excellent”.
(5) Calculating and interpreting percentages and frequencies for the creative comparisons: the percentages and frequency values of the creative comparisons were calculated under the various categories. These values belong to the entire sample. At the same time, the creative comparisons established in the study were interpreted and an attempt was made to uncover the missing information or alternative conceptions about the concepts exhibited by the prospective teachers.
The data obtained from the semi-structured interviews were analyzed in five categories. These categories were defined as “sound understanding,” “partial understanding,” “partial understanding with an alternative conception”, “alternative conceptions” and “no response.” These types of categories are widely used in studies in the literature (Abraham et al., 1992; Çalık, 2005). The categories related to the level of understanding and the content of these categories are shown in Table 3. The records of the interviews were separately coded by the two researchers and the percentage of agreement between the two series of code was calculated to be 0.88 (Miles and Huberman, 1994).
| The level of understanding | Criteria for the classification of prospective teachers' responses |
|---|---|
| Sound understanding (SU) | Scientifically complete responses and correct explanations |
| Partial understanding (PU) | Responses that included at least one of the components of an acceptable response and explanation |
| Partial understanding with an alternative conception (PUAC) | Scientifically complete responses and unacceptable explanations |
| Alternative conceptions (AC) | Completely scientifically unacceptable responses and explanations |
| No response (NR) | ‘No answer’; ‘I don't know’; ‘I have no idea’ |
Results
This section describes the results obtained from the creative comparison questionnaire and the semi-structured interviews.Creative comparison questionnaire results
The frequencies and percentages of the creative comparisons that the prospective teachers composed for the concepts of “ionic bonds, covalent bonds, polar covalent bonds, and non-polar covalent bonds (intramolecular forces)” are presented in Table 4. Table 5 shows frequencies and percentages of the creative comparisons belonging to intermolecular forces (“hydrogen bonds, van der Waals force, and, dipole–dipole force”). For each concept, creative comparisons formed by the prospective teachers have been presented according to this order: “creative comparisons including scientifically correct explanations,” “creative comparisons including some unclear points” and “creative comparisons including alternative conceptions.”| Categories | Ionic bond | Categories | Covalent bond | Categories | Polar covalent bond | Categories | Non-polar covalent bond | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creative comparisons | f | % | Creative comparisons | f | % | Creative comparisons | f | % | Creative comparisons | f | % | ||||
| Formation | Trading | 26 | 25.7 | Formation | Sharing money | 14 | 13.9 | Formation | Marriage | 20 | 19.8 | Formation | Identical twins | 27 | 26.7 |
| A magnet | 24 | 23.8 | Brotherhood | 13 | 12.9 | Lovers | 4 | 3.9 | Two friends of the same gender sharing the same house | 11 | 10.9 | ||||
| Financial aid | 9 | 8.9 | Marriage | 12 | 11.9 | Two siblings sharing a toy | 2 | 1.9 | A family company | 5 | 4.9 | ||||
| Teacher–student relationship | 9 | 8.9 | A public transportation vehicle | 9 | 8.9 | A company partnership | 2 | 1.9 | Two students sitting on the same bench | 5 | 4.9 | ||||
| The relationship between a customer and a supermarket attendant | 7 | 6.9 | A common eraser | 9 | 8.9 | Two siblings with different features | 6 | 5.9 | A sacrificial animal bought together | 5 | 4.9 | ||||
| Puzzle pieces | 7 | 6.9 | Roommates | 7 | 6.9 | A class | 2 | 1.9 | A pair of shoes | 3 | 2.9 | ||||
| Men and women | 7 | 6.9 | Public property | 5 | 4.9 | A magnet | 15 | 14.8 | An object and its mirror image | 3 | 2.9 | ||||
| Agreement between two people with opposing ideas. | 5 | 4.9 | Family relations | 5 | 4.9 | The need of two people of opposite sexes for each other | 1 | 0.9 | An asocial person | 2 | 1.9 | ||||
| Two girls | 1 | 0.9 | A joint stock company | 4 | 3.9 | Identical twins | 1 | 0.9 | People in the world | 1 | 0.9 | ||||
| Filling in the tiles on a game of Bingo | 1 | 0.9 | People | 2 | 1.9 | The others | 13 | 12.9 | Two different people holding a rope on both ends | 1 | 0.9 | ||||
| The others | 1 | 0.9 | Combination of contrast colours | 1 | 0.9 | The others | 10 | 9.9 | |||||||
| People with the same opinions | 1 | 0.9 | |||||||||||||
| The others | 12 | 11.9 | |||||||||||||
| Strength | Dumbbell weight | 1 | 0.9 | Strength | Rope | 2 | 1.9 | Sharing electrons unequally | People of the same gender but with different strengths pulling on a rope” | 17 | 16.8 | Sharing electrons unequally | Two people with equal strength pulling a rope | 17 | 16.8 |
| Concrete | 1 | 0.9 | A friendship | 2 | 1.9 | A big and a small dog pulling on the same bone | 3 | 2.9 | Two children of equal weight on a seesaw | 3 | 2.9 | ||||
| Father of the family | 1 | 0.9 | Bond inside the family | 2 | 1.9 | A big fish biting off a bigger piece” | 1 | 1.9 | Not being able to win an arm-wrestling contest” | 2 | 1.9 | ||||
| Love | 1 | 0.9 | A chain | 1 | 0.9 | Arm-wrestling with different strengths | 1 | 1.9 | Equal rights | 2 | 1.9 | ||||
| Twins pulling at two ends of a rope | 1 | 0.9 | Civil servants at the same level getting the same salary | 2 | 1.9 | ||||||||||
| The others | 12 | 11.9 | A roly-poly doll | 1 | 0.9 | ||||||||||
| A match ending in a tie | 1 | 0.9 | |||||||||||||
| Categories | Hydrogen bond | Categories | Van der Waals force | Categories | Dipole–dipole force | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Creative comparisons | f | % | Creative comparisons | f | % | Creative comparisons | f | % | |||
| Formation | A selective person | 12 | 11.9 | Strength | A feather | 16 | 15.8 | Formation | A magnet | 24 | 23.8 |
| A family | 8 | 7.9 | A Rotten rope | 10 | 9.9 | People who are diametrically opposite to each other | 11 | 10.9 | |||
| A selective permeant | 7 | 6.9 | Cotton | 9 | 8.9 | Lightning | 3 | 2.9 | |||
| An obsessed person | 6 | 5.9 | A house of cards | 7 | 6.9 | People with different strengths pulling on a rope | 7 | 6.9 | |||
| Enzyme–substrate relationship | 4 | 3.9 | A rotten piece of wood | 6 | 5.9 | Two similar people attracting each other | 3 | 2.9 | |||
| A cell membrane | 4 | 3.9 | A rotten building | 5 | 4.9 | A bag of lentils | 1 | 0.9 | |||
| Bond between twins | 4 | 3.9 | The bones of an elderly person | 2 | 1.9 | A married couple sharing a blanket | 1 | 0.9 | |||
| Some kind of article that people share | 1 | 0.9 | Two casual classmates | 2 | 1.9 | One nation becoming another nation's dominion | 1 | 0.9 | |||
| A social person | 1 | 0.9 | Magnet | 3 | 2.9 | Two balls of the same kind sticking to each other | 1 | 0.9 | |||
| The others | 13 | 12.9 | Handcuffs | 3 | 2.9 | Gravity | 1 | 0.9 | |||
| The others | 19 | 18.8 | The bond between people of the same city | 1 | 0.9 | ||||||
| The others | 10 | ||||||||||
| Strength | Friendship | 7 | 6.9 | Molecules were affected by van der Waals force | Blood vessels | 2 | 1.9 | Strength | Medium-sweet turkish coffee | 7 | 6.9 |
| Hercules | 5 | 4.9 | Energy | 2 | 1.9 | A person of medium strength | 7 | 6.9 | |||
| A rope | 5 | 4.9 | Jealousy | 1 | 0.9 | Strong person | 4 | 3.9 | |||
| Steel | 3 | 2.9 | The ability to talk | 1 | 0.9 | A vacuum cleaner's picking up dust | 3 | 2.9 | |||
| A beam or column | 3 | 2.9 | Mobile phones | 1 | 0.9 | The largest root of a tree | 1 | 0.9 | |||
| A thick branch | 1 | 0.9 | Gravity | 1 | 0.9 | A hurricane | 1 | 0.9 | |||
| The strongest individual in a society | 1 | 0.9 | A party that identical twins come to | 2 | 1.9 | A stone | 1 | 0.9 | |||
| The roots of a tree digging into the ground | 1 | 0.9 | Two similar people agreeing with each other | 2 | 1.9 | Love between romeo and juliet | 1 | 0.9 | |||
| The father in a family | 1 | 0.9 | The others | 12 | 11.9 | ||||||
| A mother's love for a baby | 1 | 0.9 | |||||||||
| A wall | 1 | 0.9 | Formation | Feeling of liking someone momentarily | 4 | 3.9 | |||||
| Wrestlers | 1 | 0.9 | |||||||||
| The weakest link in a chain | 1 | 0.9 | |||||||||
| The others | 8 | 7.9 | |||||||||
| Physical properties | Brain | 1 | 0.9 | Physical properties | A flying balloon | 1 | 0.9 | ||||
| A knot | 1 | 0.9 | Imagination | 1 | 0.9 | ||||||
| A person who's patient | 1 | 0.9 | A fat person's taking up more space than a thin person | 1 | 0.9 | ||||||
Creative comparisons including scientifically correct explanations about the concept of ionic bonds
The prospective teachers were able to form creative comparisons that included scientifically correct explanations about the concept of ionic bonds and belonged to the formation and strength categories.In the formation category, the prospective teachers used the creative comparisons “men and women,” “agreement between two people with opposing ideas,” and “magnet” to explain the attraction between oppositely charged ions in an ionic bond. Some of the statements where these creative comparisons were used are the following:
“Ionic bond is like men and women because men and women attract each other even though they are the opposite of each other. Similarly, ionic bonds are formed. The atom gives up electron or electrons to another atom. As a result, this atom is loaded with (+) charge. Another atom receives electron or electrons, and is loaded with (−) charge. The ionic bond is formed as a result of the attraction these opposite charges.” PT-81
“Ionic bond is like a magnet because opposite poles on a magnet attract each other. In an ionic bond too, the electrostatic attraction between the (+) and (−) poles causes the ions to attract one another and form a bond.” PT-85
Some prospective teachers explained the transfer of electrons and the existence of oppositely charged ions in an ionic bond by using the creative comparisons of trading and magnet. The statements that are an example of these creative comparisons are as follows:
“Ionic bond is like trading because in trading, money is exchanged whereas in ionic bonds, there is a trade of electron between metal and non-metal atoms.” PT-83
“Ionic bond is like a magnet because it has opposite poles like N and S like a magnet. Ionic bonds too have opposite poles that are cations (+) and anions (−). While an atom gives up an e−and forms a (+) pole, an atom that receives an e−forms a (−) pole.” PT-2
It can be seen in Table 4 that a code for “Other” is listed under the formation category. The “Other” code contains creative comparisons with a frequency value of 1 that are scientifically correct. One creative comparison in this category is “a friendship between a miser and a generous person.” The statement that is an example of this creative comparison is as follows:
“Ionic bond is like a friendship between a miser and a generous person because one of the electrons tends to give, displaying a generous character, while the other electron only receives, showing a miserly character.” PT-49
The prospective teachers also created creative comparisons that included scientifically correct explanations about the concept of ionic bonds in the category of strength. This category contained creative comparisons on “dumbbell weight” (0.9%), “concrete (0.9%), “father of the family” (0.9%) and “love” (0.9%). An example of the statement in this context is the following:
“Ionic bond is like love because love is a strong interaction; ionic bonds also have a strong attraction between (+) and (−) ions.” PT-25
Creative comparisons including some unclear points about the concept of ionic bonds
In the formation category, some prospective teachers used the creative comparisons of “financial aid” (8.9%), “teacher–student relationship” (8.9%), “the relationship between a customer and a supermarket attendant” (6.9%), and “puzzle pieces” (6.9%) to explain the concept of the transfer of electrons between ions in the formation of an ionic bond. Although these prospective teachers did not state clearly in their creative comparisons that ionic bonds occur as a result of the transfer of electrons, they tended to see ionic bonds as the result of the transfer of electrons (PT-10 stated this more clearly than the other prospective teachers). In other words, these prospective teachers did not take into consideration the fact that ionic bonds are formed by the electrostatic interaction of oppositely charged ions as a result of electron transfer. These results are consistent with the findings of Taber (1997b). Some examples of the statements in this context are the following:“Ionic bond is like the relationship between a customer and a supermarket attendant because the customer and the supermarket attendant exchange money between them. Here, the electrons are like money. The supermarket attendant takes money and therefore is a non-metal (takes the electron) and the customer gives the money and is therefore a metal (gives the electron).” PT-6
“Ionic bond is like puzzle pieces because one puzzle has an extra piece and another has a missing piece. The extra piece is used to take the place of the missing piece in the other puzzle and in the end, they both become whole. In an ionic bond, too, the electrons which are extra in a metal are given to the atom of a non-metal, a trade is made and a bond is formed.” PT-10
Creative comparisons including alternative conceptions about the concept of ionic bonds
It was also found from the creative comparisons in the formation category created by the prospective teachers that they had alternative conceptions as well. An example of one of these is the following:“Ionic bond is like two girls because ionic bonds are formed between two non-metal groups; we can separate the sexes into categories the same way, such as non-metal and metal. We can call girls as non-metals and boys as metals. This way, two girls are like an ionic bond.” PT-16
As can be seen from this explanation, the prospective teacher sees ionic bonds as bonds formed between non-metal atoms. This finding has been discovered in various studies (Ünal, 2003). Another creative comparison worthy of note is the creative comparison of “filling in the tiles on a game of Bingo.” This creative comparison is stated as follows:
“Ionic bond is like filling in the tiles on a game of Bingo because the electrons on both sides are trying to complete the 8.” PT-36.
It may be gathered from the statement above that the prospective teacher believes that bonds can only be formed as the result of the octet rule. Similar alternative conceptions have been reported in various other studies in the literature (Robinson, 1998, Eshach and Garik, 2001; Coll and Taylor, 2002; Coll and Treagust, 2003).
Creative comparisons including scientifically correct explanations about the concept of covalent bonds
The prospective teachers produced creative comparisons that included scientifically correct explanations about the concept of covalent bonds in both categories of formation and strength. In these creative comparisons, the prospective teachers explained the formation and strength of covalent bonds from different perspectives. For example, the prospective teachers used the creative comparison of “family relation” (4.9%) to express their belief that a covalent bond was formed between non-metal atoms. An example of this belief is the following:“Covalent bond is like family relations because it does not occur between metal and non-metal atoms but between non-metal and non-metal atoms.” PT-70
The creative comparison of “people” (1.9%) was also used regarding the formation of covalent bonds. An examination of this creative comparison reveals that the prospective teachers thought that the equal or unequal sharing of electrons in polar and non-polar covalent bonds resembled people. One of the explanations where these creative comparisons were used is the following:
“Covalent bond is like people because people can be both compliant and argumentative. A covalent bond can be of two types-a non-polar and a polar covalent bond. Non-polar covalent bonds are formed by compliant non-metals because they share the electrons equally. Polar covalent bonds are formed by argumentative non-metals. We can say that these quarrel among themselves because they all want more electrons.” PT-23
The other code set up for the formation of the covalent bond contained the creative comparisons of “tribe,” “jury,” “mutual assistance,” “a country and its people,” “two people drinking from the same glass of orange juice,” “people of the same race supporting each other,” “the friendship of two people from different countries,” “relationships based on self-interest,” “people of the same gender in a population eating the same food,” “two hungry people sharing food,” “children,” and “a pencil being shared.” One of the statements where these creative comparisons are used is the following:
“Covalent bond is like a country and its people because a covalent bond is made up of different or the same kinds of non-metal atoms. The people who make up a country can also be different or they can be of the same religion, language or race.” PT-25
In the category of the strength of covalent bonds, the creative comparisons of “a friendship” (1.9%), “bond inside the family” (1.9%) and “a chain” (0.9) were formed by the prospective teachers to compare intra- and intermolecular forces. One of the statements where these creative comparisons were used is the following:
“Covalent bond is like bond inside the family because this bond is stronger than a neighbourly bond. Similarly, covalent bond as intramolecular forces is stronger than intermolecular forces.” PT-69.
The creative comparison of “rope” (1.9%) was used by different prospective teachers to explain two different meanings. For example, PT 97 used this creative comparison to compare the strength of intra- and intermolecular forces. On the other hand, PT 20 used the same creative comparison to compare the strength of ionic and covalent bonds. This creative comparison is the following:
“Covalent bond is like rope because rope is not stronger than steel cable. Similarly, covalent bond is not stronger than ionic bond.” PT-20
Creative comparisons including some unclear points about the concept of covalent bonds
Examination of the creative comparisons shown in Table 4 shows that the prospective teachers mostly used the creative comparisons of “sharing money” (13.9%), “brotherhood” (12.9%), “marriage” (11.9%), “a public transportation vehicle” (8.9%), “a common eraser” (8.9%), “roommates” (6.9%), “public property” (4.9%) and “a joint stock company” (3.9%) to explain the formation of a covalent bond. From these creative comparisons, it was found that the prospective teachers accepted a covalent bond to be the sharing of electrons. However, it was not understood whether or not these prospective teachers accepted a covalent bond as the attraction of bonding electrons by the nuclei of covalently bonded atoms in these creative comparisons. Thus, Taber (1998) and Boo (2000) discovered that students accepted a covalent bond as a pair of electrons that were shared. Some of the sentences in this context are the following:“Covalent bond is like marriage because covalent bond is formed by sharing of electrons just like in a marriage where couples share the same house.” PT-55
“Covalent bond is like a common eraser because a covalent bond is formed by sharing of electrons among non-metals. An eraser that's the only one in a row of students is used by all. This way, both sides make up for their deficiencies.” PT-83
“Covalent bond is like a roommate because covalent bond occurs by sharing electrons. Roommates share the same house.” PT-22
Creative comparisons including alternative conceptions about the concept of covalent bonds
Some of the creative comparisons in the category of formation created by the prospective teachers contained alternative conceptions. One of the examples of this is the following statement:“Covalent bond is like the combination of contrast colours because a covalent bond is the force between metal and non-metal atoms. The color black, for instance, is the metal, and its contrast is white, the non-metal. The combination of these colors we can say is the force between the atoms of metals and non-metals.” PT-16
These explanations clearly show that the prospective teacher has confused the concepts of ionic bonds and covalent bonds. This alternative conception was reported as well in studies by Taber, 1997b; Boo, 1998, Tan and Treagust, 1999; Coll and Treagust, 2001a; Nicoll, 2001. Another interesting point is the following explanation:
“Covalent bond is like people with the same opinions coming together because a covalent bond is made up of anions of the same charge. For example, S−2and O−2in SO2.” PT-9
This explanation by this prospective teacher shows that the PT-9 not only did not understand how a covalent bond is formed but that an erroneous association has been formed about anion charges.
Creative comparisons including scientifically correct explanations about the concept of polar covalent bonds
Similar to the covalent bond, the prospective teachers formed creative comparisons including scientifically correct explanations about the formation of a polar covalent bond. One of these creative comparisons is the “magnet” creative comparison. Some prospective teachers used the “magnet” creative comparison to correctly explain the polar structure of a polar covalent bond. One of these explanations is the following:“Polar covalent bond is like a magnet because a magnet has a (+) pole and a (−) pole. There is polarization in polar covalent bond as well. One pole is partially charged (+) and the other is partially charged (−).” PT-44
Another creative comparison in this category is “two siblings with different features” (5.9%) and “a class” (1.9%). These creative comparisons were used to show that polar covalent bonds are formed between different non-metal atoms. One of the statements where these creative comparisons were used is the following:
“Polar covalent bond is like two siblings with different features because a polar covalent bond is formed by different non-metal atoms.” PT-10
The creative comparisons coded as “Other” in the formation category are the following: “different flowers,” “municipalities,” “two students using the same substance in a laboratory,” “a sister and a brother sharing the same room,” “people and animals sharing the environment,” “roommates at a dorm,” “the blood relation between two cousins,” “the teacher–student relationship,” “apartments,” “the poles in mitosis,” “two students sitting on the same bench,” “a necklace made out of black and white pearls,” and “the solidarity between a mother and father.” One of the statements where these creative comparisons are used is the following:
“Polar covalent bond is like a necklace made out of white and black pearls because this necklace has different kinds of pearls but they are still connected to each other and they are in harmony. A polar covalent bond can also bond with different non-metal atoms.” PT-25
In the category of sharing electrons unequally, the creative comparisons “people of the same gender but with different strengths pulling on a rope” (16.8%), “a big and a small dog pulling on the same bone” (2.9%), “a big fish biting off a bigger piece” (1.9%) and “arm-wrestling with different strengths” (1.9%) were statements the prospective teachers created to explain bond polarity on the basis of atom electronegativity. One of the statements where these creative comparisons were used is the following:
“Polar covalent bond is like people of the same gender but with different strengths pulling on a rope because the stronger person will pull the rope harder. In a polar covalent bond, the electronegativity of non-metal atoms is different from each other. The atom with more electronegativity will pull the electrons more toward itself and this way, the part where there are more electrons is negatively charged and the other pole is positively charged.” PT-23
Of the prospective teachers, 11.9% created creative comparisons that were coded as “Other” in the category of sharing electrons unequally. These were the creative comparisons, “competition between the beautiful and the ugly,” “the older sister and the younger sister,” “shareholding,” “the quarrel of a strong and a weak person,” “inequality,” “a scale with more weight on one side,” “two different athletes,” “students with different capacities,” “slyness,” “capitalism,” “a plump and a thin child on a seesaw,” and “civil servants of different levels being paid different salaries.” One of the statements where these creative comparisons were used is the following:
“Polar covalent bond is like civil servants of different levels being paid different salaries because civil servants are paid the different salaries of their own levels. In this bond, too, because the electronegativities of atoms are different, they will attract the electrons they share with different forces.” PT-37
Creative comparisons including some unclear points about the concept of polar covalent bonds
Many prospective teachers emphasized the sharing of electrons in the formation of a polar covalent bond as it was in the covalent bond. With this aim, the creative comparisons of “marriage” (19.8%), “lovers” (3.9%), “two siblings sharing a toy” (1.9%) and “a company partnership” (1.9%) were used. However, it was not understood that whether these prospective teachers considered nucleus–electron interactions in the formation of a polar covalent bond. Some of the statements are the following:“Polar covalent bond is like lovers because couples as individuals have different characters but they share a lot of things. A polar covalent bond is formed when non-metal atoms share electrons.” PT-22
“Polar covalent bond is like a company partnership because different people come together and make use of a common capital stock to establish a company. In other words, they are partners in the company. In this bond, too, different non-metals share electrons in partnership and form a polar covalent bond in this way.” PT-85
Creative comparisons including alternative conceptions about the concept of polar covalent bonds
Although prospective teachers composed some meaningful creative comparisons in the formation category, some of the creative comparisons also carried alternative conceptions. For instance:“Polar covalent bond is like a magnet because a magnet has two opposite poles. One is (+) and the other is (−). Covalent bonds also form between metal and non-metal atoms. Metals are (+), non-metals are (−).” PT-16
From this statement, it can clearly be understood that the prospective teacher has explained the polar covalent bond as if it were an ionic bond. The creative comparison in this category of “the need of two people of opposite sexes for each other” (0.9%) is one of the creative comparisons which show that the prospective teachers were confusing ionic and covalent bonds. The explanation for this creative comparison is as follows:
“Polar covalent bond is like the need of two people of opposite sexes for each other because each gender can fulfill the other's need. The same holds true for metals and non-metals. Non-metals fulfill their electron needs from metals and form polar covalent bonds.” PT-3
Another creative comparison, “identical twins” (0.9%), reveals how polar and non-polar covalent bond concepts have been mixed up. This statement follows:
“Polar covalent bond is like identical twins because all the features of identical twins are alike. The non-metal atoms forming the polar covalent bonds have exactly the same features because this occurs between non-metal atoms.” PT-87
Also, one alternative conception was revealed in the category of sharing electrons unequally. This creative comparison is stated as follows:
“Polar covalent bond is like twins pulling at two ends of a rope because twins will pull and hold the rope in the same strength. In polar covalent bonds, too, atoms pull electrons in the same strength.” PT-98
As can be understood from this statement, the prospective teacher has said that atoms will attract electrons with equal force in a polar covalent bond. This shows that the prospective teacher has confused the concepts of polar and non-polar covalent bonds.
Creative comparisons including scientifically correct explanations about the concept of non-polar covalent bonds
In the formation category, the creative comparisons of “identical twins” (26.7%), a pair of shoes” (2.9%), “an object and its mirror image” (2.9%) and “an asocial person” (1.9%) are creative comparisons that the prospective teachers used to explain that non-polar covalent bond was formed by the same kind of non-metal atoms. One of the statements where these creative comparisons were used is the following:“Non-polar covalent bond is like a pair of shoes because shoes are identically the same. In non-polar covalent bond too, there are the same non-metal atoms.” PT-31
Besides the creative comparisons above, the prospective teachers formed creative comparisons that fell into the “Other” code. These were the creative comparisons “two poor students with no money buying a book together,” “two women of the same character hanging out together,” “a herd of animals,” “a pearl necklace,” “a building made completely out of brick,” “a political party,” “school,” “public property,” “ants helping each other,” and “two neighbors on the same floor using the common corridor light.” One of the statements where these creative comparisons were used is the following:
“Non-polar covalent bond is like a herd of animals because the same type of animals come together to form a herd. A similar situation exists with non-polar covalent bond. It forms in the same type of non-metal atoms.” PT-29
The creative comparisons in the category of sharing electrons equally, when compared with the category of formation, show that the prospective teachers have interpreted the concept in light of electronegativity and have produced more scientific explanations The creative comparisons in this category are the following: “two people with equal strength pulling a rope” (16.8%), “two children of equal weight on a seesaw” (2.9%), “not being able to win an arm-wrestling contest” (1.9%), equal rights (1.9%), “civil servants at the same level getting the same salary” (1.9%), “a roly-poly doll” (0.9%), and “a match ending in a tie” (0.9%). The creative comparison that stands out the most in this category has a 16.8% ratio and refers to “two people with equal strength pulling a rope.” Creative comparisons resembling this one, in particular, are also found in high school textbooks. It may be for this reason that prospective teachers have been able to create this creative comparison more easily. One of the statements the prospective teachers use to explain the creative comparisons in this category is the following:
“Non-polar covalent bond is like two people with equal strength pulling a rope because even though both sides will be pulling the rope, the rope won't budge to either side; both sides will be pulling with the same amount of force. In non-polar covalent bond, because there are the same kinds of non-metal atoms, both of the atoms will attract the shared electrons with the same amount of force; their electronegativities are the same.” PT-42
Creative comparisons including some unclear points about the concept of non-polar covalent bonds
The creative comparisons, “two friends of the same gender sharing the same house” (10.9%) and “a family company” (4.9%) were used by the prospective teachers to explain that non-polar covalent bonds were formed when the same kind of non-metal atoms shared electrons. Some the prospective teachers used the creative comparisons, “two students sitting on the same bench” (4.9%) and “a sacrificial animal bought together” (4.9%), however, these prospective teachers mention that non-polar covalent bonds are formed only when atoms share electrons. In other words, it was determined that the prospective teachers exhibited no knowledge about nucleus–electron interactions in the formation of non-polar covalent bonds. Some of the statements where these creative comparisons were used are the following:“Non-polar covalent bond is like a family company because in non-polar covalent bond, the same types of non-metal atoms form the bond by sharing electrons. The same thing holds true in a family company because members of the same family pool their resources and form the organization.” PT-22
“Non-polar covalent bond is like two students sitting on the same bench because the two students use the bench together. This bond too is about sharing electrons together.” PT-4
Creative comparisons including alternative conceptions about the concept of non-polar covalent bonds
It was also found from these creative comparisons that the prospective teachers had alternative conceptions as well. Some examples of the statements in this context are the following:“Non-polar covalent bond is like the people in the world because all the people in the world have different characteristics but yet they stay together. The non-metal atoms forming this bond also have very different characteristics but they still form the bond.” PT-13
“Non-polar covalent bond is like two different people holding a rope on both ends because the non-metal atoms forming the bond are different.” PT-98
From these explanations it can be seen that the prospective teachers have confused non-polar covalent bonds with polar covalent bonds. Similar results have been reported in the literature in various studies (Nicoll, 2001; Ünal, 2007). The fact that the words polar and non-polar derive from English or perhaps the inability to fully understand the concept of electronegativity may have caused these alternative conceptions (Ünal, 2007).
Creative comparisons including scientifically correct explanations about the concept of hydrogen bonds
The prospective teachers produced creative comparisons that included scientifically correct explanations about the hydrogen bond in the categories of strength and physical properties.One of the scientifically correct explanations given by the prospective teachers was the one offered by PT-100. This prospective teacher mentions the strength of the hydrogen bond and then goes on to talk of surface tension. This prospective teacher's explanation is the following:
“Hydrogen bond is like a beam or column because in order for a building to be strong, the beams and columns have to be built on a solid structure. In the same way, hydrogen bond causes the increasing in the surface tension. As a result, needles and similar objects remain on the surface of liquids and don't sink. Just like the durability of a building. The beams and columns in this example are like a hydrogen bond.” PT-100
Also, creative comparisons including scientifically correct explanations coded as “Other” exist in the category of strength. These creative comparisons include “a sailor's knot,” “handcuffs,” “love,” “ropes,” “articulated lorry”, “boxers,” “thin people,” and “weak human relations.” One of the explanations given by the prospective teachers is the following:
“Hydrogen bond is like a sailor's knot because sailor's knots are stronger than other knots; a hydrogen bond is the strongest of the intermolecular forces.” PT-71
Some important scientific explanations about hydrogen bonds fit into the physical properties category. A review of the creative comparisons in this category reveals that the prospective teachers chose these types of creative comparisons to explain the effect of hydrogen bonds on boiling points, viscosity and other properties. One of creative comparisons is the following:
“Hydrogen bond is like a person who's patient because a patient person has more strength to cope with problems. The boiling points of molecules that contain a hydrogen bond are also higher than those without a hydrogen bond.” PT-85
Creative comparisons including some unclear points about the concept of hydrogen bonds
From the prospective teachers' creative comparisons, it was determined that there were some unclear points about the formation and strength of hydrogen bonds. Particularly, the creative comparisons in the formation category of “the selective person” (11.9%), “the family” (7.9%), “the selective permeant” (6.9%), “an obsessed person” (5.9%) the enzyme–substrate relationship” (3.9%) and the “cell membrane” (3.9%) did not clearly define whether the prospective teachers accepted a hydrogen bond as an intramolecular or as an intermolecular interaction. Some of the statements where these creative comparisons were used were the following:“Hydrogen bond is like a selective person because the hydrogen bond can't be with just any atom; the elements that it bonds with are limited. It occurs H between F, O, N atoms. A selective person doesn't become friends with everyone.” PT-49
“Hydrogen bond is like an obsessed person because people with obsessions think of only one thing, they concentrate on that; for hydrogen bond to occur, the hydrogen atom can only bond with an atom in the F, O or N group. In other words, it has become obsessed with F, O or N.” PT-4
“Hydrogen bond is like a selective permeant because hydrogen bond occurs when hydrogen bonds with F, O or N atoms.” PT-25
“A hydrogen bond is like a cell membrane because it won't work with any atom; it's a kind of selective permeant because the cell membrane has the property of selectivity.” PT-81
It can be seen from the explanations above that the prospective teachers stated the conditions under which a hydrogen bond occurred, but it was not clearly understood that the prospective teachers accepted the hydrogen bond as an intermolecular interaction. In particular, the explanations like a hydrogen bond occurs when the H atom binds to F, O or N atoms may stem from the fact that the prospective teachers have accepted that the hydrogen bond is an intramolecular bond. To clarify this, the prospective teachers were asked during the semi-structured interviews to point out the molecules that bind together in the hydrogen bond. Indeed, various studies in the literature have reported that a hydrogen bond is perceived as an intramolecular bond (Peterson et al., 1989; Peterson and Treagust, 1989; Nicoll, 2001; Ünal et al., 2002; Ünal, 2003). Possible reasons for this alternative conception in the students' minds may be that they were unable to differentiate between intramolecular and intermolecular forces, that their textbooks categorized intermolecular forces under the heading “Other Types of Bonds” and that students may have gotten the concepts of atoms and molecules confused.
Similar to the creative comparisons above, there are some unclear points in the creative comparison in the category of formation that belong to the “Other” code (12.9%). These are the following creative comparisons: “finding your mother from among the crowd,” “inmates in a prison,” “a child who's a picky eater,” “inventions,” “chatting,” “relations with friends,” “an adolescent hanging out with certain people,” “relations between a mother and her three children,” “a person with a monotonous life,” “group work with close friends,” “gangs,” “babies and baby-food,” and “the person with the most powers in a company.” One of the statements where these creative comparisons were used is the following:
“Hydrogen bond is like inmates in a prison because these people communicate with a limited number of people. A hydrogen bond can occur among hydrogen, F, O and N atoms, in other words, among a limited number of atoms.” PT-82
The other important findings were revealed in the strength category. Particularly, the creative comparisons of “friendship” (6.9%), “Hercules” (4.9%), “a rope” (4.9%), “steel (2.9%), and “a beam or column “(2.9%) stand out in the strength category. Some of the expressions in which these creative comparisons were used are the following:
“Hydrogen bond is like Hercules because Hercules is a very strong hero figure. A hydrogen bond is also much stronger than others.” PT-84
“Hydrogen bond is like a rope because a rope is very strong and won't break easily. A hydrogen bond is strong too and it can't be broken up easily.” PT-55
“Hydrogen bond is like steel because steel is very strong. Hydrogen bonds are also very strong.” PT-83
It can be clearly seen from a review of these creative comparisons and explanations that the prospective teachers perceived a hydrogen bond as strong. However, while qualifying a hydrogen bond as strong, it is not clear how they came to this conclusion whether they believe that a hydrogen bond is the strongest of the intermolecular forces or that there is a strong intramolecular as well as intermolecular force. To clear this up, the prospective teachers were asked in the interview to give examples of intra- and intermolecular forces and compare and explain these forces.
Creative comparisons including alternative conceptions about the concept of hydrogen bonds
The prospective teachers' creative comparisons indicated that they had some alternative conceptions about hydrogen bonds. For example, the creative comparisons of “the bond between twins” (3.9%), “some kind of article that people share” (0.9%), and “a social person” (0.9%) in the formation categories were significant in that they uncovered the alternative conceptions that the prospective teachers had. One of the statements where these creative comparisons were used is the following:“Hydrogen bond is like the bond between twins because each wears similar things, they are the same. A hydrogen bond, as in (H2), can only occur between two identical atoms.” PT-31
As can be seen from the explanation above, the prospective teachers used creative comparison of the bond between twins to express their perception of a hydrogen bond as a bond occurring between hydrogen atoms inside a molecule. One of the most important reasons for this may have been the way the concept of hydrogen bonds is expressed. Many students accept a hydrogen bond as a chemical bond because the expression contains the word “hydrogen;” they think it is some kind of chemical bond between the atoms of hydrogen. This finding is consistent with similar results obtained in the study in the literature (Ünal, 2003). Another explanation includes:
“Hydrogen bond is like some kind of article that people share because a hydrogen bond is a covalent bond, and it occurs as a result of sharing of electrons.” PT-12
The explanation of PT-12 above indicates that the prospective teacher has completely misunderstood the concept of hydrogen bond and has qualified the hydrogen bond as a covalent bond. The findings of Ünal (2003) are similar.
Besides, some prospective teachers had alternative conceptions about the strength of hydrogen bonds. Examples of these explanations are the following:
“Hydrogen bond is like a thick branch because it is hard to break. A hydrogen bond is just like that. Hydrogen bond is stronger than ionic and covalent bonds.” PT-2
“Hydrogen bond is like the strongest individual in a society because the hydrogen bond is the strongest of all bonds.” PT-52
“Hydrogen bond is like the roots of a tree digging into the ground because tree roots hang on very strongly to the earth and are very difficult to pull up. A hydrogen bond is the strongest of all bonds.” PT-77
“Hydrogen bond is like wrestlers because wrestlers are stronger than athletes in other branches of sports. A hydrogen bond too is the strongest of the all bonds.” PT-22
As can be seen from these explanations, the prospective teachers interpreted the concept as “the hydrogen bond is the strongest of the intermolecular forces” and qualified the hydrogen bond as being even stronger than intramolecular bonds. Similar findings have been reported in different studies in the literature (Nicoll, 2001; Ünal, 2003, 2009).
Creative comparisons including scientifically correct explanations about the concept of van der Waals force
The prospective teachers gave scientifically correct explanations about van der Waals force in four categories: “strength”, “formation”, “molecules were affected by van der Waals force”, and “physical properties”. In the strength category, the creative comparisons of “a feather” (15.8%), “a rotten rope” (9.9%), “cotton” (8.9%), “a house of cards” (6.9%), “ a rotten piece of wood” (5.9%), “a rotten building” (4.9%), “bones of an elderly person” (1.9%), and “two casual classmates” (1.9%) showed that the prospective teachers were able to make a correct and scientific comparison of the van der Waals force and other forces. Some statements that exhibited examples of these creative comparisons are the following:“The van der Waals force is like a rotten rope because a rotten rope is very weak and can break easily. The van der Waals force is weaker compared to the other intermolecular forces and it is easier to break.” PT-55
“The van der Waals force is like cotton because cotton can easily break by using only slight force. The van der Waals force is also very easily broken because it's weak.” PT-83
“The van der Waals force is like the bones of an elderly person because the bones of an elderly person can very easily break. The van der Waals force isn't strong either, it will easily break.” PT-12
In addition, creative comparisons including scientifically correct explanations in the “Other” code exist in the strength category. These creative comparisons include “a travel companion,” “the weakest member of the household,” “newborn babies,” “pencil tip,” “a weak person,” “a mobile phone line,” “music turned down low,” “nationalism,” “two friends who haven't seen each other for many years,” “dough,” “a distant relative,” “a strand of hair,” “the fans of some football teams,” “schoolmates,” “two rods tied together with a weak string,” “a family having weak relations with other relatives,” “a little child who is surrounded by only adults,” “people who are weak in their personal relations,” and “breaking a piece of chalk.” One of the statements where these creative comparisons were used is the following:
“The van der Waals force is like the weakest member of the household because this, meaning the hydrogen bond and dipole–dipole force, is the weakest of the intermolecular forces.” PT-25
The prospective teachers explained the formation of the van der Waals force in detail by using creative comparison of “the feeling of liking someone momentarily” (3.9%). In this creative comparison, it was stated that the van der Waals force occurs when molecules or noble gases get close to each other and the symmetry of the electron cloud is distorted and momentary dipoles are produced. An example of the statement related to this is the following:
“The van der Waals force is like the feeling of liking someone momentarily because a woman and a man may see each other and feel a sudden attraction between them. A similar situation exists in the van der Waals force. As molecules or noble gases get close to each other, when electrons are more on one side, momentary dipoles, in other words, + and − charges, will be produced. These momentary charges will affect the other molecules as well. The attraction between momentary charges is the van der Waals force.” PT-81
In the category of “molecules were affected by van der Waals force”, the prospective teachers explained that the van der Waals force has an effect on all kinds of molecules, both polar and non-polar. The creative comparisons include “blood vessels” (1.9%), “energy” (1.9%), “jealousy” (0.9%), “the ability to talk” (0.9%), and mobile phones (0.9%). Some of the statements where these creative comparisons were used are the following:
“The van der Waals force is like blood vessels because even though people are very different from each other (in the context of weight, height and other factors), everybody has blood vessels. Polar and non-polar molecules have van der Waals force.” PT-4
“The van der Waals force is like the ability to talk because everybody has it but some don't have anything else. Some decorate it with intelligence and creativity, that is, they have other capabilities. A similar situation exists in molecules. There is only van der Waals force between non-polar molecules. Polar molecules, however, have dipole–dipole force beside the van der Waals force. Depending on the condition, they may also have hydrogen bonds.” PT-18
There are some striking creative comparisons about the van der Waals force that fit into the creative comparison category of “physical qualities.” These creative comparisons are the following: “a flying balloon” (0.9%), “imagination” (0.9%), and “a fat person's taking up more space than a thin person” (0.9%). These creative comparisons of the prospective teachers in particular were found to explain the effect of molecular masses on the van der Waals force, and the effect of the van der Waals force on the boiling point. One of the creative comparisons in this category is the following:
“van der Waals forces are like the imagination because as the imagination develops, a person's brain power also grows. As the van der Waals forces increase, the boiling point of molecules will rise.” PT-85
Creative comparisons including some unclear points about the concept of van der Waals force
In the category of “molecules were affected by van der Waals force,” PT-13 used the creative comparison of “gravity.” This creative comparison and its explanation are presented as follows:“The van der Waals force is like gravity because gravity is a force affecting all objects. The van der Waals force similarly affects all atoms.” PT-13
From the explanation above, it can be seen that the prospective teachers perceive the van der Waals force as a force that affects atoms. This explanation, however, does not clearly indicate whether what is meant is the force of attraction that holds inert atoms together in a solid or liquid or the attraction between atoms in non-polar and polar molecules. Indeed, various studies in the literature report the alternative conception that the van der Waals force exists between atoms in non-polar molecules (Ünal, 2003, 2007). To clarify this situation, the prospective teachers were asked about the van der Waals force in their interviews.
Creative comparisons including alternative conceptions about the concept of van der Waals force
The creative comparison created by the prospective teachers, and the explanations given, showed that there were some alternative conceptions about the strength of the van der Waals force. Some of the examples of these explanations are the following:“The van der Waals force is like handcuffs because handcuffs can break only with difficulty. Similarly, it's very difficult to break this force.” PT-22
“The van der Waals force is like a magnet because force of magnet is very powerful. van der Waals is a very powerful force.” PT-31
As can be seen from the explanations above, the prospective teachers described the van der Waals force as very powerful. The reason the prospective teachers had this alternative conception was perhaps because they had not fully understood the concept of intermolecular attraction. In many studies in the literature, it is reported that students have a weak understanding of intermolecular attraction (Treagust, 1988; Peterson and Treagust, 1989; Peterson et al., 1989; Tan and Treagust, 1999, Ünal, 2003, 2007).
Also, the prospective teachers had alternative conceptions in the category of “molecules were affected by van der Waals force.” Creative comparisons in the category of “two similar people agreeing with each other” (1.9%) and “a party that identical twins come to” (1.9%) are significant in that they show the alternative conceptions that the prospective teachers seem to have. The statement is as follows:
“The van der Waals force is like two similar people agreeing with each other because people who are similar attract each other and agree. The van der Waals force is the force that is created between two non-metal atoms.” PT-79
This statement shows that the prospective teacher believes that the van der Waals force is a force between atoms. This alternative conception, as mentioned before, may derive from the fact that the prospective teachers have not fully understood intramolecular and intermolecular forces. This finding has also been reported in various studies in the literature (Ünal, 2003, 2007).
Creative comparisons including scientifically correct explanations about the concept of dipole–dipole force
The creative comparisons formed by the prospective teachers, with respect to dipole–dipole force, fall into two categories: “formation” and “force”. The prospective teachers produced creative comparisons including scientifically correct explanations in these categories. The creative comparisons of “a magnet” (23.8%) in the formation category, as well as “people who are diametrically opposite to each other” (10.9%) and “lightning” (2.9%) are creative comparisons that were used to describe the dipole–dipole force between opposite poles of polar molecules. Some of the statements where these creative comparisons were used are the following:“Dipole–dipole force is like a magnet because the (+) and (−) poles of a magnet attract each other when they are brought near to each other. A similar situation is seen between polar molecules. Because the attraction occurs between the partial positive side of polar molecules and partial negative side of another polar molecules. This attraction is called as dipole–dipole force.” PT-55
“Dipole–dipole force is like people with different strengths pulling on a rope because the people on the ends of the rope with different strengths represent the molecules' (+) and (−) poles. In polar molecules, the atoms that have more electronegativity are negatively charged and the other atoms are charged positively. The attraction between the negative side of the polar molecule and the positively charged part of the other molecule constitutes the dipole–dipole force. Dipole–dipole force is between molecules.” PT-46
Similar to other concepts, some of the creative comparisons in the formation category were coded as “Other” (9.9%). The creative comparisons contained in this code are the following: “selective force,” “grounding,” “tractors trying to pull each other,” “relations between two people,” “the cement between two bricks,” “two lovers hugging each other,” “two stubborn goats quarrelling,” “two people who have missed each other very much running to each other,” “love between people of the same gender,” and “a charged glass rod picking up pieces of paper.” One of the statements where these creative comparisons were used is the following:
“Dipole–dipole force is like two stubborn goats quarrelling because goats will oppose each other but they'll still attract each other. Dipole–dipole force too occurs between the positive and negative poles of neighboring polar molecules.” PT-24
At the same time, some creative comparisons were used correctly to compare the other intermolecular forces and dipole–dipole forces. These creative comparisons were: “medium-sweet Turkish coffee” (6.9%) and “a person of medium strength” (6.9%). One of the statements where these creative comparisons were used is the following:
“Dipole–dipole force is like medium-sweet Turkish coffee because medium-sweet coffee is neither sweet nor unsweetened. It's in-between. Dipole–dipole force is the same way; it's a medium-strong attraction. In other words, it's weaker than a hydrogen bond but stronger than van der Waals force.” PT-100
As in the formation category, the strength category too has creative comparisons coded as “Other.” These creative comparisons are the following: “hairs on the body,” “a sick person,” “wavy hair,” “neighborly bond,” “plastic,” “a laundry rope,” “a two-year-old child,” “being second in the class,” “a middle-level civil servant,” “being cousins,” “an adolescent,” and “10th wedding anniversary.” Some of the statements where these creative comparisons were used are the following:
“Dipole–dipole force is like being second in class because it's a position that's between first and third. The dipole–dipole force is a force that is weaker than the hydrogen bond in intermolecular forces and it's stronger than van der Waals force. In other words, it's second in terms of force.” PT-25
“Dipole–dipole force is like a neighborly bond because neighbors form bonds too but it's not as strong as the bonds inside the family. Because dipole–dipole force is an intermolecular bond, it's not as strong as inside the molecule.” PT-85
Creative comparisons including some unclear points about the concept of dipole–dipole force
In the strength category, creative comparisons of “strong person” (3.9%) and “a vacuum cleaner's picking up dust” (2.9%) were striking. Although the explanations for the creative comparisons “strong person” and “a vacuum cleaner's picking up dust” are correct, the creative comparisons themselves may lead to misunderstandings. For instance:“Dipole–dipole force is like a strong person because dipole–dipole force is strong force. And it is stronger than van der Waals force.” PT-59
“Dipole–dipole force is like a vacuum cleaner's picking up dust because just like the vacuum cleaner, this force is a strong force. When compared with van der Waals force, dipole–dipole force is strong too.” PT-65
In both statements above, dipole–dipole force has been correctly expressed as being stronger than the van der Waals force. However, when the creative comparisons are carefully read, it can be seen that the prospective teachers have characterized dipole–dipole attraction as a strong force. This may be an indication that the prospective teachers perceived dipole–dipole attraction to be a force that is stronger than intramolecular bonds.
Creative comparisons including alternative conceptions about the concept of dipole–dipole force
In the formation category, the creative comparison of “people of different strengths pulling a rope” was striking since some prospective teachers explained the formation of dipole–dipole force scientifically by using this creative comparison; however, some of them interpreted it differently. For instance:“Dipole–dipole force is like people with different strengths pulling on a rope because somebody who is weak can't pull on the rope very much while those who are strong can pull it toward themselves. Similarly, dipole–dipole force occurs between atoms with different electronegativity, and the atom with more electronegativity pulls electrons toward itself.” PT-83
From the statements above, it can be seen that the prospective teachers accepted dipole–dipole force as a force between atoms. This belief of the prospective teachers may stem from the fact that they had not completely understood the concept of polar covalent bonds. At the same time, the following creative comparisons in the formation category show that the prospective teachers' conceptions are not consistent with scientific knowledge: “two similar people attracting each other” (2.9%), “a bag of lentils” (0.9%), “a married couple sharing a blanket” (0.9), “one nation becoming another nation's dominion” (0.9%), “two balls of the same kind sticking to each other” (0.9%), “gravity” (0.9%) and “the bond between people of the same city.” Some of the statements where these creative comparisons were used are the following:
“Dipole–dipole force is like two similar people attracting each other because people who are similar are affected by each other and there is interaction between them. This leads to their being together. Dipole–dipole force too occurs between non-polar molecules.” PT-31
“Dipole–dipole force is like two balls of the same kind sticking to each other because dipole–dipole force occurs between atoms and these attract each other.” PT-76
From the statement of prospective teacher 31, it can be seen that the prospective teacher explained dipole–dipole forces as intermolecular forces; however, they believed that this force is effective between non-polar molecules. Another prospective teacher (PT-76) stated that dipole–dipole force is an interaction between the atoms in a molecule.
At the same time, creative comparisons including alternative conceptions in the strength category were determined. These creative comparisons include “the largest root of a tree” (0.9%), “a hurricane” (0.9%), “a stone” (0.9%), and “the love between Romeo and Juliet” (0.9%). The statements containing the creative comparisons are as follows:
“Dipole–dipole force is like the largest root of a tree because it is the more enduring bond compared to ionic and covalent bonds.” PT-70
“Dipole–dipole force is like a hurricane because it's very strong. This force of attraction is stronger than all the other bonds.” PT-93
Results from semi-structured interviews
Prospective chemistry teachers' responses in each category for the first interview question are shown in Table 6.| Category | High level | Middle level | Low level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT 49 | PT 55 | PT 81 | PT 85 | PT 4 | PT 18 | PT 25 | PT 84 | PT 12 | PT 22 | PT 31 | PT 83 | ||
| SU | I. | ||||||||||||
| II. | |||||||||||||
| III. | ✓ | ✓ | |||||||||||
| PU | I. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| II. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| III. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| PUAC | I. | ✓ | |||||||||||
| II. | |||||||||||||
| III. | |||||||||||||
| AC | I. | ✓ | |||||||||||
| II. | ✓ | ||||||||||||
| III. | ✓ | ||||||||||||
| NR | I. | ||||||||||||
| II. | ✓ | ||||||||||||
| III. | |||||||||||||
Question 1.
I. CH3OCH3
II. KNO3
III. MgCl2
What type of intramolecular forces is formed in the given compounds? Please explain how intramolecular forces are formed in the compounds?
Question 1 aims to determine whether or not prospective teachers could predict the type of intramolecular forces within these compounds. In addition, this question investigates prospective teachers' ideas about how covalent and ionic bonds are formed.
As can be seen in Table 6, none of prospective teachers' responses about intramolecular forces in dimethyl ether were classified in the category of sound understanding. Mostly, prospective teachers explained that a covalent bond is formed by sharing of pairs of electrons between the atoms (C–H, C–O) within dimethyl ether, however, they couldn't explain that a covalent bond is the attraction of bonding electrons by the nuclei of covalently bonded atoms. As a result of this, most of the prospective teachers' answers were classified in the category of partial understanding. This result was not surprising because prospective teachers' creative comparisons about a covalent bond did not state that a covalent bond was the attraction of the bonding electrons by the nuclei of atoms. For example, PT 55 as a high level prospective teacher just explained sharing of electrons in a covalent bond in her “marriage” creative comparison. A similar result was observed in the interview with her. A sample quotation of the prospective teacher's responses in the interview is as follows:
PT 55: covalent bonds are formed between carbon–hydrogen and carbon–oxygen.
Researcher: How is a covalent bond formed between the carbon–hydrogen and carbon–oxygen in dimethyl ether?
PT 55: carbon and hydrogen atoms share their single electrons to have more stable configuration. As a result, they have full outer shell and obtain octet and doublet structures. Carbon obtains octet structure, hydrogen obtains doublet structures.
Researcher: Okay. When carbon–hydrogen and carbon–oxygen atoms share their single electrons, how is a covalent bond formed? Could you explain your answer?
PT 55: These atoms are non-metals. They want to have a more stable configuration. For this reason, they share their single electrons with each other. Consequently, these atoms bond together with covalent bond.
Researcher: Okay. Is there any force to hold atoms together?
PT 55: I do not know.
These responses showed that the prospective teachers preferred to explain the formation of covalent bonds using the octet rule. Interestingly, the prospective teacher could not associate a covalent bond with the electrostatic force between the nucleus and shared electrons of the two atoms. At the same time, some studies indicated that students tended to retain and use the octet rule even after they had studied bond energies and thermodynamic considerations on more advanced courses (Taber, 2002). Wang and Barrow (2013) stated that once the octet rule is perceived by a student as the main explanation for why atoms share or transfer electrons to form chemical bonds, it is difficult for students to reconcile it with later learned scientific explanations.
Another important finding belongs to PT 22 as a low level prospective teacher. The prospective teacher produced “roommate” creative comparison to explain the formation of a covalent bond. In this creative comparison, the prospective teacher accepted that a covalent bond resulted from the sharing of electrons. In addition, findings from the interview indicated that the prospective teacher could not explain a covalent bond by the nucleus–electron interactions and he described the covalent bond as a pair of shared electrons. For this reason, the prospective teacher's answers were classified in the category of partial understanding with an alternative conception. A sample quotation of the prospective teacher's responses in the interview is as follows:
PT 22: All atoms in the dimethyl ether are non-metals. If we examine atoms one by one, bonds between carbon and hydrogen atoms are covalent bonds. Similarly, bonds between carbon and oxygen atoms are covalent bonds.
Researcher: How is a covalent bond formed between the carbon–hydrogen and carbon–oxygen in dimethyl ether?
PT 22: Carbon, and oxygen atoms want to complete their octet. Also, hydrogen wants to complete its doublet. For this reason, they share electrons to complete their octet and doublet. Thus, a covalent bond is formed. A covalent bond is a pair of shared electrons.
Researcher: Could you show me the covalent bonds in dimethyl ether? And could you explain in more detail the formation of covalent bonds?
PT 22: Yes. carbon–hydrogen and carbon–oxygen bonds are covalent bonds because these bonds are single bonds. For example, carbon–hydrogen atoms share a pair of electrons to complete their octet. So, a pair of shared electrons forms a single bond. This single bond is covalent bond.
From the prospective teacher's explanations, it was seen that prospective teacher could not understand exactly the nature of covalent bond and was not aware of nucleus–electron interactions. One of the reasons for this may be the line-bond structures of the compounds. In the line-bond structures, a single covalent bond is where one pair of shared electrons is represented by a line drawn between atoms. In addition, Boo (2000) stated that some statements found in textbooks such as “one pair of shared electrons constitute a single covalent,” and “two pairs of shared electrons constitute a double bond” may cause students to consider the sharing of electrons as the ‘force’ instead of nucleus–electron interactions.
A different result was revealed from PT 83's creative comparison and interview as a low level prospective teacher. The prospective teacher emphasized sharing of electrons in the covalent bond by using “a common eraser” as a creative comparison. On the other hand, the same prospective teacher confused inter- and intramolecular forces in the interview. This result indicated that the prospective teacher knew the meaning of a covalent bond but was not able to apply this knowledge on the compounds. Her responses to the interview are as follows:
PT 83: Firstly, there is van der Waals force in the molecule. Hydrogen bond does not occur in this molecule because this compound does not have hydrogen atom attached to oxygen atom. For this reason, only van der Waals force occurs.
Researcher: Do you mean that van der Waals force is intramolecular force?
PT 83: Yes.
Researcher: Okay. How is van der Waals force formed in dimethyl ether?
PT 83: This molecule is non-polar. In other words, dipole moment is zero for this molecule. As a result, only van der Waals force is formed.
Researcher: How do you understand that dipole moment is zero for this molecule? Could you show this?
PT 83: Two methyl groups are attached to oxygen atom in the molecule. So, direction of dipole moment points toward a carbon atom and the net dipole moment is zero. For this reason, dimethyl ether is non-polar (Fig. 1).
These answers indicated that PT 83 had difficulties in determining the polarity of the molecule. Thus, she did not consider the electronegativity of atoms, and drew incorrectly the direction of dipole moment. As a result, she accepted dimethyl ether as a non-polar molecule. Also, she held an alternative conception, thinking that van der Waals force is an intramolecular force. Linguistic similarity of the terms (intermolecular and intramolecular forces) may be a cause of this alternative conception (Ünal et al., 2006)
Similar to dimethyl ether, the prospective teachers did not give responses that fell within the “sound understanding category” to explain intramolecular forces in potassium nitrate. The prospective teachers' responses usually fell into the partial understanding category. In this category, a larger proportion of the prospective teachers emphasized that an ionic bond was formed only as a result of electron transfer in potassium nitrate. While the prospective teachers stated that potassium and nitrate ions occurred through electron transfer, they did not mention an electrostatic attraction between these positively charged and negatively charged ions. In addition, the prospective teachers could not explain covalent bonds in the nitrate ion. Only, two prospective teachers (PT 81 and PT 85) explained that both ionic and covalent bonds occurred in potassium nitrate. Also, these prospective teachers expressed that electrostatic attraction existed between potassium and nitrate ions. On the other hand, they could not give satisfactory explanations about the formation of covalent bonds in the nitrate ion. This result showed that the prospective teachers believed that ionic bonds were electrostatic in nature not the covalent bond. Boo (2000) reported that the textbooks merely mentioned that a covalent bond is electrostatic in nature, as a result of this, many students believed that only an ionic bond is electrostatic in nature. Besides, when the prospective teachers' creative comparisons were analyzed, it was seen clearly that the prospective teachers did not produce any creative comparisons to explain nucleus–electron interactions in the formation of covalent bonds. However, they produced creative comparisons such as “magnet” and “women–men” to explain electrostatic attraction in the formation of ionic bonds. For example, PT 85, as a high level prospective teacher, explained an electrostatic attraction between the oppositely charged ions in ionic bonds by using “magnet” creative comparison. The same prospective teacher only mentioned sharing of electrons for covalent bonds through “joint stock company” creative comparison. A sample quotation of the PT 85's responses in the interview is as follows:
Researcher: What type of intramolecular forces is formed in potassium nitrate?
PT 85: Ionic bond is formed between potassium and nitrate ions.
Researcher: How is ionic bond formed between potassium and nitrate ions?
PT 85: As a result of electron transfer, potassium and nitrate ions occur. These ions are oppositely charged. So, electrostatic attraction was formed between these oppositely charged ions. This attraction is ionic bond.
Researcher: Is there only ionic bond in potassium nitrate?
PT 85: No. Covalent bond is formed between nitrogen and oxygen atoms in nitrate ion.
Researcher: How is covalent bond formed in nitrate ion?
PT 85: Nitrogen and oxygen atoms share their electrons with each other to complete their octets. Consequently, covalent bond is formed.
As another example, PT 25, who was one of the middle level prospective teachers, produced “love” and “a country and its people” creative comparisons for ionic and covalent bonds. The prospective teacher's creative comparisons revealed that she could not understand exactly the electrostatic nature of ionic and covalent bonds, which was consistent with her expressions during the interviews. A sample quotation of the PT25's responses in the interview is as follows:
PT 25: Ionic bond.
Researcher: How is ionic bond formed in potassium nitrate?
PT 25: Potassium and nitrate ions are oppositely charged. Potassium ion is positive, nitrate ion is negative. ionic bond is formed between potassium and nitrate ions.
Researcher: You said that ionic bond is formed between potassium and nitrate ions. So, how are potassium and nitrate ions formed?
PT 25: With the electron transfer. One electron is transferred from the potassium to the nitrate group. As a result, potassium cation and nitrate anion occurs. This causes the formation of ionic bond.
Researcher: Is there another intramolecular force in potassium nitrate?
PT 25: No.
As seen in Table 6, one prospective teacher's (PT 83) responded in the category of alternative conceptions for this interview question. PT 83 used “trading” as a creative comparison to explain electron transfer in an ionic bond. But, she explained the bond between potassium and nitrate ions as dipole–dipole attractions in the interview. Her responses to the interview are as follows:
PT 83: Dipole–dipole attractions.
Researcher: How are dipole–dipole attractions formed in potassium nitrate?
PT 83: Nitrate ion is negative, potassium ion is positive. As a result of attraction between the oppositely charged ions, dipole–dipole attractions occurs.
Researcher: Could you draw the dipole–dipole attractions in potassium nitrate?
PT 83: It should be like this (Fig. 2).
Table 6 shows that most of the prospective teachers gave responses in the category of partial understanding to explain intramolecular forces in magnesium chloride. Similar to potassium nitrate, only two prospective teachers (PT 81 and PT 85) stated that an ionic bond in magnesium chloride was formed by the means of the attraction due to the opposite charge. This result was identified in their creative comparisons about ionic bonds. Similar to PT 85, PT 81 explained electrostatic attraction between oppositely charged ions in the formation of an ionic bond by using “men and women” creative comparison. Thus, the findings from the interview supported this creative comparison. PT 81's explanations in the interview are as follows:
Researcher: What type of intramolecular forces is formed in Magnesium chloride?
PT 81: It is ionic bond.
Researcher: Could you explain this in more detail?
PT 81: Ionic bond is formed between the magnesium and chloride ions in MgCl2. Magnesium atom transfers two electrons to chlorine atoms. Consequently, oppositely charged ions occur. As a result of the electrostatic force of attraction between the oppositely charged ions, ionic bond occurs.
From Table 6, it was understood that prospective teachers from a high level to a low level mostly gave responses that fell into the partial understanding category. In these responses, the prospective teachers explained an ionic bond based on the transfer of electrons, however, they did not mention the attractions of the ions. For instance, PT 49, who is one of the high level prospective teachers, produced “a friendship between a miser and a generous person” creative comparison for an ionic bond. From this creative comparison, it was understood that this prospective teacher explained the formation of an ionic bond by considering the transfer of electrons. A similar result was identified in the interview. His responses to the interview are as follows
PT 49: Ionic bond occurs.
Researcher: How do you understand the formation of ionic bond?
PT 49: I understand because magnesium is a metal, chloride is a non-metal. This means that magnesium loses two electrons as a result, cation occurs. Chlorine atoms each gain an electron. In this way, anions occur. This causes the formation of ionic bond.
One prospective teacher (PT 83) gave responses with an alternative conception in this question. This prospective teacher explained dipole–dipole attractions between magnesium and chlorine atoms like potassium nitrate. Her responses are as follows:
PT 83: Dipole–dipole attractions occurs.
Researcher: How are dipole–dipole attractions formed in magnesium chloride?
PT 83: Magnesium ion is positive, chlorine ion is negative. Attraction between the oppositely charged ions causes dipole–dipole (Fig. 3).
Question 2.
I. C2H4 (OH)2
II. CH4
III. HCl
What types of intermolecular forces are formed in the given compounds? Please explain how intermolecular forces are formed in the compounds?
Question 2 was prepared to reveal prospective teachers' understanding regarding intermolecular forces. It was seen from Table 7 that more prospective teachers in high and middle levels gave responses that fell into the category of sound understanding comparing question 1. On the other hand, only one prospective teacher's (PT 81) responses about 1,2-ethanediol were classified in the category of sound understanding. PT 81 explained hydrogen bonds in the compound considering nonbonding electron pairs. She stated that a hydrogen bond is an attraction between a hydrogen atom bonded to strong electronegative atoms such as F, O, and N and nonbonding electron pairs on the other electronegative atom. Actually, when her creative comparison about a hydrogen bond (a cell membrane) was analyzed, it was revealed that she understood that some conditions were necessary for hydrogen bonding to occur. However, it was not clear which conditions were necessary in this creative comparison. This uncertainty was removed with the interview. A sample quotation of the PT 81's responses in the interview is as follows:
| Category | High level | Middle level | Low level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT 49 | PT 55 | PT 81 | PT 85 | PT 4 | PT 18 | PT 25 | PT 84 | PT 12 | PT 22 | PT 31 | PT 83 | ||
| SU | I. | ✓ | |||||||||||
| II. | ✓ | ||||||||||||
| III. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| PU | I. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| II. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| III. | |||||||||||||
| PUAC | I. | ✓ | |||||||||||
| II. | |||||||||||||
| III. | ✓ | ✓ | |||||||||||
| AC | I. | ✓ | ✓ | ✓ | |||||||||
| II. | ✓ | ✓ | |||||||||||
| III. | ✓ | ||||||||||||
| NR | I. | ✓ | |||||||||||
| II. | ✓ | ✓ | |||||||||||
| III. | ✓ | ||||||||||||
PT 81: Firstly, I should write another molecule next to this molecule to show intermolecular forces between them. This molecule is a polar molecule. Indeed, there are dipole–dipole and van der Waals forces between molecules. However, hydrogen bonds are more effective than the others.
Researcher: Could you show hydrogen bonds in these molecules.
PT 81: OK. Hydrogen bond occurs between oxygen atom and hydrogen atom in the other molecules.
Researcher: Well, Could you explain the formation of hydrogen bonds in more detail?
PT 81: Well, Hydrogen bonds do not occur with every atom. Some atoms are able to form hydrogen bonds, such as fluorine, oxygen and nitrogen, since these atoms have high electronegative and nonbonding electron pairs. Firstly, in the compound, a hydrogen atom should bond to these electronegative atoms such as fluorine, oxygen and nitrogen. As a result of this, hydrogen is positively polarized. The attraction occurs between nonbonding electron pairs of electronegative atom and positively polarized hydrogen atom of the other molecule. This is a hydrogen bond.
When another high level prospective teacher's (PT 49) creative comparison about hydrogen bonds (selective person) was analyzed, there was some uncertainty in this creative comparison, like PT81. In this creative comparison, he explained clearly which atoms could form a hydrogen bond, but it was not clear whether he accepted hydrogen bonds as intermolecular forces. The findings from the interview removed this uncertainty. In the interview, he showed correctly hydrogen bonds as intermolecular forces, but he did not mention the effects of nonbonding electron pairs of an electronegative atom. For this reason, his answers were classified as partial understanding. Also, some studies reveal that students rely on rote memorization to tell them which elements could be involved in a hydrogen bond. As a result of this, they are not able to fully reason through it, such as nonbonding electron pairs of an electronegative atom (Henderleiter et al., 2001). PT 49's explanation in the interview is below:
PT 49: This compound is polar. Therefore, there are dipole–dipole forces in this compound. Also, van der Waals forces occur in the compound since all compounds have van der Waals forces. At the same time, a hydrogen atom bonded to oxygen. For this reason, this hydrogen atom forms a hydrogen bond with an oxygen atom of the other compound.
Researcher: Could you show hydrogen bond in the compound? And could you explain how hydrogen bond is formed in more detail?
PT 49: Yes. This attraction between hydrogen and oxygen is called hydrogen bond; because, a hydrogen atom bonded to oxygen. Oxygen is more electronegative than hydrogen. So, hydrogen is positively polarized. Similarly, there is an oxygen atom on the neighbour compound. Therefore, this oxygen atom is negatively polarized. This situation causes an attraction between oxygen and hydrogen atoms. This is hydrogen bond.
Table 7 indicates that most of the middle level prospective teachers (PT4, 18, and 25) gave responses of partial understanding regarding intermolecular forces in 1,2-ethanediol. Similar to high level prospective teachers, these prospective teachers did not consider nonbonding electron pairs of an electronegative atom in the formation of a hydrogen bond. At the same time, all of these prospective teachers formed creative comparisons related to a hydrogen bond in the category of formation (respectively, an obsessed person, enzyme–substrate relationship, and a family). Although, in these creative comparisons, it was determined that these prospective teachers were aware of some conditions needed for the formation of a hydrogen bond, they did not give any explanations about nonbonding electron pairs of an electronegative atom. A similar result was identified in the interview.
Another middle level prospective teacher's (PT 84) responses in the interview showed that he understood that hydrogen bonds could occur between a partially positively charged hydrogen atom and a partially negatively charged oxygen atom. However, he accepted hydrogen bonds as intramolecular forces. This situation is not surprising since a similar alternative conception was identified in some studies (Peterson and Treagust, 1989; Peterson et al., 1989; Tan and Treagust, 1999; Nicoll, 2001). Thus, Taber (2011) stated that the students commonly supposed hydrogen bonds as intramolecular forces since the attraction between a partially positively charged hydrogen atom and a partially negatively charged atom on another molecule does not fit their notion of a bond. Besides, it was not concluded whether PT 84 could understand the formation of a hydrogen bond or not, since his creative comparison (Hercules) was related to the strength of a hydrogen bond. A sample quotation of the PT 84's responses in the interview is as follows:
PT 84: In this compound, hydrogen bonds occur between hydrogen and oxygen.
Researcher: Could you show hydrogen bonds in the compounds
PT 84: Okay. This (Fig. 4).
Researcher: How does hydrogen bond form in this compound?
PT 84: Oxygen is more electronegative than hydrogen. While, oxygen is partial negatively charged, hydrogen is partial positively charged. This causes the formation of hydrogen bonds.
Particularly, when low level prospective teachers' creative comparisons related to hydrogen bonds were analyzed, it was understood that they had difficulties in understanding the formation or strength of hydrogen bonds. For example, PT 12 explained a hydrogen bond as a covalent bond in her creative comparison (some kind of article that people share). Another prospective teacher (PT 31) stated a hydrogen bond as a non-polar covalent bond by using “the bond between twins” creative comparison. Thus, similar results were identified in the interview. For PT 22 and PT 83, their level of understanding about the formation of a hydrogen bond could not be decided, as they formed creative comparisons about the strength of a hydrogen bond (respectively, wrestlers and steel). PT 12's explanations in the interview are below:
PT 12: I think there are a lot of hydrogen bonds in this compound.
Researcher: How do you understand hydrogen bonds in this compound?
PT 12: The bond between carbon and hydrogen is hydrogen bond.
Researcher: Could you explain the formation of hydrogen bond in detail?
PT 12: The hydrogen bond occurs a result of sharing electrons because hydrogen bond is also covalent bond.
Researcher: Do you mean that carbon and hydrogen share electrons with each other to form hydrogen bond?
PT 12: Yes.
Researcher: Could you show hydrogen bonds in the compound?
PT 12: Yes (Fig. 5).
Researcher: Does hydrogen bond occur only between carbon and hydrogen atoms?
PT 12: Yes
From Table 7, it was seen that only one prospective teacher (PT 81) gave responses regarding the second compound (methane) that fell into the sound understanding category. Also, this prospective teacher stated clearly the formation of van der Waals force with “the feeling of liking someone momentarily” creative comparison. The results of the interview were similar to her explanation in this creative comparison. A sample quotation of the PT 81's responses in the interview is as follows:
PT 81: Methane is non-polar molecule. For this reason, only, van der Waals force is effective.
Researcher: Okay. How do van der Waals forces form in the compound?
PT 81: This compound is non-polar, but momentary dipoles occur. When two non-polar molecules get close to each other, electron clouds repulse each other. As a result of this, momentary dipoles form. Namely, one side of molecule is positive, other side is negative. These momentary dipoles affect the other molecule, and the attraction forms between the molecules.
Although other high level prospective teachers stated that methane was non-polar molecule, and van der Waals forces were effective in this compound, they did not explain how van der Waals force formed. Primarily, these prospective teachers did not form any creative comparisons to explain the formation of van der Waals. They produced creative comparisons considering the strength of van der Waals, and physical properties. For example, PT 49 and PT 85 explained the effects of van der Waals forces on the boiling points by using “flying balloon” and “imagination” creative comparisons. PT 55 formed “rotten rope” creative comparisonto compare the strength of van der Waals forces. One of these prospective teachers' (PT 85) explanations in the interview is below:
PT 85: If we examine this compound, we can say that the bond between carbon and hydrogen is polar covalent, but the compound is a non-polar molecule. Therefore, we do not mention that dipole–dipole force is effective in this compound. There are only van der Waals forces.
Researcher: How do van der Waals forces form in the compound?
PT 85: The attractions occur between non-polar molecules. Also, polar molecules have van der Waals forces. In other words, van der Waals forces form in all molecules.
Researcher: Okay. Could you explain the formation of van der Waals forces?
PT 85: I think positive and negative charges cause this attraction. But I am not sure.
Table 7 shows that all of the middle level prospective teachers' responses about the second compound were classified into partial understanding. Similar to high level prospective teachers, they stated correctly that methane is a non-polar compound, but they did not mention the formation of van der Waals forces. This situation was not surprising since they did not form creative comparisons in the category of formation of van der Waals forces. PT 4, 18 and 84 stated that van der Waals forces were effective in all compounds through “blood vessels, “ability to talk” and “mobile phone” creative comparisons. PT 25 compared correctly the strength of van der Waals forces, dipole–dipole interactions and hydrogen bonds with the “weakest member of the household” creative comparison. PT 25's explanations in the interview are below:
PT 25: When I see methane, I can say that methane has a tetrahedral shape, As a result of this, methane is non-polar. In this compound, hydrogen bond does not occur because of the non-polar compound. Similarly, dipole–dipole force does not form. Only, van der Waals forces are effective in this compound.
Researcher: Why?
PT 25: Because all compounds have van der Waals forces.
Researcher: How do van der Waals forces form in the compound?
PT 25: I know that when the compounds get close to each other, this force occurs. But I do not know in detail.
An important result identified in this question belonged to PT 12 as one of the low level prospective teachers. PT 12 stated the strength of van der Waals forces with “bones of an elderly person” creative comparison, but she described the bond between carbon and hydrogen as a hydrogen bond like 1,2-ethanediol in the interview. She did not express the existence of van der Waals forces in this compound. Similarly, PT 83 explained correctly the strength of van der Waals forces by using “cotton” creative comparison. On the other hand, she did not express the existence of intermolecular forces in this compound in the interview. As for the other low level prospective teachers (PT 22 and PT 31), it was revealed that they had alternative conceptions about the strength of van der Waals forces in their creative comparisons (respectively, “handcuffs”, “magnet”). At the same time, the results of the interview pointed out that they had difficulties in understanding the identification and formation of intermolecular forces. In light of these results, it can be seen that these prospective teachers' levels of understanding about the strength of van der Waals forces were particularly good, but their levels of understanding about the formation and identification of intermolecular forces were not satisfactory. One of these prospective teachers' (PT 31) explanations in the interview are below:
PT 31: Covalent bonds.
Researcher: Do you mean that covalent bonds are intermolecular forces?
PT 31: Yes. That's what I mean.
Researcher: How do covalent bonds form between the compounds?
PT 31: These bonds occur as a result of sharing electrons. Namely, C and H atoms share electrons and covalent bond occurs.
Researcher: Could you show these bonds in the compound?
PT 31: Okay (Fig. 6).
As can be seen from Table 7, all of the high level prospective teachers' responses regarding the third compound fell into the sound understanding category. Similar results were identified in their creative comparisons about dipole–dipole attractions. Particularly, PT 55, and PT 81 clarified the formation of dipole–dipole attractions by producing the same creative comparison (“magnet”). A sample quotation of the PT 55's responses in the interview is as follows:
PT 55: Firstly, this compound is a polar compound. In other words, the chlorine atom attains partial negative charge; and the hydrogen atom attains partial positive charge. Similarly, another compound has partial positive and negative charges. As a result, attractions occur between these compounds. These attractions are called as dipole–dipole attractions.
The other high level prospective teachers (PT 49 and PT 85) formed creative comparisons about the strength of dipole–dipole attraction (respectively, a person of medium strength, neighborly bond), however, they could explain exactly the formation of dipole–dipole attractions in the interview. One of these prospective teachers (PT 49) explanations from the interview is below:
PT 49: In this compound, dipole–dipole attractions are effective.
Researcher: How do you understand that dipole–dipole attractions are effective in the compound?
PT 49: Because it is a polar compound. A chlorine atom is more electronegative than a hydrogen atom. So, a chlorine atom has a negative charge and a hydrogen atom has positive charge. When two compounds get closer to each other, the attraction occurs between negative and positive charges. Also, van der Waals forces occur between these compounds.
All of the middle level prospective teachers were able to give responses that fell into the sound understanding category only in this question. Indeed, their creative comparisons about dipole–dipole forces revealed that most of them could explain exactly the formation of dipole–dipole forces. Only PT 25 formed a creative comparison (being second in class) about the strength of dipole–dipole force. The other prospective teachers' (PT 4, PT 18, and PT 84) creative comparisons were related to the formation of dipole–dipole forces (respectively, people who are diametrically opposite to each other, magnet). The findings from the interview supported their creative comparisons. One of these prospective teachers' (PT 25) explanations in the interview is below:
PT 25: In this compound, the side of hydrogen is positive; the side of chloride is negative since chloride is more electronegative than hydrogen. Namely, there is polarization in this compound. Similar polarization occurs in other molecules. In this way, two compounds attract each other. The name of this attraction is dipole–dipole.
From Table 7, it was seen that none of the low level prospective teachers' responses fell within the sound understanding category. Only two prospective teachers (PT 83 and PT 12) could give responses with partial understanding with alternative conceptions. These results were similar to their creative comparison about dipole–dipole forces. PT 83 formed “people with different strengths pulling on a rope” creative comparison to explain dipole–dipole forces, but she stated dipole–dipole forces as intramolecular forces. At the same time, the same prospective teacher explained ionic bonds as dipole–dipole forces in the interview. These results indicated that PT 83 had difficulties in understanding intra- and intermolecular forces. A sample quotation of PT 83's responses in the interview is as follows:
PT 83: In this compound, while hydrogen has a positive charge, chlorine has a negative charge. So, attraction between the oppositely charged ions causes dipole–dipole forces.
Researcher: Could you show dipole–dipole forces in the compound?
PT 83: I can show it like this (Fig. 7).
Similar to PT 83, PT 12 explained dipole–dipole forces as intramolecular forces. Thus, her creative comparison about dipole–dipole forces (people with different strengths pulling on a rope) revealed that she accepted dipole–dipole force as a force between atoms. Also, she was trying to explain bond polarity as dipole–dipole force.
Question 3.
Could you compare the strength of ionic bond, covalent bond, van der Waals forces, dipole–dipole forces and hydrogen bond? Please explain your answer.
Question 3 was designed to reveal whether the prospective teachers compared correctly the strength of inter- and intramolecular forces or not.
Table 8 indicates that most of the responses grouped under the sound understanding category belonged to high level prospective teachers. In this category, the prospective teachers determined the inter- and intramolecular forces and also compared correctly their strength. In addition, when these prospective teachers' creative comparisons were analyzed, it was shown that the creative comparisons used included correct explanations about the strength of inter- and intramolecular forces. For example, PT 49 compared correctly the dipole–dipole and van der Waals forces by using “a person of medium strength” creative comparison. Moreover, his responses in the interview supported this conclusion. A sample quotation of PT 49's responses in the interview is as follows:
| Category | High level | Middle level | Low level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT 49 | PT 55 | PT 81 | PT 85 | PT 4 | PT 18 | PT 25 | PT 84 | PT 12 | PT 22 | PT 31 | PT 83 | |
| SU | ✓ | ✓ | ✓ | |||||||||
| PU | ✓ | ✓ | ✓ | |||||||||
| PUAC | ✓ | ✓ | ✓ | |||||||||
| AC | ✓ | ✓ | ✓ | |||||||||
| NR | ||||||||||||
PT 49: Among the intermolecular forces, hydrogen bonds are the strongest. In other words, hydrogen bond is stronger than dipole–dipole. Also, van der Waals forces are weak compared to dipole–dipole forces because van der Waals forces occur as a result of temporary fluctuating dipoles. When ionic and covalent bonds as intramolecular forces were compared, an ionic bond is stronger than a covalent bond. This is because electrostatic attraction between opposite charges in ionic bonds is more effective than the covalent bond.
Researcher: If you compare all of them, how do you put them in order in the context of strength?
PT 49: Ionic bond is the strongest. The second is covalent bond. Ionic and covalent bonds are intramolecular forces. Intramolecular forces are stronger than the intermolecular forces. Third is hydrogen bond since it is intermolecular force. Afterwards, dipole–dipole and van der Waals forces.
An important result was determined from the interview conducted with PT 55. PT 55 used “rope” creative comparison to explain the strength of hydrogen bonds. It was not understood clearly, however, how the prospective teacher compared the strength of hydrogen bonds in this explanation. The same prospective teacher compared correctly the strength of van der Waals force and the other intermolecular forces by using “rotten rope” creative comparison. The result of the interview indicated that the prospective teacher identified correctly intra- and intermolecular forces, compared correctly the strength of van der Waals force and the other intermolecular forces, but believed that a hydrogen bond was stronger than intramolecular forces. Some studies stated that over-emphasis of the strength of hydrogen bonds was one of the reasons for this alternative conception (Peterson and Treagust, 1989; Tan and Treagust, 1999). A similar finding was revealed in the interview of PT 55. A sample quotation of PT 55's responses in the interview is as follows:
PT 55: The strongest are hydrogen bonds.
Researcher: Could you explain this in more detail?
PT 55: Hydrogen bonds are stronger than others. As a result of this, a substance with hydrogen bonds will have higher boiling point.
Researcher: What do you think about the others?
PT 55: Ionic bonds are stronger than covalent bonds because electrostatic attraction is more effective in the formation of ionic bonds. van der Waals forces and dipole–dipole forces are weaker than ionic and covalent since these forces are intermolecular forces. If we compare the strength of van der Waals and dipole–dipole forces, van der Waals is weaker than the and dipole–dipole forces.
Researcher: Consequently, how do you put them in order in the context of strength?
PT 55: From strongest to weakest: Hydrogen bond, ionic bond, covalent bond, dipole–dipole and van der Waals.
As can be seen in Table 8 three middle level prospective teachers' (PT 4, PT 18 and PT 25) responses were classified in the category of partial understanding. Also, these prospective teachers' creative comparisons mostly related to the formation of intra- and intermolecular forces. Only PT 25 formed a creative comparison about formation and strength of inter- and intramolecular forces. She used “love” creative comparison for ionic bonds, “the weakest member of the household” creative comparison for van der Waals force, and “being second in class” for dipole–dipole force. Particularly, from “the weakest member of the household” and “being second in class” creative comparisons, it was understood that PT 25 compared correctly the strength of intermolecular forces. In addition, she stated ionic bonds as a strong attraction in the “love” creative comparison, but she could not compare the strength of ionic bonds with the other forces. Similar results were obtained in the interview of PT 25. Her responses to the interview are as follows:
PT 25: Ionic and covalent bonds are generally stronger than the others since they are intramolecular forces. Among the intermolecular forces, van der Waals forces are the weakest. Dipole–dipole is stronger than van der Waals, however, hydrogen bond is the strongest of intermolecular forces. On the other hand, I do not know the differences of strength of ionic and covalent bonds. I am confused about this topic.
Another important finding belongs to PT 84. The prospective teacher usually produced creative comparisons taking the formation of intra-and intermolecular forces into consideration. For example, “magnet” for ionic bonds, “sharing money” for covalent bonds, “an asocial person” for non-polar covalent bonds, and “magnet” for dipole–dipole are creative comparisons produced by PT 84 in the category of formation. The same prospective teacher produced “mobile phone” creative comparison about van der Waals forces. In this creative comparison, he explained that the molecules were affected by van der Waals forces. For hydrogen bonds, he produced “Hercules” creative comparison, and he accepted a hydrogen bond as a strong bond. On the other hand, it was not clear how he compared the strength of hydrogen bonds. Thus, the findings from the interview showed that the PT 84 believed that a hydrogen bond is the strongest of the intra-and intermolecular forces. His responses to the interview are given below:
PT 84: Hydrogen bonds are the strongest. After hydrogen bonds, ionic bonds and dipole–dipole forces are the next strongest. After the dipole–dipole forces, covalent bonds and van der Waals forces.
Researcher: Why? Could you explain this?
PT 84: Ionic bonds and dipole–dipole forces are based on attraction between opposite poles. For this reason, ionic bonds and dipole–dipole forces are stronger than covalent bonds and van der Waals forces. On the other hand, this attraction between opposite poles in ionic bond is more effective than dipole–dipole forces. If we examine hydrogen bonds, hydrogen bonds are the strongest of all them because it is very difficult to break hydrogen bond. van der Waals forces are very weak. As a result of this, van der Waals forces break very easily.
When Table 8 was examined, it can be seen that none of the low level prospective teachers' responses were classified into sound understanding or partial understanding. These results were not surprising since these prospective teachers produced creative comparisons that included alternative conceptions about the strength of intermolecular forces. For example, in regard to the strength of hydrogen bond, PT 22 formed “wrestlers” creative comparison, and emphasised that hydrogen bonds are the strongest of the bonds. Besides, PT 22 formed “handcuffs” creative comparison, and she stated that van der Waals forces were hard to break. Similarly, PT 31 explained that van der Waals forces are powerful forces by using “magnet” creative comparison. The other prospective teacher (PT 83) produced “steel” creative comparison to explain hydrogen bonds. In this creative comparison, it was not understood clearly if she believed that a hydrogen bond is the strongest of the intermolecular forces or that there is a strong intramolecular as well as intermolecular force. This uncertainty was removed with the interview. Sample quotations of the prospective teachers' responses in the interview are as follows:
PT 83: The strongest are ionic bonds because it depends on a transfer of an electron. Second are hydrogen bonds. Hydrogen bonds are strong bonds. For example, water. Third are dipole–dipole forces, because dipole–dipole forces are polar. At the same time, there are covalent bonds and van der Waals forces. If we compare these, we can say that covalent bonds are stronger than van der Waals forces. van der Waals forces are the weakest.
As can been seen in this explanation, PT 83 compared the strength of ionic bonds correctly, but that could not be said for covalent bonds and dipole–dipole forces. In addition, she stated that hydrogen bonds are strong bonds, as in her creative comparison. For this reason, PT 83' explanation was grouped under the PUAC. Another prospective teacher's explanation is below:
PT 22: Hydrogen bonds are the strongest because the compounds that include the hydrogen bond are stronger than others. Covalent bonds are second since covalent bonds occur as a result of sharing electrons. Dipole–dipole forces, van der Waals forces, and ionic bonds are all strong. Also, polar molecules are important for dipole–dipole forces. Namely, the ranking is as follows: hydrogen bond, covalent bond, van der Waals force, dipole–dipole forces, and ionic bond.
It can be understood from this explanation that PT 22 accepted hydrogen bonds as the strongest. This result was coherent with PT 22's creative comparison (wrestlers). In addition, PT 22 accepted that van der Waals force was stronger than dipole–dipole forces and ionic bonds. Thus, PT 22's responses were in the category of AC. Similarly, PT 22 explained with the “handcuffs” creative comparison that van der Waals forces are hard to break.
Question 4.
I. C6H6
II. C6H5Cl
III. C6H5Br
IV. C6H5OH
Could you compare the boiling points of the compounds above? Please explain your answer.
Question 4 aims to determine how intermolecular forces affect the boiling point of compounds. Table 9 shows that all of the high level prospective teachers gave responses in the category of SU. According to these results, it can be said that these prospective teachers can discriminate intermolecular forces from each other and compare their effects on the boiling point of compounds. Thus, these prospective teachers mostly produced creative comparisons about the strength of intermolecular forces.
| Category | High level | Middle level | Low level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT 49 | PT 55 | PT 81 | PT 85 | PT 4 | PT 18 | PT 25 | PT 84 | PT 12 | PT 22 | PT 31 | PT 83 | |
| SU | ✓ | ✓ | ✓ | ✓ | ||||||||
| PU | ✓ | ✓ | ||||||||||
| PUAC | ✓ | ✓ | ✓ | |||||||||
| AC | ✓ | ✓ | ||||||||||
| NR | ✓ | |||||||||||
In addition, it was revealed that PT 49 and PT 85 produced creative comparisons about van der Waals forces and hydrogen bonds that belonged to the physical properties category. For example, PT 49 produced the “flying balloon” creative comparison about van der Waals force, and he emphasized that compounds only had van der Waals forces as intermolecular forces, leading to a low boiling point. Similarly, PT 85's creative comparisons about van der Waals forces, hydrogen bonds (respectively, imagination, a person who's patient) indicated that he could relate intermolecular forces to the boiling point of molecules. Thus, this situation was supported by the findings from the interview. Sample quotations of PT 85's responses in the interview are as follows:
PT 85: The strongest one is fourth, because this compound included hydrogen bonds as intermolecular forces. For the compounds that included chlorine and bromine, I think the compound that included bromine's boiling point was higher than the other. This is because the atomic mass of bromine is higher than chlorine
Researcher: Well, how does atomic mass of bromine effect the boiling point?
PT 85: Because if atomic mass increases, the number of electrons increases. As a result of this, van der Waals forces between the molecules are strong strongly.
Researcher: Consequently, how do you order these compounds in the context of boiling points?
PT 85: From highest to lowest: Fourth, third, second and first.
Researcher: Why is first compound the lowest?
PT 85: Because it has only van der Waals forces between the molecules. The other molecules have both van der Waals and dipole–dipole forces.
From Table 9, it can be seen that none of middle level prospective teacher' responses lie in the SU category. Thus, it was not clearly understood whether these prospective teachers could relate intermolecular forces to the physical properties of the compound or not according to their creative comparisons. These prospective teachers mostly formed creative comparisons about the formation and strength of intermolecular forces. In these creative comparisons the prospective teachers did not mention the physical properties of the compound such as the boiling point. These unclear points about the understanding of prospective teachers were clarified with the interviews. Sample quotations of the prospective teachers' responses in the interview are as follows:
PT 84: The fourth compound has the highest boiling points since it includes hydrogen bond. Hydrogen bonds are the strongest. It is very difficult to break these bonds.
Researcher: What about the other compounds?
PT 84: The smallest is benzene.
Researcher: Why?
PT 84: Benzene does not include other kinds of atoms, groups such as bromine and hydroxyl. This group may affect the boiling points of compounds. But, I am not sure. I cannot say anything about the boiling points of other compounds.
These explanations above indicated that PT 84 first gave correct explanations based on the strength of hydrogen bonds, but he did not present any explanation about the other compounds considering dipole–dipole and van der Waals forces. In light of this result, it can be said that PT 84 could not understand the effects of intermolecular forces on the boiling points of compounds. The other prospective teacher's (PT 18) responses to the interview are given below:
PT 18: I think boiling points of the fourth compound is higher than the others, because the fourth compound includes hydroxyl group. As a result of this, hydrogen bonds occur, and, hydrogen bonds leads to increasing boiling point. When the second and third compounds were compared, dipole–dipole forces are effective in these compounds. Electronegativity of chlorine is higher than bromine. As a result of this, polarity, and dipole–dipole forces are more effective in the second compound. The boiling points of the second compound are higher than the third compound's.
Researcher: Well. What do you think about the first compound?
PT 18: I think the boiling point of first compound is the smallest since it does not include different atoms such as chlorine and bromine.
Similarly PT 84 and PT 18 identified correctly that the fourth compound has the highest boiling point since it includes hydrogen bonds. On the other hand, she did not explain the effects of van der Waals force on the boiling points of the other compounds. Thus, the “the ability to talk” creative comparison about the van der Waals force formed by PT 18 indicated that she was unaware of the effects of van der Waals force. At the same time, PT 18 could not compare correctly the boiling points of the other compounds since she did not consider van der Waals force.
In this interview question, it was learned that low level prospective teachers' creative comparisons provided evidence about their conceptual understanding. Findings from creative comparison analysis showed that these prospective teachers had alternative conceptions about formation or strength of intermolecular forces. For example, PT 12 explained a hydrogen bond as a covalent bond by using “some kind of article that people share” creative comparison. A similar finding was identified in the interview. Her responses to the interview are as follows:
PT 12: The first compound has the highest boiling point since it includes hydrogen bond. The second, third and fourth compounds include hydrogen bonds. However, these compounds do not contain as many hydrogen bonds as the first compound.
Researcher: Why? Can you explain this?
PT 12: Because, the first one includes more bonds between carbon and hydrogen atoms than the fourth one. In the fourth compound, one carbon and hydrogen bond broke, as a result of this carbon–hydroxyl, carbon–bromine, and carbon–chlorine bonds occurred. Thus, the number of hydrogen bonds decreased.
Researcher: Do you mean that carbon and hydrogen bonds are hydrogen bonds?
PT 12: Yes.
Researcher: What about the boiling points of the other compounds?
PT 12: In my opinion, the boiling points of other compounds are the same
Researcher: Why?
PT 12: These compounds include the same number of hydrogen bonds
Conclusions and implications
The present study aims to identify prospective chemistry teachers' creative comparisons towards “the basic concepts of inter- and intramolecular forces and to reveal the relationship between these creative comparisons and their conceptual understanding. In this context, firstly, a creative comparison questionnaire was applied to reveal prospective teachers' creative comparisons used to explain the basic concepts of inter- and intramolecular forces.The findings from creative comparison analysis drew attention to several important points. For example, when the prospective teachers' creative comparisons about ionic bonds were investigated (see Table 4), it was understood that, for the most part, ionic bonds are created as a result of transfer of electrons. Only, in the “magnet”, “the agreement between two people with opposing ideas” and “men and women” creative comparisons, the formation of ionic bonds was explained as the electrostatic interaction of opposite charge ions. One possible reason for this is due to the representation of ionic bonds in the textbooks. In particular, secondary school chemistry textbooks published before 2008 in Turkey explained ionic bonds as a result of electron transfer. However, secondary school chemistry curriculum in Turkey was modified in the 2008–2009 academic year. New chemistry curriculum and chemistry textbooks were implemented for all of ninth grade beginning in the 2008–2009 academic year. In the new curriculum and textbooks, emphasis is on the electrostatic interaction in the formation of ionic bonds However, most of the prospective teachers in this study graduated from secondary school before 2009. For this reason, many of them may explain the formation of an ionic bond as a result of transfer of an electron. Thus, some studies have indicated that prior knowledge is highly resistant to change (Driver, 1989; Ozdemir and Clark, 2007). In addition, similar results were determined in some studies that examined the representation of ionic bonds in the textbooks. For example, Bergqvist et al. (2013) determined that all typical examples used in the section concerning ionic bonding, in all textbooks which were analyzed by them, represent ionic bonding as the result of electron transfer.
Another important finding was revealed from creative comparisons about covalent, non-polar covalent and polar bonds. None of the prospective teachers stated the electrostatic force between the nucleus and shared electrons in the formation of covalent bonds (see Table 4). The prospective teachers consider the sharing of electrons as the ‘force’ instead of nucleus–electron interactions. One possible reason for this is the line-bond structures of the compounds which were used commonly by chemistry teachers in Turkey. In the line-bond structures, a single covalent bond is where one pair of shared electrons is represented by a line drawn between atoms. This model could lead to conception that the shared electron pair in itself is the bond. Another reason may be related to representation of covalent bonds in the textbooks. Thus, Boo (2000) stated that some statements in textbooks like one pair of shared electrons constitute a single covalent may cause students to accept the shared electron pair in itself as the bond. Also, many textbooks do not use the term electrostatic force or even attraction force to explain covalent bonds (Bergqvist et al., 2013). Similar results were observed in the secondary school chemistry textbooks (9th grades) in Turkey. In addition, some of the creative comparisons indicated that the prospective teachers had some alternative conceptions. For example, “combination of contrast colours” creative comparison for covalent bonds, “magnet” and “the need of two people of opposite sexes for each other” creative comparisons for polar covalent bonds revealed that the prospective teachers confused ionic and covalent bonds. Similarly, “identical twins”, and “twins pulling at two ends of a rope” creative comparisons for polar covalent bonds, “the people in the world” and “two different people holding a rope on both ends” creative comparisons for non-polar covalent bonds showed that prospective teachers had difficulties in the discrimination of polar and non-polar covalent bonds.
Findings from creative comparisons about intermolecular forces are important to detect what clear points and what alternative conceptions exist in the minds of the prospective teachers. For instance, some creative comparisons about the formation of hydrogen bonds such as “selective person”, “obsessed person”, “selective permeant”, and “cell membrane” revealed that these prospective teachers stated the conditions under which hydrogen bonds occurred. However, from these explanations, it was not understood clearly whether the prospective teachers accepted hydrogen bonds as intramolecular or intermolecular forces. For this reason, semi-structured interviews were conducted with the prospective teachers. In addition, “some kind of article that people share”, and “the bond between twins” creative comparisons are important because these creative comparisons showed that the prospective teachers identified hydrogen bonds as covalent bonds or, they believed that hydrogen bonds occur between atoms. Thus, some researchers stated that the concept of “bond” could cause the learners to accept hydrogen bonds as intramolecular forces (Ünal, 2003; Taber, 2011). When the creative comparisons about the strength of hydrogen bonds were investigated, it was seen that these creative comparisons could be classified into three categories. In the first category, the prospective teachers compared correctly the strength of hydrogen bond with other intermolecular forces or intramolecular forces. For example, “a sailor's knot”, and “thin people” creative comparisons were included in the first category. In the second category, the prospective teachers stated that hydrogen bonds are strong bonds by using “friendship”, “Hercules”, “rope”, and “steel” creative comparisons. But, from these creative comparisons, it was not clearly understood how prospective teachers compared the hydrogen bond. In the last category, it was determined that the prospective teachers had alternative conceptions about the strength of hydrogen bonds according to their creative comparisons such as “thick branch”, “the strongest individual in a society”, and “the roots of a tree digging into the ground”. Some studies pointed out that over-emphasis of the strength of hydrogen bond could lead the learners to accept hydrogen bonds as the strongest (Peterson and Treagust, 1989; Tan and Treagust, 1999). At the same time, some creative comparisons such as “brain”, “knot”, and “person who's patient” indicated that the prospective teachers could explain the effects of hydrogen bonds on the physical properties of compounds.
Opposite to the other concepts, minimum creative comparisons in the category of formation were determined for van der Waals forces (see Table 5). Only, one kind of creative comparison (“feeling of liking someone momentarily”) was formed by the prospective teachers. From this result, it can be understood that very few prospective teachers could explain scientifically the formation of van der Waals force. The prospective teachers mostly produced creative comparisons regarding the strength of van der Waals force. While the prospective teachers made scientific comparisons of van der Waals force and other forces in many creative comparisons such as “feather”, “rotten rope”, “house of cards”, and “the bones of an elderly person”, some alternative conceptions about the strength of van der Waals force in some creative comparisons were revealed (“handcuffs”, and “magnet”). Different from the other concepts, a category about molecules in van der Waals force was identified. In this category, the prospective teachers were able to clarify that van der Waals forces had effects on both polar and non-polar molecules by using “blood vessels”, “energy”, “jealousy”, and “the ability to talk” creative comparisons. Even, with the “jealousy”, and “the ability to talk” creative comparisons, the prospective teachers could explain that the other intermolecular forces were more effective than van der Waals force in polar molecules. Similar to hydrogen bonds, in the category of physical properties, the prospective teachers could explain the effects of van der Waals forces on the boiling point of compounds by using “flying balloon”, “imagination”, and “a fat person taking up more space than a thin person” creative comparisons.
From the creative comparison analysis of dipole–dipole forces, striking points were determined. One of them is “people with different strengths pulling on a rope” creative comparison. This creative comparison contained different meanings. For example, PT 46 presented scientifically acceptable explanations by using this creative comparison. On the other hand, PT 12 and PT 83 explained dipole–dipole force as a force between atoms, and they tried to clarify bond polarity. This situation was not surprising since a similar pictorial creative comparison was used in a Turkish high school Chemistry textbook (10th grade) to explain bond polarity. At the same time, the prospective teachers could scientifically explain the formation of dipole–dipole forces through creative comparisons such as “people who are diametrically opposite to each other”, “magnet”, “two stubborn goats quarrelling” and “charged glass rod picking up pieces of paper”. In addition to these scientific explanations, “two similar people attracting each other” and “a bag of lentils” creative comparisons revealed the prospective teachers' belief that dipole–dipole forces occur between the non-polar molecules. Also, it was determined that the prospective teachers accepted dipole–dipole force as a force between the atoms through “a married couple sharing a blanket”, “two balls of the same kind sticking to each other”, “the bond between people of the same city” creative comparisons. In the strength category, from some creative comparisons such as “medium-sweet Turkish coffee”, “a person of medium strength”, and “being second in class”, it was understood that these prospective teachers could compare correctly the strength of dipole–dipole forces and other intermolecular forces. Besides, some creative comparisons such as “the largest root of a tree”, “hurricane”, and “the love between Romeo and Juliet” pointed out that the prospective teachers accepted dipole–dipole forces as very strong.
In light of creative comparison analysis, it can be said that creative comparisons can help instructors to identify learners' alternative conceptions. On this point, instructors should be careful in acknowledging which creative comparisons used by learners contained the alternative conceptions. Acknowledging which creative comparisons lead to alternative conceptions will hinder the use of similar creative comparisons in courses or textbooks. On the other hand, the prospective teachers could scientifically explain many concepts with creative comparisons in this study. For this reason, instructors can use creative comparisons identified in this study to present the basic concepts of intra- and intermolecular forces to their students. However, they should not use the same creative comparison to explain different concepts in the same topic. Also, student-generated creative comparisons could be used for formative assessments to help instructors become aware of students' ideas when they come into the classroom (Lancor, 2014b). Although the use of creative comparisons can be useful to determine learners' alternative conceptions and scientific explanations, some creative comparisons used by the prospective teachers in the study contained points of uncertainty. The possible reason for this uncertainty may be that some prospective teachers were not sure how to articulate his or her ideas using a creative comparison. In this context, asking learners about the limits of their creative comparisons can be helpful in removing this uncertainty. At the same time, when some uncertainty is revealed in learners' creative comparisons, a technique such as drawing and/or interviews should be used to remove uncertainty.
In this study, semi-structured interviews were used for two aims. The first aim is to remove uncertainty points in prospective teachers' creative comparisons. The second aim is to determine whether there is a relationship between prospective teachers' creative comparisons and conceptual understanding or not. Based on these aims, the prospective teachers were classified into three categories according to their creative comparisons, and four prospective teachers in each category were selected randomly for an interview. With the interview results, points of uncertainty were removed. For example, it was not understood clearly from the creative comparisons belonging to PT 49 and PT 81 that they accepted hydrogen bonds as intermolecular forces. However, the result of interview indicated that they described hydrogen bonds as intermolecular forces. As another example, PT 55 could be given. PT 55 explained the strength of hydrogen bonds by using the “rope” creative comparison; however, it was not understood clearly how she compared the strength of hydrogen from this creative comparison. This uncertainty was removed with the interview. It was determined that she, in fact, believed that hydrogen bonds were stronger than intramolecular forces in the interview. When the levels of prospective teachers' understanding were examined, it was found that the prospective teachers' conceptual understanding was not tightly linked to the complexity of their creative comparisons. However, it was seen that the creative comparisons submitted by the prospective teachers could be used to infer their conceptual understanding. For example, none of the prospective teachers from a high level to a low level in the interview could explain the formation of covalent bonds based on nucleus–electron attraction. Similar results were seen in their creative comparisons. For ionic bonds, only two prospective teachers' creative comparisons (PT 81 and PT 85) were related to electrostatic attraction. Thus, the same prospective teachers were able to explain ionic bonds according to electrostatic attraction in the interview. Similarly, if any alternative conceptions were identified in the prospective teachers' creative comparisons, the same result was found in the interview. For example, PT 12 identified hydrogen bonds as covalent bonds in “some kind of article that people share” creative comparison. The same statements were identified in the interview. Another prospective teacher (PT 22) used “wrestlers” and “handcuffs” creative comparisons for hydrogen bonds and van der Waals forces, and it was determined that she has alternative conceptions about the strength of hydrogen bonds and van der Waals forces. The results of the interview supported these alternative conceptions. On the other hand, all prospective teachers' creative comparisons were not coherent with their conceptual understanding. For instance, PT 83 used the “trading” creative comparison for ionic bonds, and “a common eraser” creative comparison for covalent bonds. In these creative comparisons, an alternative conception was not determined. However, PT 83 confused intra- and intermolecular forces in the interview, and she explained ionic bonds as dipole–dipole forces, covalent bonds as van der Waals forces. This result suggests that the creative comparison analysis should be combined with some techniques like the semi-structured interview. Even the use of think-aloud activity might be helpful in identifying how learners generate creative comparisons. Moreover, this study did not examine prospective teachers' creative comparisons concerning metallic bonds. For this reason, future studies may be carried out to investigate learners' creative comparisons about metallic bonds and their conceptual understanding.
Acknowledgements
The author wish to thank the referees, and Dr Keith S. Taber for their valuable suggestions which have improved the manuscript.References
- Abraham M. R., Grzybowski E. B., Renner J. W. and Marek E. A., (1992), Understandings and misunderstandings of eight grades of five chemistry concepts found in textbooks, J. Res. Sci. Teach., 29(2), 105–120.
- Amin T. G., (2009), Conceptual metaphor meets conceptual change, Human Dev., 52, 165–197.
- Amin T. G., Jeppsson F., Haglund J. and Strömdahl H., (2012), Arrow of time: metaphorical construals of entropy and the second law of thermodynamics, Sci. Educ., 96(5), 818–848.
- Ashworth P. D. and Lucas U., (1998), What is the world of phenomenography? Scan. J. Educ. Res., 42(4), 415–431.
- Aubusson P. J., Harrison A. G. and Ritchie S. M., (2006), Metaphor and analogy in science education, Dordrecht: Springer.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: What changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Bergqvist A., Drechsler M., Jong O. D. and Rundgren S. C., (2013), Representations of chemical bonding models in school textbooks – help or hindrance for understanding, Chem. Educ. Res. Pract., 14, 589–606, DOI: 10.1039/c3rp20159g.
- Birk J. P. and Kurtz M. J., (1999), Effect of experience on retention and elimination of alternative conceptions about molecular structure and bonding, J. Chem. Educ., 76(1), 124–128.
- Bodner G. and Domin D., (1998), Mental models: The role of representations in problem solving in chemistry, Paper presented at international council for association in science education, Summer Symposium, Proceedings.
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetic of chemical reactions, J. Res. Sci.Teach., 35, 569–581.
- Boo H. K., (2000), Pre-service teachers' content weaknesses concerning chemical bonds and bonding, in Goh N. K., Chia L. S., Boo H. K., Tan S. N. and Tsoi M. F. R. (ed.), Chemistry Teachers' Network, Singapore: Singapore National Institute of Chemistry, pp. 60–63.
- Brookes D. T. and Etkina E., (2007), Using conceptual metaphor and functional grammar to explore how language used in physics affects student learning, Phys. Rev. Spec. Top.–Phys. Educ. Res., 3, 1–16.
- Cameron L., (2002), Metaphors in learning of science: a discourse focus, Br. Educ. Res. J., 28(5), 673–688.
- Chiappe D. L., Kennedy J. M. and Chiappe P., (2003), Aptness is more important than comprehensibility in preference for metaphors and similes, Poetics, 31, 51–68.
- Christidou V., Koulaidis V. and Christidis T., (1997), Children's use of metaphors in relation to their mental models: the case of ozone layer and its depletion, Res. Sci. Educ., 27(4), 541–552.
- Coll R. K., (2006), The role of models, mental models and analogies in chemistry teaching, in Aubusson P. J., et al. (ed.), Metaphor and Analogy in Science Education, Dordrecht: Springer, pp. 65–77.
- Coll R. K. and Taylor N., (2001), Alternative conceptions of chemical bonding held by upper secondary and tertiary students, Res. Sci. Technol. Educ., 19(2), 171–191.
- Coll R. K. and Taylor N., (2002), Mental models in chemistry: Senior chemistry students' mental models of chemical bonding, Chem. Educ.: Res. Pract. Eur., 3, 175–184.
- Coll R. and Treagust D. F., (2001a), Learners' mental models of chemical bonding, Res. Sci. Educ., 31, 357–382.
- Coll R. K. and Treagust D. F., (2001b), Learners' use of analogy and alternative conceptions for chemical bonding, Aust. Sci. Teach. J., 48(1), 24–32.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486.
- Collette A. T. and Chiappetta E. L., (1994), Science instruction in the middle and secondary schools, 3rd edn, New York: MacMillan.
- Çalık M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- De Posada J. M., (1997), Conceptions of high school students concerning the internal structure of metals and their electric conduction: structure and evolution, Sci. Educ., 81, 445–467.
- Dogru M. and Sarac E., (2013), Metaphors of primary school students relating to the concept of global warming, Educ. Res. Rev., 8(21), 2071–2082.
- Driver R., (1989), Students' conceptions and the learning of science, Int. J. Sci. Educ., 11, 481–489.
- Duit R., (1991), On the role of analogies and metaphors in learning science, Sci. Educ., 75(6), 649–672.
- Erdoğan T. and Erdoğan Ö., (2013), A metaphor analysis of the fifth grade students' perceptions about writing, Asia-Pacific Educ. Res., 22(4), 347–355. Retrieved from http://link.springer.com/article/10.1007/s40299-012-0014-4, DOI: http://10.1007/s40299-012-0014-4.
- Eshach H. and Garik P., (2001), Students' conceptions about atoms and atom-bonding, available online at: http://www.bu.edu/smec/qsad/ed/QM-NARST-finalpg.pdf, accessed 15 December, 2005.
- Fleiss J. L. and Levin B. L., (1981), Statistical methods for rates and proportions, New York: Wiley.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: A look to the future, J. Chem. Educ., 76, 548–554.
- Gentner D., (1988), Metaphor as structure mapping: the relational shift, Child Dev., 59(1), 47–59.
- Gentner D. and Jeziorski M., (1993), The shift from metaphor to analogy in western science, in Ortony A. (ed.), Metaphor and Thought, Cambridge: Cambridge University Press, pp. 447–480.
- Gilbert S. W., (1989), An evaluation of the use of analogies and metaphors in learning science, J. Res. Sci. Teach., 26(4), 315–327.
- Glynn S. M., (1995), Conceptual bridges: using analogies to explain scientific concepts, Sci. Teach., 62(9), 25–27.
- Griffiths A. and Preston K., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Griffiths A. K. and Preston K. R., (1999), Grade-12 students' alternative conceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 2611–2628.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in Grade 11 chemistry, Sci. Educ., 84, 352–381.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding, J. Chem. Educ., 78(8), 1126–1130.
- Herron J. D., (1996), The chemistry classroom: Formulas for successful teaching, Washington: American Chemical Society.
- Israel M., Harding J. R. and Tobin V., (2004), On Simile, in Achard M. and Kemmer S. (ed.), Language, Culture, and Mind, Stanford: CSLI, pp. 123–135.
- Jeppsson F., Haglund J., Amin T. G. and Strömdahl H., (2013), Exploring the use of conceptual metaphors in solving problems on entropy, J. Learn. Sci., 22, 70–120.
- Justi R. and Gilbert J., (2006), The role of analog models in the understanding of the nature of models in chemistry, in Aubusson P. J., et al. (ed.), Metaphor and Analogy in Science Education, Dordrecht: Springer, pp. 119–130.
- Kanthan R. and Mills S., (2006), Using metaphors, analogies and similes as aids in teaching pathology to medical students, J. Int. Assoc. Med. Sci. Educ., 16(1), 19–26.
- Lakoff G. and Johnson M., (1980), Metaphors we live by, Chicago: Chicago University Press.
- Lancor R., (2014a), Using metaphor theory to examine conceptions of energy in biology, chemistry, and physics, Sci. Educ., 23, 1245–1267, DOI: http://10.1007/s11191-012-9535-8.
- Lancor R. A., (2014b), Using student-generated analogies to investigate conceptions of energy: a multidisciplinary study, Int. J. Sci. Educ., 36(1), 1–23.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91, 579–603.
- Lodico M. G., Spaulding D. T. and Voegtle K. H., (2010), Methods in Educational Research From Theory to Practice, 2nd edn, San Francisco: Wiley.
- Marton F. M., (1994), Phenomenography, in Husén T. and Postlethwaite T. N. (ed.), The international encyclopedia of education, Oxford: Pergamon, 2nd edn, vol. 8, pp. 4424–4429.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis, Thousand Oaks: Sage.
- Miller G. A., (1993), Images and models, similes and metaphors, in Ortony A. (ed.), Metaphor and Thought, Cambridge: Cambridge University Press, pp. 357–400.
- Nakipoğlu C. and Taber K. S., (2013), The atom as a tiny solar system: Turkish high school students' understanding of the atom in relation to common teaching analogy, in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Innovations in Science Education and Technology, Dordrecht: Springer, vol. 19, pp. 169–198.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Özdemir G. and Clark D. B., (2007), An overview of conceptual change theories, Eurasian J. Math., Sci. Technol. Educ., 3(4), 351–361.
- Özder A., (2013), How do high-school students perceive the concept of ‘map’: A case study from Istanbul, Educ. Res. Rev., 8(16), 1392–1398.
- Patton M. Q., (1987), How to use qualitative methods in evaluation, Newbury Park, CA: Sage.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students' misconceptions of covalent bonding and structure, J. Chem. Educ., 66, 459–460.
- Peterson R. F., Treagust D. F. and Garnett P. J., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314.
- Robinson W. R., (1998), An alternative framework for chemical bonding, J. Chem. Educ., 75(9), 1074–1075.
- Rompayom P., Tambunchong C., Wongyounoi S. and Dechsri P., (2011), Using Open-Ended Questions to Diagnose Students' Understanding of Inter- and Intramolecular Forces, US-China Educ. Rev. B, 1(1), 12–23.
- Saban A., (2004), Prospective classroom teachers' metaphorical images of selves and comparing them to those they have their elementary and cooperating teachers, Int. J. Educ. Dev., 24, 617–635.
- Saban A., (2008), Primary school teachers' and their students' mental images about the concept of knowledge, Elementary Education Online, 7(2), 421–455.
- Saban A., (2011), Prospective computer teachers' mental images about the concepts of “school” and “computer teacher”, Educ. Sci.: Theory Pract., 11(1), 435–446.
- Saban A., Kocbeker B. N. and Saban A., (2006), An investigation of the concept of teacher among prospective teachers through metaphor analysis, Educ. Sci.: Theory Pract., 6(2), 461–522.
- Seung E., Park S. and Narayan R., (2011), Exploring elementary pre-service teachers' beliefs about science teaching and learning as revealed in their metaphor writing, J. Sci. Educ. Technol., 20, 703–714.
- Taber K. S., (1995), Development of student understanding: A case study of stability and lability in cognitive structure, Res. Sci. Technol. Educ., 13, 87–97.
- Taber K. S., (1997a), Understanding chemical bonding—the development of A level students' understanding of the concept of chemical bonding, Unpublished PhD thesis, University of Surrey.
- Taber K. S., (1997b), Student understanding of ionic bonding: molecular versus electrostatic framework? School Sci. Rev., 78(285), 85–95.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (2002), Chemical misconceptions - prevention, diagnosis and cure:Theoretical background, London: Royal Society of Chemistry, vol. 1.
- Taber K. S., (2011), Models, Molecules and Misconceptions: A Commentary on “Secondary School Students' Misconceptions of Covalent Bonding”, J. Turkish Sci. Educ., 8(1), 3–18.
- Taber K. S., (2013), Upper secondary students' understanding of the basic physical interactions in analogous atomic and solar systems, Res. Sci. Educ., 43, 1377–1406.
- Taber K. S., (in press), Asking gifted sicence learners to be creative, in Demetrikopoulos M. Pecore J. (ed.) (Forthcoming), Interplay of Creativity and Giftedness in Science, The Netherlands: Sense Publishers.
- Taber K. S. and Coll R., (2002), Bonding, in Gilbert J., De Jong O., Justi R., Treagust D. and Van Driel J. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 213–234.
- Taber K. S., Tsaparlis G. and Nakiboğlu C., (2012), Student conceptions of ionic bonding: patterns of thinking across three european contexts, Int. J. Sci. Educ., 34(18), 2843–2873.
- Tan K. D. and Treagust D. F., (1999), Evaluating students' understanding of chemical bonding, School Sci. Rev., 81, 75–83.
- Thomas G. P., (2006), Metaphor, students' conceptions of learning and teaching, and metacognition, in Aubusson P. J., Harrison A. G. and Ritchie S. M. (ed.), Metaphor and Analogy in Science Education, Dordrecht: Springer, pp. 105–117.
- Treagust D. F., (1988), The development and use of diagnostic instruments to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10, 159–169.
- Ünal S., (2003), Lycee 1 and lycee 3 studentsgh school students' understanding levels and misconceptions on, Unpublished Master thesis, Turkey: Karadeniz Technical University.
- Ünal S., (2007), A New Approach on Teaching of “Chemical Bonds and Intermolecular Forces”: The Effects of CAI and CCT on Conceptual Change, Unpublished Doctoral Dissertation, Turkey: Karadeniz Technical University.
- Ünal S., Calik M., Ayas A. and Coll R. K., (2006), A review of chemical bonding studies: Needs, aims, methods of exploring students' conceptions, general knowledge claims and students' knowledge claims and students' alternative conceptions, Res. Sci. Technol. Educ., 24, 141–172.
- Ünal S., Coştu B. and Ayas A., (2010), Secondary school students' misconceptions of covalent bonding, J. Turkish Sci. Educ., 7(2), 3–29.
- Ünal S., Özmen H., Demircioğlu G. and Ayas A., (2002), A Study for determining high school students' understanding levels and misconceptions on chemical bonds. Presented at the Fifth Conference on Science and Mathematics Education, Ankara, METU.
- Vanderstoep C. W. and Johnston D. D., (2009), Research Methods for Everyday Life: Blending Qualitative and Quantitative Approaches, San Francisco: Wiley.
- Vosniadou S., (2009), Conceptual Metaphor Meets Conceptual Change: Yes to Embodiment, No to Fragmentation, Human Dev., 52, 198–204.
- Wang C. Y. and Barrow L H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract., 14, 130–146.
- Yener Y. and Özkadif S., (2010), The suggested metaphors regarding on the concept of “cell” by teacher candidates of biology, science and primary, Procedia Soc. Behav. Sci., 2, 1107–1113.
- Yob I. M., (2003), Thinking constructively with metaphors, Stud. Philos. Educ., 22, 127–138.
- Zheng H. and Song W., (2010), Metaphor analysis in the educational discourse: A critical review, US–China Foreign Language, 8(9), 42–49.
| This journal is © The Royal Society of Chemistry 2014 |