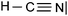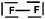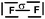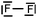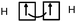Conceptual integration of covalent bond models by Algerian students
Hazzi
Salah
a and
Alain
Dumon
*b
aEcole Normale Supérieure – Chemistry, Kouba, Algeria
bESPE d'Aquitaine, Pau, France. E-mail: alain.dumon@neuf.fr
First published on 24th July 2014
Abstract
The concept of covalent bonding is characterized by an interconnected knowledge framework based on Lewis and quantum models of atoms and molecules. Several research studies have shown that students at all levels of chemistry learning find the quantum model to be one of the most difficult subjects to understand. We have tried in this paper to analyze the extent to which Algerian students, at the end of their training, have integrated the covalent bonding theories based on the quantum model of atom theory and are able to interpret Lewis structures using the quantum model. The analysis of the responses to a written questionnaire showed that this integration was not achieved by our students and that they are not able to correctly describe covalent bonds in a Lewis structure using the concepts of the quantum model. They have a “quantum box” conception of atomic or hybrid orbitals. This conception acts as a “pedagogical learning impediment” to the integration of the geometrical representations of atomic and hybrid orbitals, the conditions of their overlapping to give bonds and consequently the description of covalent bonds using the quantum model. So, the students use an alternative conceptual framework based on the use of Lewis model paired valence electrons to form covalent bonds that we have named the: “electrons pair framework”. Furthermore, the students restricted the denomination of a covalent bond to the sharing of one electron (either s or p but not spn) from each atom to give one “electron pair σ”, and thought that σ bonds are only formed in single bonds.
Introduction
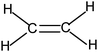
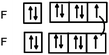
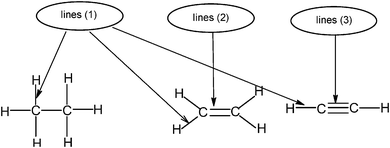
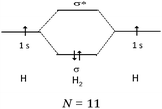
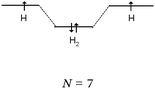
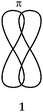
The usual representation of molecules is based on the Lewis model. The atoms, represented by their symbols, are connected by single, double or triple lines, supposed to represent the sharing of two, four or six valence electrons by the two bonded atoms. Let us consider the Lewis representation of ethene:An experienced chemist is able to interpret this representation in terms of single and double covalent bonds, six bonding electrons pairs, and to make a link between the concepts and representations of the atom and molecule quantum models (atomic or hybrid orbitals overlap to form σ and π bonds). These are abstract concepts that student have to master in order to interpret such representations. It is therefore not surprising that they encounter difficulties in understanding the concepts and symbolism used to represent molecules (Taber, 2009).
Solving problems in organic chemistry requires articulation of these different models depending on the particular facts that we want to explain or predict (molecular geometry, inductive and mesomeric effects, reactivity, etc.). Without a correct understanding of the models and the meaning of the representations which are associated with them, students will have difficulty perceiving the implications of the structural aspects of a molecule in its reactivity. It is the coordination of these multiple representations that allows students to give meaning to models and to favor the conceptual integration of knowledge.
The theoretical framework for analysis
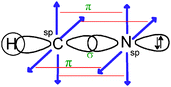
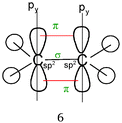
Consider again the Lewis representation of ethene. To interpret its reactivity it is necessary to consider that the two dashes (or bonding pairs) between the carbon atoms do not symbolize bonds of the same type. To differentiate it is necessary to use the “Valence Bond Theory” quantum model. The two carbon atoms are sp2 hybridized; one of the dashes symbolizes a σ bond resulting from the overlap of the axial sp2 hybrid orbitals (HO) of the carbons, the other symbolizes the π bond resulting from the lateral overlap of the p atomic orbitals (AO) of the two carbon atoms. This results in a molecule with planar geometry and angles of 120° between the links. To make this reasoning, students must be able to link the concepts and representations of both models.Generally to give meaning to complex knowledge of this type, students will, from the information that they have been taught during various teaching sessions, perform their “conceptual integration” (Taber, 2005a) by linking the different concepts involved and thus create a personal “knowledge structure” (Champagne et al., 1981; Tsai, 1998; Taber, 2005b; Nakiboglu, 2008).
One of the characteristics of science is that it produces a highly interconnected and mostly coherent knowledge framework “(Taber, 2005a; here the Lewis and quantum models). The conceptual integration is seen as the source of our ability to give meaning (Turner, 2000). It implements an integration mechanism which is based on a dynamic approach in which the partial information or apparently contradictory insights can be reformulated so that these elements can be combined into an harmonious piece of information with more value (Fauconnier and Turner, 1998). According to Taber (2005a), the requirements for conceptual integration are that “the knowledge structures of an individual are organized in such a way that there is strong linking between different ‘areas’, and that, generally speaking, there is consistency between different parts of the person's personal knowledge.” A future teacher, at the end of their university studies, should have built for themselves an efficient knowledge structure that best reflects the appropriation of the “target” knowledge set by teaching.
Several researchers have noted that the covalent bonding theories based on quantum mechanical models are amongst the most difficult subjects for students at all levels of chemistry learning to understand (Gold, 1988; Zoller, 1990; Tsaparlis, 1997, Taber, 2001, 2002a,b; Tsaparlis and Papaphotis, 2002; Nakiboglu, 2003; Papaphotis and Tsaparlis, 2008). The concepts involved are complex and too abstract for many students. In this study our research questions were: To what extent have Algerian students integrated the concepts of Molecular Orbital Theory (MOT) to describe covalent bonding? To what extent do students link Lewis structures and concepts of Valence Bond Theory (VBT) when describing single, double or triple covalent bonds in terms of σ and π bonds?
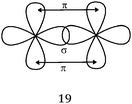
Context of study
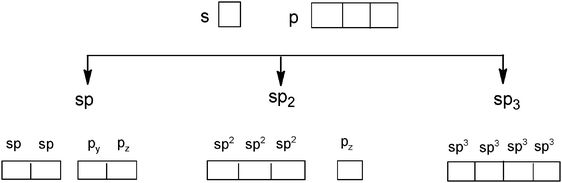
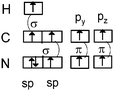
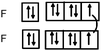
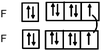
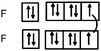
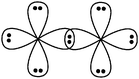
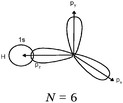
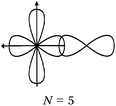
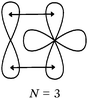
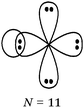
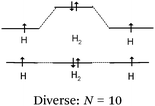
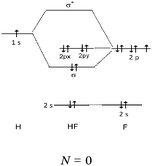
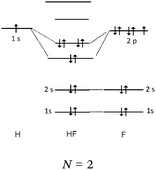
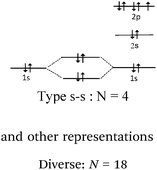
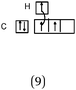
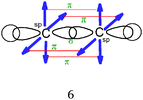
Students intending to teach physical sciences (the subjects of our study) and currently studying for physical sciences bachelor degrees at Kouba (Algeria) Superior Institute of Education (Ecole Normale Supérieure in French) are taught about covalent bonding during the first two years of their courses as a common part of the curriculum in the chapter on chemical bonding within the unit of general chemistry. Covalent bonding is first described using the Lewis model and then the quantum model theories: VBT and MOT. The axial (σ bond or MO σ bonding) or lateral (π bond or MO π bonding) overlap of hybrid or atomic orbitals leads to another interpretation of electron sharing during the formation of covalent bonds. Note that in order to give a visual representation of the combination of orbitals and electron distribution (paired or not) resulting from hybridization, a representation of the hybridized and non-hybridized orbitals in quantum boxes is used, as shown in Fig. 1.The description and representation of organic structures are then organized around the modeling of covalent bonds involving the use of different concepts related to the Lewis model (shared or lone pair, electronic deficiency, covalence, octet or duet rule, etc.) as well as that of the quantum model (s and p AO, orbital overlap, hybridization sp3, sp2 and sp, etc., see examples in Table 1). It is during their use in organic or inorganic chemistry that students are asked to link the concepts of the different models.
Previous research on the difficulties in understanding covalent bonding models based on the quantum model
The atomic orbital concept (AO)
There is much confusion regarding the understanding of atomic orbitals; the concept of the atomic orbital is often considered to be equivalent to those of an orbit, shell or sub-shell, quantum box (Cervellati and Perugini, 1981; Taber, 1997, 2002a; Nicoll, 2001; Tsaparlis and Papaphotis, 2002; Nakiboglu, 2003; Stefani and Tsaparlis, 2009) or to an energy level (Cervellati and Perugini, 1981; Taber, 2002a; Nakiboglu, 2003).Taber (2001) has suggested that learning the atomic structure in terms of electron shells may hinder learning in terms of orbitals and that the emphasis of teaching the rules of filling orbitals (s, p, d) is understood by students as being a “puzzle” that needs to be solved but does not enable the understanding of different concepts (Taber, 2002a). For example, Nakiboglu (2008) has shown that if the concept of the atomic orbital is strongly linked to that of the electron, it is weakly related to other concepts in the atom quantum model such as quantum numbers or the designation of the orbital types (s, p, d, f).
The hybridization concept
Taber (2002b) has stated that in the minds of students the formation of hybrid orbitals takes place in three steps: the starting point is the electronic configuration of the atom in its ground state, followed by seeking of a new set of orbitals (electronic configurations) that are best suited for recovery (hybridization), then they consider the formation of molecular orbitals or bonds (Papaphotis and Tsaparlis, 2008b). In a previous study we showed that almost all of the students involved in the study (Hazzi and Dumon, 2011) adopted the above reasoning to talk about hybridization. Thus hybridization was considered to be the formation of bonds between atoms by pairing of their single electrons (the lone pairs are forgotten). If the number of unpaired electrons does not allow the formation of the identified number of bonds, then a state of hybridization by reorganization/mixing or excitation of electrons has to be considered, otherwise it is not useful. Furthermore, the meaning of the hybrid orbitals symbolism (sp, sp2, sp3) was not understand. For example the carbon atom in CH4 is sp3 in conformity with 3 sH–pC bond pairs and does not have a linear combination of s orbitals with the 1, 2, or 3 p orbitals of the same atom.As has already been reported by other authors (Dumon and Sauvaitre, 1995; Taber, 2002a,b; Stefani and Tsaparlis, 2009) there is much confusion between the formation of (spn) hybrid equivalent orbitals (combined AO belonging to the same atom) and that of molecular orbitals (combined AO belonging to two different atoms) leading to the formation of bonds. However, the formation of σ and π bonds is not directly related to the hybridization state of atoms but to the nature (single, double or triple) of the bond to be formed. Moreover, as the lone pairs are not taken into account when considering a hybridization state, it is systematically associated with the number of σ bonds which can be formed: 4σ bonds to sp3, 3σ and one π for sp2, 2σ and 2π for sp.
The quantum models for covalent bonds
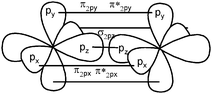
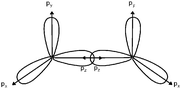
A significant proportion of students gave a rather poor definition of the molecular orbital (“a linear combination of atomic orbitals”) resulting in confusion between the mathematical modeling of the formation of MO based on approximations (LCAO method) and the orbitals themselves (Dumon and Merlin, 1988; Dumon and Sauvaitre, 1995; Tsaparlis, 1997; Taber, 2002b). Dumon and Merlin (1988) and Dumon and Sauvaitre (1995) show that the MO conditions of formation, σ MO by axial overlapping and π MO by lateral overlapping, are well known, but that the relationship between the signs of the wavefunction appearing in the lobes of the AO polar representation which overlap and the bonding or antibonding character of the MO is not achieved. Cervellati and Perugini (1981) have suggested that students tend to understand MO (or AO) as being energy levels.The understanding of what a π bond represents seems to pose some particular problems for students. Taber (2001) has reported that some students consider π bonds to be π hybridization, probably for the reason that they consider an atom to be hybridized (sp2 or sp) before the lateral overlap of unhybridized p orbitals? Other students consider one π bond to correspond to two bonds (Taber, 2001, 2002b). This can be explained by the practice of orbital representation by probability envelopes suggesting two unconnected areas of the presence probability (Taber, 1997).
Methodology
Subjects
Undergraduate students from the Kouba (Algeria) Superior Institute of Education studying for a physical sciences bachelor diploma were divided into three options (physics, chemistry and technology) depending on which branch of teaching they had chosen and the level they wished to teach (higher level or secondary school). All the students of the physical chemistry and chemistry options (intending to teach in high schools) were asked to participate in the study and gave their consent. These included the same 140 third year students (98 females and 42 males, 21 or 22 years old) who had followed the same course of physical chemistry (in the first two years at university) and organic chemistry (in the third year at university) as those involved in the study of hybridization (Hazzi and Dumon, 2011). The period between the end of the teaching of the concept of quantum models and the collection of data after teaching organic chemistry was 8 months.Collection of data
Data were collected using a paper and pencil questionnaire on the concepts taught during the common part of the curriculum and frequently used in organic chemistry. The questions were written out after dialogue between the two authors, then submitted to two other chemistry teachers to assess whether the students would be able to answer the questions in view of the teaching given. The questionnaire was then tested on a control group of 18 students (chosen from the group studied) to assess their interpretation of the meaning of the questions. The aim was to obtain the students' descriptions of the formation of single and multiple bonds using the concepts of covalent bonding theories. In order to promote the emergence of the knowledge that had been integrated by the students we decided to include two open questions (Table 2). The first question analyzed the extent to which the students were able to use MOT concepts to describe the formation of single covalent bonds in the case of simple polyatomic molecules (first research question). The second question concerned the description, using the concepts of atom and molecule quantum models, of different Lewis representations of molecules containing two carbon atoms linked by single or multiple bonds (second research question). Note that, as the students used the concept of VBT appropriately to answer this question, but not that of MOT, it might be sensible to revisit the inappropriate wording of question 2 if the work were to be repeated/developed. Students had to answer the questions during a 30 minute session. The nature of the questionnaire was made clear to the students (it was anonymous, not used for assessment, seeking personal conceptions).It should be noted that the students received teaching in Arabic and that it is this language which was used for the collection of data. The questions and the answers were translated into French then into English for this report.
Data analysis
We expected the students to describe the formation of bonds as written sentences. If the covalent bonding theories based on the quantum model of atoms had been correctly integrated, the students should have been able to write similar sentences to those detailed below.– The bond F–F is a single covalent bond. It results from an axial overlap of 2pz AO of two fluorine atoms to give a σz MO representing a bonding electron pair. Each of the two atoms provides a single electron from its outer shell (eventually: other non-bonding electron pairs correspond to lone pairs of the Lewis diagram).
– The H–F bond is a single covalent bond. It results from the axial overlap of the 1s AO of H with the 2pz AO of fluorine to generate a σ MO. Each of the two atoms provides a single electron from the outer shell (eventually: other non-bonding electron pairs represent lone pairs of the Lewis diagram; possibly: the energy levels of the 1s AO (H) and 2p (F) are neighbors, assuming that the z-axis is the interatomic axis, both AO qualify for an axial overlap and the formation of an MO).
Line 2 represents one covalent double bond. The axial overlap of two sp2 HO belonging to each of the carbon atoms leads to the formation of a σ bond representing a bonding electron pair. The lateral overlap of the two unhybridized py orbitals of each atom leads to the formation of a π bond representing a second bonding electron pair (eventually: this lateral overlap is possible only if their axes are parallel).
Line 3 represents one covalent triple bond. A σ bond is formed from the axial overlap of two sp HO of two carbon atoms, each containing one electron. Both π bonds are formed from the lateral overlap of the unhybridized px and py AO present on each hybridized carbon which have parallel axes (eventually: their symmetry planes are orthogonal and intersect at the bond axis).
– for written sentences, identification of key words appearing in descriptions. These key words were those included in the above-mentioned expected sentences or were other significant words. Note that key words (some words, Greek symbols) could also appear in other short sentences included in the various representations;
– for QB, AOO and EL representations, identification of similar diagrams appearing in descriptions.
In a few cases there was disagreement on the categorization, therefore, discussions took place to arrive at a common categorization. An answer was considered to be correct or acceptable if it conformed to the information that had been taught. In the Discussion section the analysis of the data is compared with the same type of data obtained for other topics (hybridization and drawing overlapping orbitals) and presented in the thesis work of Hazzi (2012).
Results
Students' description of the single bonds formation (Question 1)
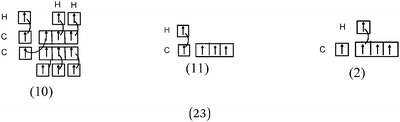
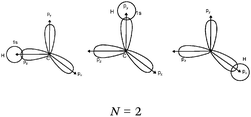
119 students (85%) responded to this question. The other students gave no answers or gave answers that were difficult to interpret. To describe the formation of single bonds, the students used various representations and, 40 students (28%) also included a Lewis Representation (LR) of the molecules. In the description given by one student multiple types of representations were used (see example in Table 3).We report in Table 4 the total number of different categories of description identified in all of the students' answers and, in italics, the number of other presentations with which they are associated.
| Categorization | WS | QB | AOO | EL | LR | NR + others |
|---|---|---|---|---|---|---|
| Number of presentations | 14 (with 10 QB, 4 AOO, 8 LR) | 72 (with 10 WS, 14 AAO, 8 EL, 29 LR) | 26 (with 4 WS, 14 QB, 4 EL, 3 LR) | 32 (with 8 QB, 4 AOO, 6 RL) | 40 | 21 |
| Total % of students | 10 | 51 | 19 | 24 | 28 | 15 |
In Table 5 are summarized the frequencies and percentages of keywords found in all the written bond descriptions given by the 119 students who responded.
| Keywords | H2 | F2 | HF | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Covalent | 79 | 66 | 85 | 71 | 21 | 18 | |
| σ | 54 | 45 | 53 | 45 | 40 | 34 | |
| Overlap (s–s) and axial overlap (p–p and s–p) | 37 | 31 | 42 | 35 | 32 | 27 | |
| MO | 2 | 2 | 2 | 2 | 2 | 2 | |
| Other keywords | Single | 13 | 11 | 15 | 13 | 0 | 0 |
| Bonding electron pair | 18 | 15 | 15 | 13 | 9 | 8 | |
| Sharing a pair of e− | 23 | 19 | 28 | 24 | 0 | 0 | |
| Duet/octet | 18 | 15 | 40 | 34 | 4 | 3 | |
| Hydrogen bond | 7 | 6 | 0 | 0 | 3 | 3 | |
| Non-covalent | 0 | 0 | 0 | 0 | 18 | 15 | |
The first finding was that when students were asked to describe the formation of bonds in terms of MO, nearly all (98%) of the descriptions did not refer to them. Considering the number of occurrences of keywords it was possible to simulate the description of bonds made by the students. For H2 and F2 molecules the bonds between atoms were mainly (69% of the students) stated to be covalent, 45% of the students stated that they were of σ-type, and about 32% of the students stated that they were obtained by overlapping of the s orbitals of the hydrogen atoms or by axial overlapping of the p orbitals of fluorine atoms. In contrast, in the case of HF, 34% of the students stated that the bond was of σ-type and 27% of the students stated that the bonds occured by axial overlapping of the s orbital of the hydrogen atom and the p orbital of the fluorine atom. The bonding was considered by 18% of the students to be covalent and by 15% of the students to be non-covalent due to the difference in electronegativity between the two atoms constituting the molecule.
According to the keywords in the expected sentence description we noted that:
– The term single bond is not often mentioned (by about 12% of students);
– The nature of the AO involved and the symmetry of their overlapping is not always described.
The reference to the Lewis model (bonding electron pair, sharing a pair of electrons; duet or octet rules) was present in some written forms (24% of the keywords on average), principally for H2 and F2, associated with QB or AOO: for example, “In hydrogen bond, each H participates in the formation of the σ bond by an electron so as to respect the duet rule”; “F–F bond: each fluorine participates with an unpaired electron to form a σ bond respecting the octet rule”; “Axial overlap of two fluorine AO pzto form one bonding electron pair representing a covalent bond σ”. These sentences show that these students have constructed a hybrid model (quantum and Lewis).
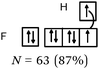
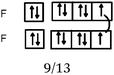
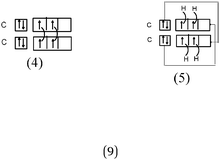
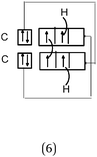
Bond H–H. In all of the representations we noted the connection between the H QB by a curved line (or arrow). Furthermore, the majority of the students mentioned the participation of one electron from each hydrogen atom in the bond formation. In 29% of the cases the bonding pair was not mentioned, but was shown in the accompanying Lewis structure representation in the QB. Finally, in the sentences the bond was called “σ covalent because it is s–s type.”
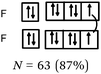
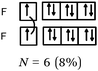
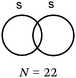
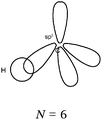
Bonds F–F and H–F. In the case of the F2 molecule representations in QB, 87% of the students represented pairing between two p electrons by using a curved line (or arrow) to connect the two unpaired p boxes. The participation of an electron from each atom in the bond formation was often given in parallel to this diagram. However, the valence shell of the fluorine atom was misrepresented by some, as it was represented schematically by only 3p AO. This suggests that they did not take into account the sub s shell, and this representation only showed two lone pairs for fluorine.
Some (8%) of the students called the bond ‘σ covalent type s–s’ as for H2. To represent it schematically, they presented the shift of an electron from the sub s shell to the sub p shell by using arrows in such a way as to present a single electron in the s box and a full sub p shell (some mentioned ‘sp hybridization’). Two s electrons of the two fluorine atoms were then paired. Thus, the term covalent seems to be qualified as one (s–s) type bond as in H2.
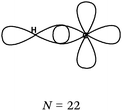
Bond H–H. This was predominantly (22 out of 26 students) represented by the participation of two s AO, schematically represented more or less correctly with spherical symmetry. But the four other schematic representations of the σ bond involved the overlap, either axial or lateral, of two s AO with axial symmetry (p-type).
Bonds F–F and H–F. In the case of H–F, the s orbitals were mostly represented with spherical symmetry, none of the students represented the p orbitals correctly. They were represented either as three half orthogonal atomic orbitals, or in most cases, as four identical pseudo orbitals arranged in a plane and in an orthogonal manner.
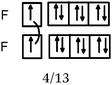
In the case of the F–F bond, in 13 representations out of 21, these orbitals were accompanied by two types of representations in the QB:
As can be from example 4 in Table 3, these representations were generally accompanied by a written commentary making reference to the Lewis model. So, we considered that the purpose of these representations was to show that, in accordance with the Lewis representation, the overlapping of two orbitals leads to a bonding electron pair and that each of the remaining orbitals contains lone pairs. There were also eleven such representations in the case of the H–F bond. Although some students mentioned the overlapping of pz orbitals (see Table 3, example 4), it seems that for these students the four “pseudo orbitals” represented in fact the four QB.
The F–F bond is a σ bond and the axial overlapping of pz/pz between the two fluorine atoms was represented in the majority of cases. In other cases, the description seemed to be schematically represented by axial overlapping and lateral overlapping of the two p AO (or 4 pseudo orbitals?), the third was forgotten.
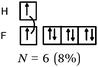
The H–F bond is a σ bond. In some representations (N = 6), the axial s/pz overlapping between the H and F atoms was mentioned. In eight other representations s AO have an axial symmetry and fluorine only represented by two orthogonal p AO (or 4 pseudo orbitals?). As in the previous case the overlapping between the orbitals was either axial or lateral.
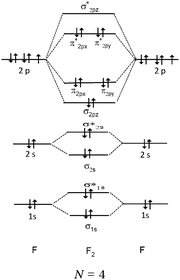
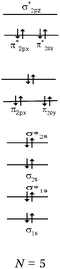
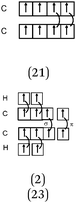
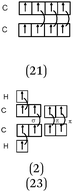
Descriptions in the form of energy level correlation diagrams (EL). Among the 32 students using such descriptions (24%), 28 represented the energy diagram of the H2 molecule, 26 that of F2 and 20 that of HF. Seven other students gave only the electronic structure of the molecules. Examples of different kinds of typical representations are summarized in Table 8.
In the case of the H2 molecule, 11 out of the 28 diagrams were represented correctly. For seven representations, the energy level of the bonding MO was correctly positioned but the antibonding MO was not represented. We considered this representation to be partially correct. In contrast, 10 of the representations were incorrect either because the energy level of the bonding MO was the same as that of the AO (so there was no stabilization by overlap of the AO) or it was superior to it (thus destabilizing the system). The principle of forming a bonding σ MO resulting in stabilizing nucleus–electrons attraction was far from being understood.
There were, for F2, a few correct representations (N = 4) of the type listed in the table. We noted a second type of representation (N = 5) which was not totally correct because the energy level of the bonding σz MO was not correctly positioned. This may be due to an analogy made by students to the N2 diagram for which there is an inversion of the energy level of the bonding σz MO and that of the πx and πy MO. These representations were considered to be partially correct. In contrast, for the 17 other representations, the relative positioning of the AO and MO energy levels were totally unclear.
In the case of HF none of the representations were correct. In two of the representations, overlapping of the hydrogen 1s AO with that of the fluorine 2p AO was considered. However, the electron pairs seemed to be placed on the p AO of fluorine which would be stabilized from an energetic point of view. Another type of representation only involved the s AO of the two atoms for the formation of MO. This confirmed the idea already mentioned that the bonds involving the fluorine atom were of “s–s” type.
Representations only in the form of electronic structures of molecules given by seven students were satisfactory only for H2 (1σ)2. In the case where fluorine was involved, the representations were incorrect; for example for F2, 1σ2 1σ*2, 2σ2 2σ*2, 2πx2 2πx*2 (πy2 πy*2, πz1 πz*1) instead of (2σ)2 (2σ*)2 (3σ)2 (or σ2pz2) (πx)2 (πy)2 (πx*)2 (πy*)2. We note that students included the electrons of the internal shell which were not involved in the formation of bonds.
Several difficulties were noted in the understanding of the relationship between the σ bond formation and the energy levels associated with the formation of an MO. The positioning of the energy level of MO assumed to be bonding, unchanged or superior to that of AO's that overlap, shows that the principle of stabilization (bonding MO) or destabilization (antibonding MO) of the system is not taken into account. Also, for molecules containing fluorine, there was generally inconsistent positioning of the energy levels related to the AO and MO, and a lack of association of the lone pairs of the Lewis representation with those present on the σ, σ* and π, π* MO, generally non-bonding (in the case of F2), or unchanged 2s and 2p AO of fluorine (in the case of HF).
Description of the formation of bonds corresponding to lines 1, 2 and 3 (Question 2)
For this question, the non-response rate was high (34%). Out of the 93 students who responded, 55 (59%) gave a description in the form of QB and 38 (41%) represented their answer schematically in the form of AOO. These representations were sometimes accompanied by written sentences.| Keywords | C–H | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C C |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Covalent | 21 | 23 | 0 | 0 | 0 | 0 | |
| σ | 14 | 15 | 0 | 0 | 0 | 0 | |
| σ + nπ | 0 | 0 | 10 | 11 | 13 | 14 | |
| Axial overlap | 25 | 27 | 44 | 47 | 46 | 49 | |
| Whose axial overlap (p–p or s–p) | (25) | (27) | (27) | (29) | (32) | (34) | |
| Lateral overlap (p–p) | 0 | 0 | 23 | 25 | 44 | 47 | |
| Other keywords | Single | 05 | 5 | 0 | 0 | 0 | 0 |
| Sharing a pair of e− | 02 | 2 | 0 | 0 | 0 | 0 | |
| Hydrogen bond | 06 | 6 | 0 | 0 | 0 | 0 | |
Simulated descriptions were written based on the percentage of keywords used by 93 students, and are as follows:
– In ethane, the C–H bond is covalent (23%), obtained by axial overlap (s–p) of the s orbital of H with the p orbital of C (27%), of σ type (15%). In contrast, this bond was not described in the cases of ethylene and acetylene.
– In the description of multiple bonds, the term ‘covalent’ did not appear. Line 2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) was obtained by an axial overlap (47%) of type p–p or s–p (29%) and a lateral overlap between two p orbitals (25%) leading to a (σ + π) type bond (11%). Line 3 (C
C) was obtained by an axial overlap (47%) of type p–p or s–p (29%) and a lateral overlap between two p orbitals (25%) leading to a (σ + π) type bond (11%). Line 3 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) resulted from the axial overlap (49%) of type p–p or s–p (34%) and the lateral overlap of two p AO (47%). It corresponds to a (σ + 2π) type bond (14%).
C) resulted from the axial overlap (49%) of type p–p or s–p (34%) and the lateral overlap of two p AO (47%). It corresponds to a (σ + 2π) type bond (14%).
According to the key words in expected descriptions we noted that:
– When the term single bond was not mentioned often for the C–H bond (by approximately 5% of students), there was no mention of double and triple bonds.
– If reference was made to the symmetry of the axial overlap leading to the formation of σ bonds in the case of multiple bonds, the nature of the AO (s/spn) and (spn/spn) involved was not cited by the students.
– For the category we called “hybridization”, the redistribution of electrons corresponding to different hybridization states of carbon was most often described in the form of 4 equivalent QB, which according to the information taught was only correct for the sp3 state (N = 23). It seems that for these students, the priority, in the all cases, was to find the four unpaired electrons necessary for the formation of four bonds. However, two students gave a representation in QB conforming to the information taught: 3 equivalent boxes for sp2 and a p unhybridized box, two equivalent boxes for sp and two unhybridized p boxes.
– A category referred to as “carbon excitement s–p”, where the hybridization state corresponds to the transfer or the excitation of an electron from an s subshell to a p subshell in such a way as to obtain a number of unpaired electrons in accordance with the tetravalence of the carbon (N = 23 for ethane or ethylene and 26 for acetylene).
– A category referred to as the “initial C structure”, for which the representation of the carbon electronic structure was not modified, because it possesses two unpaired electrons (N = 9 then 6).
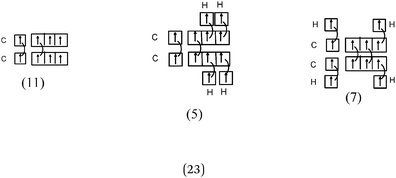
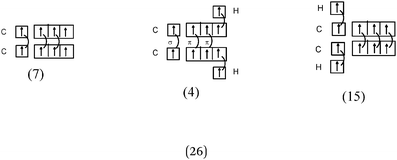
C–H bond (line 1) description. Regarding the “hybridization” category, the C–H bond was represented schematically by all only in the case of ethane, whereas carbon–carbon bonds were shown by all in the ethylene and acetylene representations.
For ethylene and acetylene, the formation of C–H bonds by the overlap of a carbon sp2 or sp HO with an s AO of hydrogen was shown schematically only by the two students who gave a correct representation. It may be assumed that for other molecules, such as ethane, the C–H bonds were obtained by sharing a 1s electron of hydrogen with one electron from an unused “hybrid orbital”.
In the case of carbon “excitement” of s–p category, the C–H bonds were represented for the three molecules as resulting from the pairing of a hydrogen 1s electron with either one 2s electron of carbon (type s/s: 7 occurrences), or with one 2p electron of carbon (type s/p: 12 occurrences). The same was true for the single C–C bond resulting from the sharing of one 2s electron of carbon with one 2p electron from the other carbon (10 occurrences).
Finally, when the representation in the form of QB of the carbon initial electronic structure was kept, the C–H bond was represented by combining the 1s electron of hydrogen with one unpaired electron in the 2p subshell of carbon. The carbon was not hybridized as having unpaired electrons, and for these students, hybridization “is the fusion between the s and p AO to give a σ bond.” It is worth noting that in the case of ethane such a representation does not allow for the interpretation of the carbon tetravalence.
Multiple bonds carbon–carbon (lines 2 and 3) description. Among the 23 “hybridization” category representations, only two explicitly indicated the formation of a σ bond between two carbon atoms by the overlap of HO (sp2 or sp) and one or two π bonds by the overlap of unhybridized p AO. The other students represented the formation of double and triple bonds using descriptions in the QB of the sp3 hybridization state in the form of four equivalent AO, each containing an unpaired electron. From there they built bonds by pairing the electrons of the two carbon atoms; 2 electrons of each of the carbon atoms were combined to form a C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and 3 to form a triple bond C
C double bond and 3 to form a triple bond C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C. This reflects a lack of mastery of the hybridization concept and its representation in QB and a failure to take into account the overlapping conditions for the formation of σ and π bonds.
C. This reflects a lack of mastery of the hybridization concept and its representation in QB and a failure to take into account the overlapping conditions for the formation of σ and π bonds.
For 23 students (26 in the case of acetylene), hybridization was summed up as the excitation of an electron in an s subshell to a p subshell, without distinction between the different possible states. For the excited state of carbon, 16 of the students for the case of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and 11 students for the case of C
C, and 11 students for the case of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, describe the formation of a bond of this kind as “s/s” linking of the 2s boxes of two carbon atoms and bonds of the kind px/px (case C
C, describe the formation of a bond of this kind as “s/s” linking of the 2s boxes of two carbon atoms and bonds of the kind px/px (case C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and py/py (case C
C) and py/py (case C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) by connecting the unpaired electrons of the two-carbon atoms p boxes. Although this appears explicitly in just a few schematic representations, in the written form (σ + π) for C
C) by connecting the unpaired electrons of the two-carbon atoms p boxes. Although this appears explicitly in just a few schematic representations, in the written form (σ + π) for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and (σ + 2π) for C
C and (σ + 2π) for C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, we can assume that for these students, the s/s type bond corresponds to a σ bond and p/p type bonds to π bonds. The other 7 students (for C
C, we can assume that for these students, the s/s type bond corresponds to a σ bond and p/p type bonds to π bonds. The other 7 students (for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) considered that multiple bonds were obtained only by the sharing of electrons in the p subshells. The pairing between 1s (H) electrons and 2s (C) was restricted to the formation of a C–H bond. In the case of acetylene, presumably because the number of unpaired p electrons enabled the interpretation of the formation of a triple bond, 16 students adopted this point of view. Here too there was a misinterpretation of the overlapping symmetry conditions (axial, lateral) leading to the formation of a σ or π bond.
C) considered that multiple bonds were obtained only by the sharing of electrons in the p subshells. The pairing between 1s (H) electrons and 2s (C) was restricted to the formation of a C–H bond. In the case of acetylene, presumably because the number of unpaired p electrons enabled the interpretation of the formation of a triple bond, 16 students adopted this point of view. Here too there was a misinterpretation of the overlapping symmetry conditions (axial, lateral) leading to the formation of a σ or π bond.
Some students (9 for ethylene and 6 for acetylene) considered that carbon does not require to be hybridized. They started with the electronic configuration of the two carbon atoms in their ground state. The schematic representation of bond formation was subsequently achieved by using two methods: either connection of the unpaired px/px and py/py electron boxes of the two carbons by a curved line (4 students in the case of ethylene) to form the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, forgetting the C–H bonds or involvement of dative bonds, the s pairs of one carbon were considered to be a donor while the vacant pz AO of the other carbon atom was considered to be an acceptor (5 for ethylene and 6 for acetylene). In this case the C–H bonds were represented by pairing the 1s electron of hydrogen with one 2p electron of carbon. The third bond for acetylene resulted from the sharing of the two p electrons of carbon.
C double bond, forgetting the C–H bonds or involvement of dative bonds, the s pairs of one carbon were considered to be a donor while the vacant pz AO of the other carbon atom was considered to be an acceptor (5 for ethylene and 6 for acetylene). In this case the C–H bonds were represented by pairing the 1s electron of hydrogen with one 2p electron of carbon. The third bond for acetylene resulted from the sharing of the two p electrons of carbon.
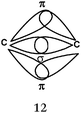
C–H bond (line 1). The description of the C–H bond appears in all of the representations given for ethane (Table 11) and only in the six correct representations which explicitly mention the overlap between C HO and H AO for ethylene and acetylene (see Table 12).
In the case of ethane, only six students correctly represented the overlap of the sp3 HO of C and the s AO of H.
In the category of representation chosen by 22 students, we found that the representation identified in question 1 of 4, identical “pseudo orbitals” were arranged in a plane and were orthogonal. It was not clear whether they were supposed to represent four equivalent carbon “hybrid” sp3, two orthogonal p AO or the four QB? The C–H bond can result from the overlap of any of these orbitals (or p AO 1/2 lobe) with the s AO of hydrogen. It was noted that the s orbital was represented as a p orbital with axial symmetry.
For five students, it seemed that it was the axial overlap of a 1s AO with a p AO that lead to the formation of a σ bond. The sp3 hybridization of the carbon was not taken into account. This was also the case for both of the students who represented the formation of σ bonds (C–H) by an axial overlap of the hydrogen s AO with the px, py and pz AO of carbon. It was noticed that, as in the case of fluorine, the p orthogonal orbitals were represented by only half a lobe and the carbon was schematically represented by three atomic orbitals (the s subshell was omitted in the representation.) And therefore the fourth bond was ignored.
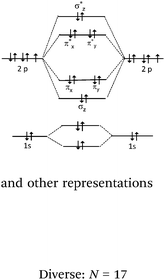
Multiple bonds carbon–carbon (lines 2 and 3) description. In 6 of the 38 representations given by the students, overlapping was clearly identified (hybrid carbon sp2 or sp HO and unhybridized p AO), correctly represented, and the axial overlapping of HO leading to the formation of a σ bond and lateral unhybridized p AO to form π bonds was explicitly reported (Table 12).
We found that the representation category identified the C–H and F–F bonds where the 4 equivalent AO of carbon were shown as being orthogonal in a plane. Multiple bonds resulting from the formation of a σ bond by axial overlapping of two pseudo orbitals and of one or two π bonds by lateral overlapping of two half lobes was used to represent p AO. It seemed that for these students the nature of AO which overlap to form a covalent bond is of little importance (they can be hybrids as well as p or s). What seems important is that the axial or lateral overlapping of two of these orbitals leads to the pairing of the two electrons required for the formation of bonds (σ + π) and (σ + 2π), each of the remaining orbitals contain a single electron which can be paired with the s electron of hydrogen to form a C–H bond. For ethylene, there results in the incorrect representation of the angles between the σ bonds and therefore of the molecular geometry.
In other representations (14 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and 12 of C
C and 12 of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) the axial overlapping leading to the formation of σ bonds, and the lateral overlapping required for the formation of π bonds were shown schematically, but with disregard to the parallelism of the symmetry axes of p AO. Finally, some representations only described the lateral overlapping of two p orbitals for π bond formation. One may wonder whether these students have confused multiple bonds with π bonds or whether this was simply due to a misunderstanding of the question asked. If in the case of the double bond the limitation of the schematic representation to only one bond may be explained (line 2 considered as the π bond), it is not the same thing for the triple bond which requires the formation of two π bonds.
C) the axial overlapping leading to the formation of σ bonds, and the lateral overlapping required for the formation of π bonds were shown schematically, but with disregard to the parallelism of the symmetry axes of p AO. Finally, some representations only described the lateral overlapping of two p orbitals for π bond formation. One may wonder whether these students have confused multiple bonds with π bonds or whether this was simply due to a misunderstanding of the question asked. If in the case of the double bond the limitation of the schematic representation to only one bond may be explained (line 2 considered as the π bond), it is not the same thing for the triple bond which requires the formation of two π bonds.
Discussion
Integration of concepts of atomic or hybrid orbitals
We noted an unsatisfactory integration of what an orbital represents. The majority of QB representations used by students reflected a “quantum box” conception of AO or HO. This conception has already been highlighted by other authors (Cervellati and Perugini, 1981, Taber, 1997, 2002a; Nicoll, 2001; Tsaparlis and Papaphotis, 2002; Nakiboglu, 2003; Stefani and Tsaparlis, 2009). Concerning the HO of carbon, we found again the conception that hybridization is an operation that results in a new electronic configuration for the carbon atom (transfer/excitation of an electron from an s subshell to a p subshell;Dumon and Sauvaitre, 1995; Taber, 2002b; Nakiboglu, 2003; Stefani and Tsaparlis, 2009, Hazzi and Dumon, 2011) leading to a number of unpaired electrons in agreement with the tetravalence of carbon in order to form σ and π bonds (Hazzi and Dumon, 2011).The s orbital was predominantly represented with spherical symmetry, several students represented s AO with axial symmetry (p-type). A correct representation of the p orbitals was rarely achieved. The few representations of three 1/2 orthogonal orbitals demonstrated the misconception that a p AO has two lobes of opposite signs oriented with axial symmetry. More often, students drew representations of two orthogonal p OA (the third having been forgotten) or a representation in the form of four identical pseudo orbitals arranged in a plane in an orthogonal manner. This same representation was found predominantly to represent four hybrid sp3 AO conforming to the tetravalence of carbon. As this type of representation is often associated with a representation in QB (see Table 3 and analysis of the F–F bond description in the form of AOO), we hypothesized that this representation was connected to the “quantum box” conception of AO.
Integration of molecular orbital concept
To describe single covalent bonds in the case of simple polyatomic molecules, students didn’t use the MO concept. They only remembered that a σ bond involves the pairing of and/or p electrons, and result from an axial overlap of AO.In the case of QB representations, this pairing was illustrated by curved lines linking the QB for the s or/and p of the two bonded atoms. In the descriptions of bonds represented schematically in the form of overlapping AO, it seemed that for many students the overlapping of two atomic or “pseudo-orbitals” leads to the pairing of two electrons (see examples of representations in Tables 3 and 7). We deduced that these students thought that the two electrons required for the formation bonds are localized in AO. This is close to the observation made by Taber (2005b) in another context: “for students the bonding electrons are in atomic orbitals rather than molecular orbitals”.
The use of energy level correlation diagrams in place of MO descriptions by some students provided evidence that, as has been previously reported by Cervellati and Perugini (1981), students have a tendency to associate an MO (or AO) with an energy level. This is probably a results of the fact that teaching gives too much importance to correlation diagrams of energy levels and their utilization (Dumon and Sauvaitre, 1995). Moreover, the incorrect information presented for the relative positioning of AO and MO energy levels in energy level correlation diagrams shows that the stabilization (bonding MO) or destabilization (antibonding MO) principles of the system are not considered.
Connection between Lewis model and concepts of covalent bonding theory VBT
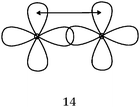
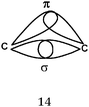
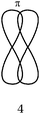
When asking students to describe the various bonds figuring in Lewis representations, what seems important for us is to show, in accordance with the Lewis representation, the formation of electron pairs by using QB representations of orbitals (s, p or “pseudo hybrid”) or representations of AO overlap.In the case of σ bonds, when the nature of the orbitals was specified, only s or p orbitals were mentioned, even in the case of multiple bonds where overlapping of type s/spn or spn/spn were only mentioned by very few students. Regarding the formation of π bonds, the lateral overlapping of orbitals was simply seen as the overlapping of two “half-orbitals” (they can be p or “pseudo-orbital”) regardless of their orientation, It should be noted that similar behavior was noted when another population of students was explicitly asked to schematize the Lewis structures in the form of orbital overlapping (Hazzi, 2012). Students don't seem to know that the overlapping symmetry conditions lead to the formation of bonds. As pointed out by Taber (2001, 2002b) they have not integrated that the π bond is represented by two lobes, separated by a nodal plane, resulting from the overlap of two p orbitals of axial symmetry whose axes are parallel.
So, it seems that for these students the nature of orbitals which overlap to form the various covalent bonds is not important (they can be hybrids, p, s or “pseudo orbital). What seems important, as has already been highlighted in a previous study on hybridization (Hazzi and Dumon, 2011), is that the axial or lateral overlapping of two orbitals leads to the pairing of the two electrons required for the formation of bonds. Thus we can hypothesize that the students have not integrated the covalent bond models but have used a hybrid model composed of a mixture of quantum and Lewis models.
It should be noted that the term single bond was not used often and only to describe the bond between two identical atoms. For many students, this bond was thought to be a σ bond obtained by the axial overlapping of orbitals, the nature of the orbitals was not always specified and the formation of a σ bond was thought to occur only in the case of single bonds.
Integration of covalent bond concept
We found that the students had the misconception that a covalent bond is restricted to the sharing of an electron from each atom to give a bonding σ pair as in the case of H–H, F–F and C–H bonds. It is a misconception already highlighted, in previous studies by Cokelez and Dumon (2005) with high school students and Hazzi and Dumon (2011) with university students, concerning the description of lines 1, 2 and 3 using the Lewis model. In addition, some students, termed covalent bonds as “s–s type”. Double and triple dashes were in no way associated with the term covalent, nor with two or three bonds of different natures and characteristics. They were simply termed (σ + π) or (σ + 2π) bonds.Conclusion
It has been shown that the concepts of AO and HO were not integrated by many of the students studied, as demonstrated by the fact that the spherical symmetry of the s AO was not considered by some students, that the two lobes of opposite signs of the p AO seemed to be absent from the minds of the students, that some have associated with QB, whatever their nature, identical orbitals. Furthermore, the AOO representations showed poor control of the linking of the symbolization of orbitals (s, p or hybrid spn) to their directional aspect (Zoller, 1990).Concerning the conceptual integration of models of covalent bonds, this study shows that our students have neither integrated the MO concept nor established the correct connection between the Lewis model and covalent bonding theories (VBT and MOT). They have only acquired a small amount of knowledge (Taber, 2005b) and have not linked the information that they have learned, as a consequence they have become mixed up between the use of Lewis models and quantum models.
In the absence of the integration of the concepts of AO and HO, they have used an alternative knowledge structure, a “quantum box” conception of orbitals to describe covalent bonds. We can consider that this knowledge structure results from the idea of sharing a pair of electrons in the Lewis model of a covalent bond. So, students search to form electron pairs, it doesn't matter much where the electrons come from to form this pair. This is an alternative conceptual framework that in our study relative to hybridization (Hazzi and Dumon, 2011) we have named: the “electron pair framework”.
Following on from the “octet framework” (Taber, 1999) we can consider that this framework is a “pedagogical learning impediment” (Taber, 2005b) in the sense that this idea represents a cognitive structure derived from the visual representation of the atomic electronic structure in each HO and AO in QB used in Algerian teaching. The linear combination of AO to form MO, the overlapping of orbitals to form MO or σ and π bonds, the symmetry conditions of overlap are not presented in this representation; it merely promotes the idea of the formation of electron pairs to make bonds.
To improve the conceptual integration of covalent bonding theories (MOT and VBT) based on the quantum model of atom theory, it is necessary at first not to use in teaching the representation of AO or HO in QB as this only promotes the development of the “quantum box” conception of orbitals. Furthermore, using this form of representation the sharing of two valence electrons represents the formation of a covalent bond, which results in the development of the “electron pair framework”. The focus should be on explaining the meaning of models by providing clear explanations of the nature of the concepts involved. On the other hand, in order to prevent students from falling into the mechanical and superficial learning of models, students should be encouraged to think about the links between concepts and proceed at once to distinguish between different models.
For example:
– by making a clear differentiation between the overlap of two atomic orbitals which leads to the formation of σ or π MO, bonding and antibonding (MOT), and overlap of an HO with another HO or AO to form a σ or π bond depending upon the type of orbital (VBT);
– by explaining the relationships between the shared and lone electron pairs in a Lewis model with the occupation of the MO energy level by electron pairs. For example, in the case of H–F, the electron pair of the bonding σ MO can be associated with the shared electron pair and the three electron pairs of the 2s, 2px and 2py non-bonding orbitals with the three lone pairs surrounding the F atom.
– by insisting on detailing the symmetry of the overlap of orbitals to form MO or bonds.
In addition, it would be interesting to use a web-based learning environment combined with cooperative learning to enhance the conceptual integration of covalent bonding theories, as was done by Frailich et al. (2007) to enhance the concept of chemical bonding.
Finally, to complete this study, we envisage submitting the questionnaire to another population of students and to interview some of them using a semi-structured interview to confirm our hypothesis on students' knowledge structures.
References
- Cervellati R. and Perugini D., (1981), The understanding of the atomic orbital concept by Italian high school students, J. Chem. Educ., 58, 568–569.
- Champagne A. B., Klopfer L. E., Desena A. and Squires D. A., (1981), Structural representations of student's knowledge before and after science instruction, J. Res. Sci. Teach., 18, 97–111.
- Cokelez A. and Dumon A., (2005), La liaison chimique: du savoir de référence au savoir appris au lycée, BUP, 99, 1011–1023.
- Dumon A. and Merlin A., (1988), Difficulties with molecular orbitals, Educ. Chem., 25, 49–52.
- Dumon A. and Sauvaitre H., (1995), Comment les étudiants s'approprient-ils le modèle quantique de la liaison chimique? Actual. Chim., 4, 13–22.
- Fauconnier G. and Turner M., (1998), Conceptual integration networks, Cognit. Sci., 22, 133–187.
- Frailich M., Kesner M. and Hostein A., (2007), The influence of web-based chemistry learning on students' perceptions, attitudes and achievements, Res. Sci. Technol. Educ., 25, 179–197.
- Gold M., (1988), Chemical education: an obsession with content, J. Chem. Educ., 65, 780–781.
- Hazzi S., (2012), Study of conceptual integration difficulties of knowledge concerning the covalent bond modeling in organic compounds by Algerian students intending to teach physical sciences, Unpublished PhD thesis (in French), Kouba, Algeria: Superior Institute of Education.
- Hazzi S. and Dumon A., (2011), Conceptual integration of hybridization by Algerian students intending to teach physical sciences, Chem. Educ. Res. Pract., 12, 443–453.
- Nakiboglu C., (2003), Instructional misconceptions of Turkish prospective chemistry teachers about atomic orbitals and hybridization, Chem. Educ. Res. Pract., 4, 171–188.
- Nakiboglu C., (2008), Using word associations for assessing non major science students' knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9, 309–322.
- Nicoll G. J., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23, 707–730.
- Papaphotis G. and Tsaparlis G., (2008), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts, Part 1: Statistical analysis of a quantitative study, Chem. Educ. Res. Pract., 9, 323–331.
- Stefani C. and Tsaparlis G., (2009), Students' levels of explanation, models and misconceptions in basic quantum chemistry: a phenomenographic study, J. Res. Sci. Teach., 46, 520–536.
- Taber K. S., (1997), Understanding chemical bonding, Unpublished PhD thesis, Faculty of Education, Roehampton Institute University of Surrey.
- Taber K. S., (1999), Alternative frameworks in chemistry, Educ. Chem., 36, 135–137.
- Taber K. S., (2001), Building the structural concepts of chemistry: some consideration from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2002a), Conceptualizing quanta: illuminating the ground state of student understanding of atomic orbitals, Chem. Educ. Res. Pract., 3, 145–158.
- Taber K. S., (2002b), Compounding quanta: probing the frontiers of student understanding of molecular orbitals, Chem. Educ. Res. Pract., 3, 159–173.
- Taber K. S., (2005a), Conceptual integration and science learners – do we expect too much? Invited seminar paper presented at the Centre for Studies in Science and Mathematics Education, University of Leeds.
- Taber K. S., (2005b), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89, 94–116.
- Taber K. S., (2009), Learning at the symbolic level, in Gilbert J. K., Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, New York: Springer Verlag, pp. 75–105.
- Tsai C. C., (1998), An analysis of Taiwanese eight graders' science achievement, scientific epistemological beliefs and cognitive structure outcomes after learning basic atomic theory, Int. J. Sci. Educ., 20, 413–425.
- Tsaparlis G., (1997), Atomic orbitals, molecular orbitals and related concepts: conceptual difficulties among chemistry students, Res. Sci. Educ., 27, 271–287.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students? Chem. Educ. Res. Pract., 3, 129–144.
- Turner M., (2000), La perspicacité et la mémoire. Conférence lue au Collège de France, à Paris. Disp. sur: http://www.inform.umd.edu/EdRes/Colleges/ARHU/Depts/English/englfac/MTurner/cdf/cdf3.html.
- Zoller U., (1990), Students' misunderstandings and misconceptions in college freshman chemistry (General and Organic), J. Res. Sci. Teach., 27, 883–903.
| This journal is © The Royal Society of Chemistry 2014 |